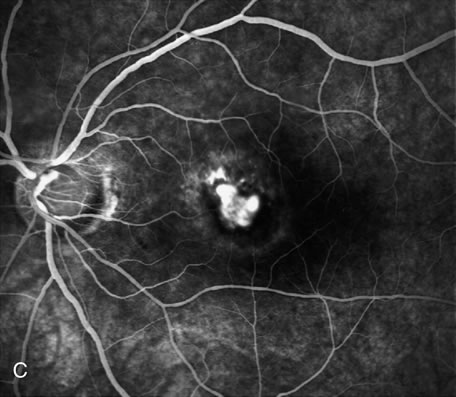
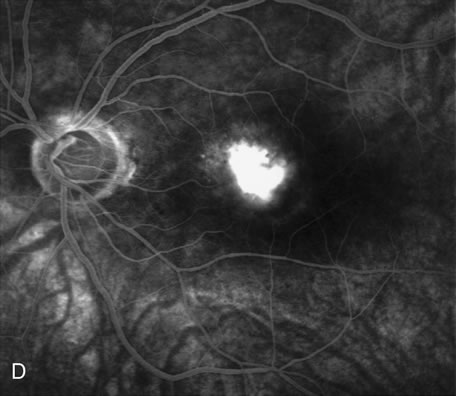
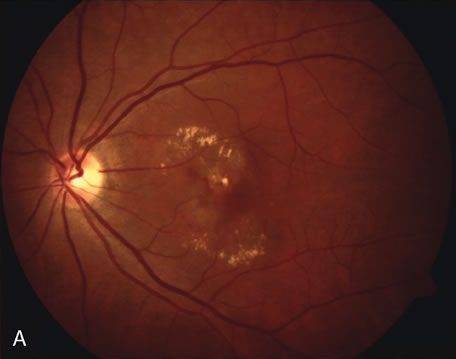
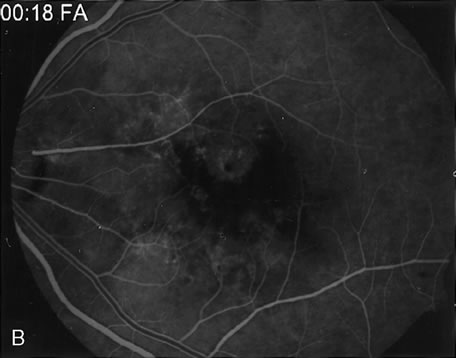
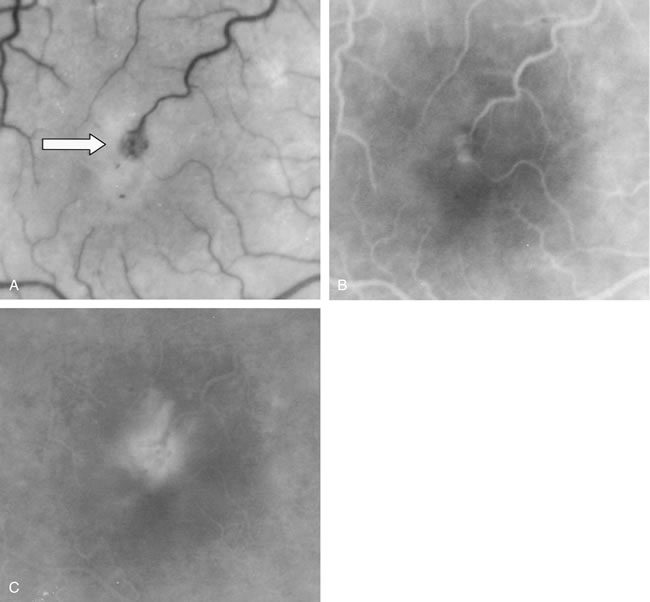
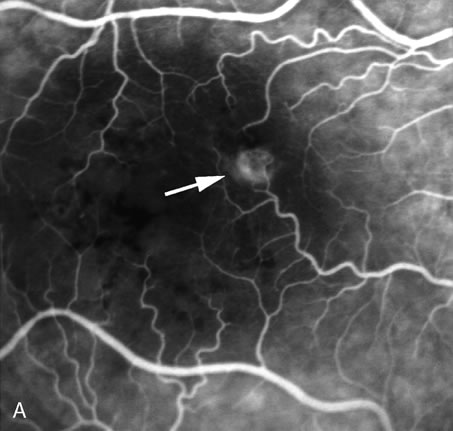
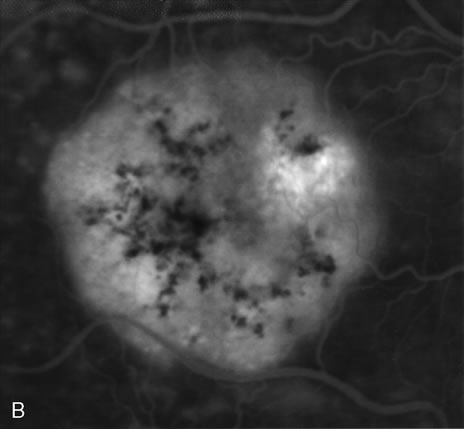
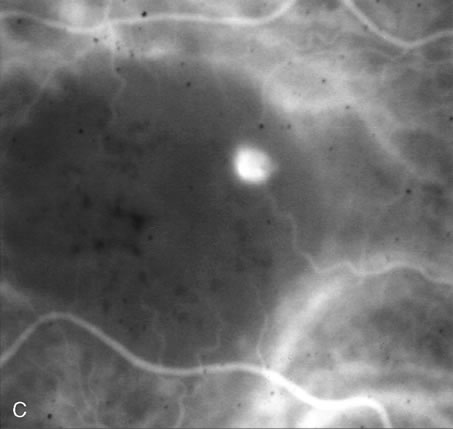
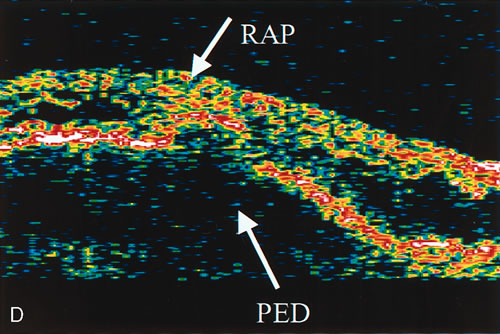
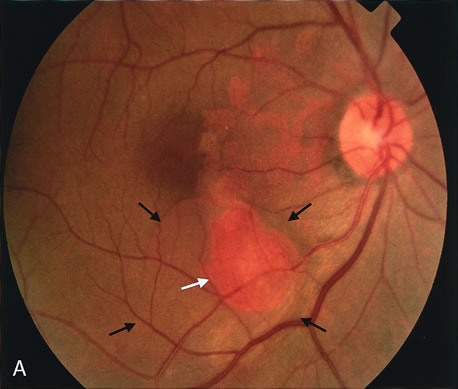
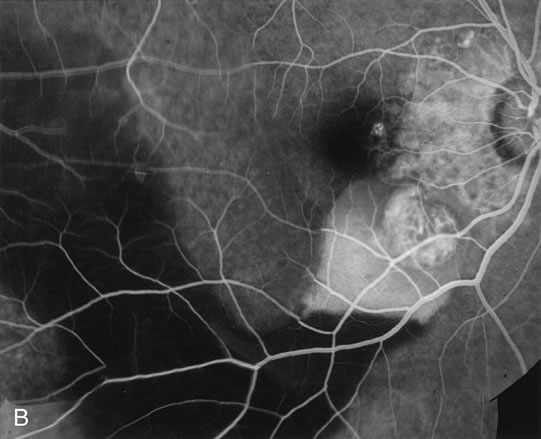
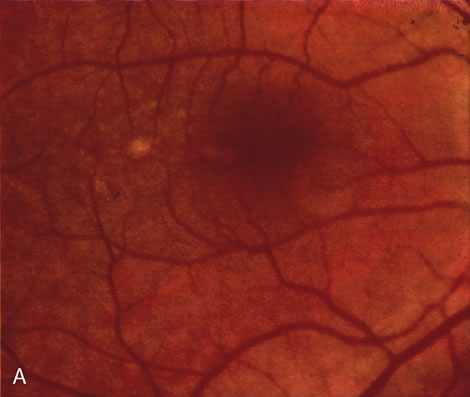
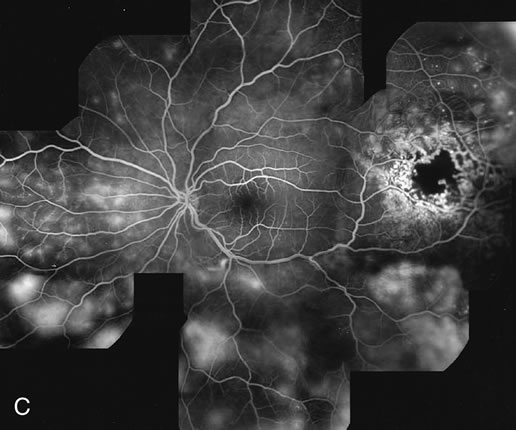
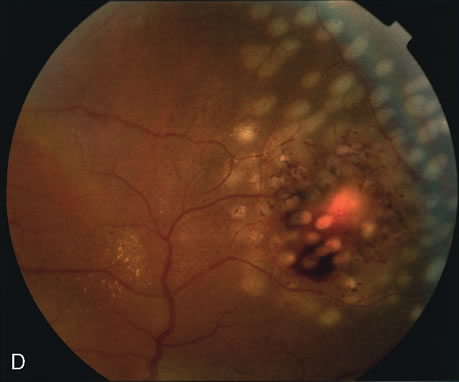
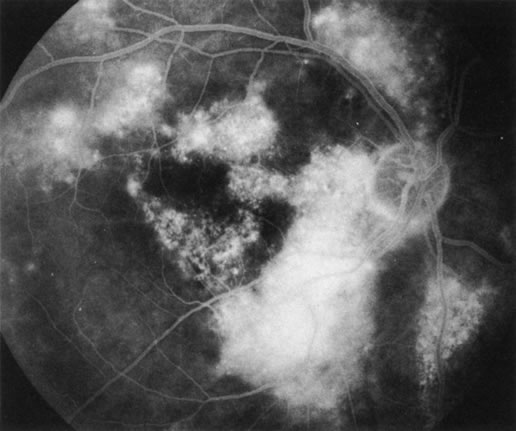
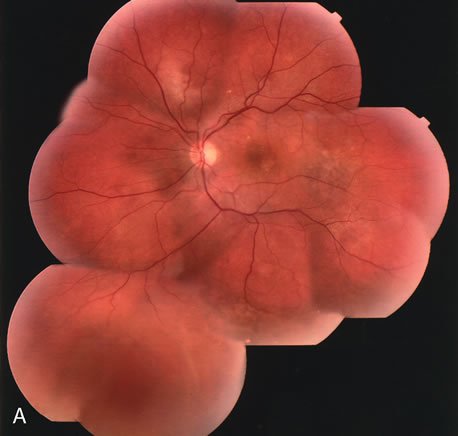
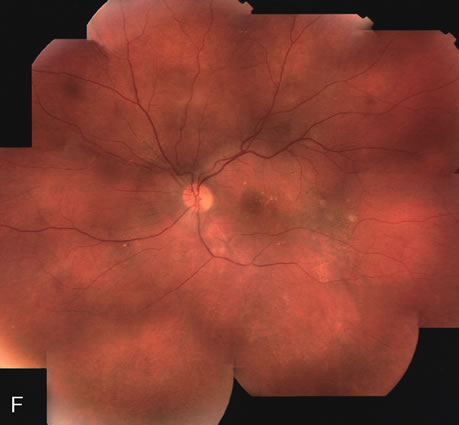
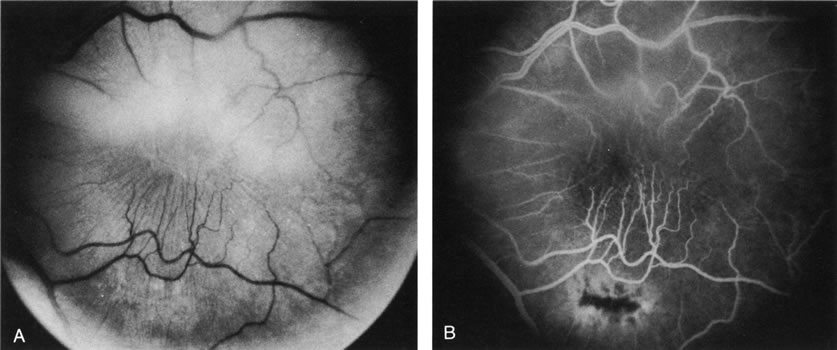
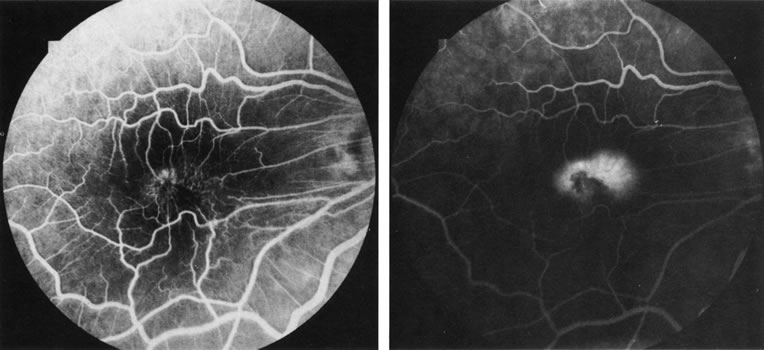
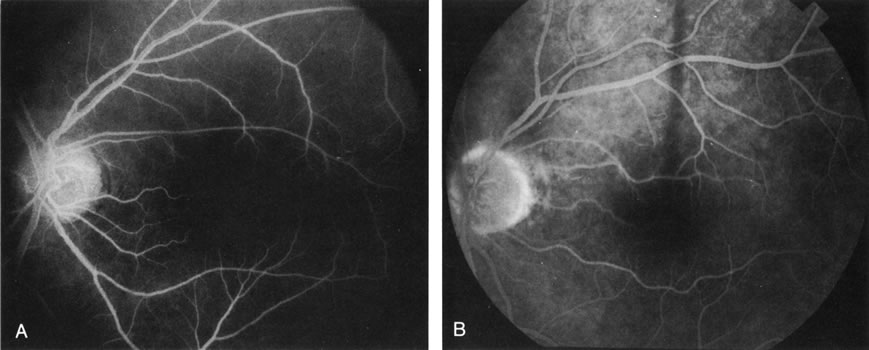
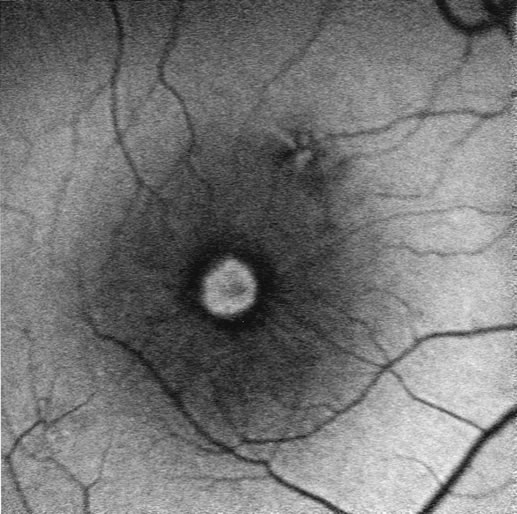
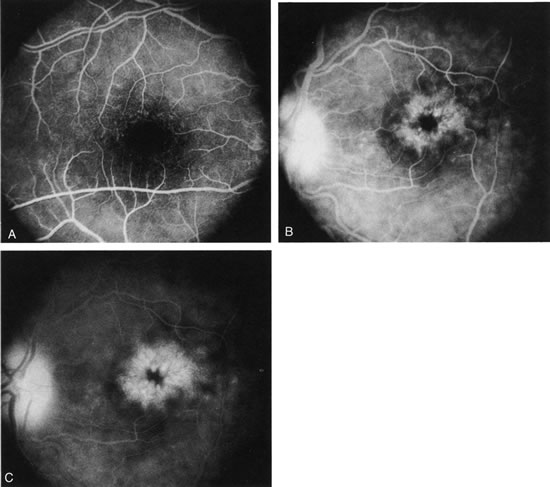
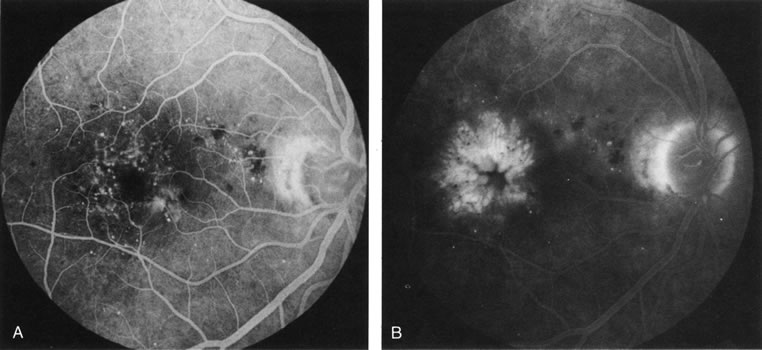
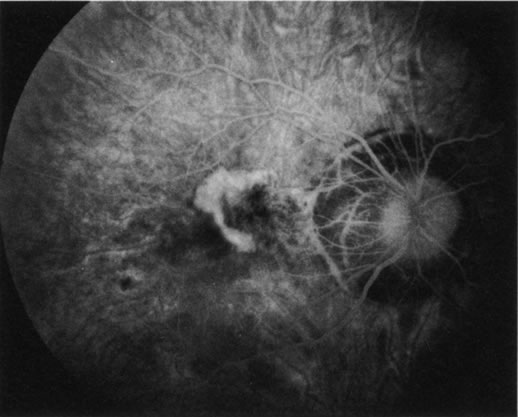
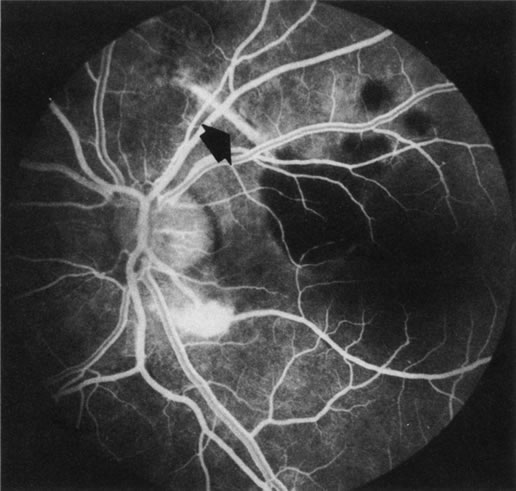
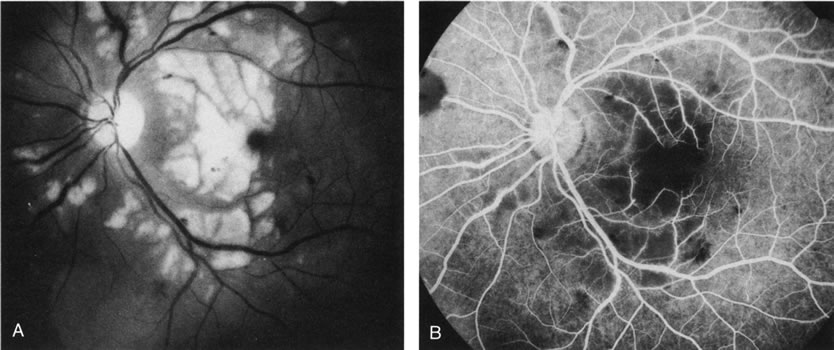
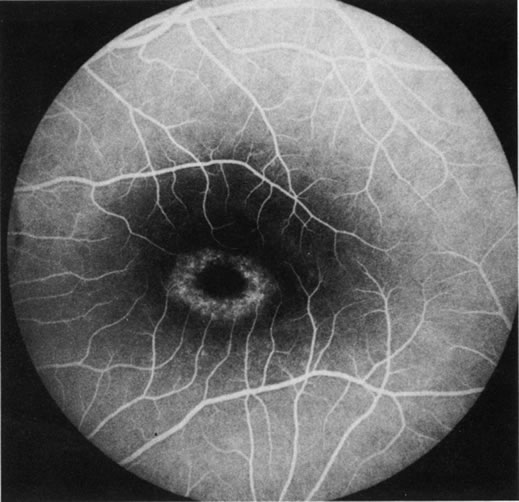
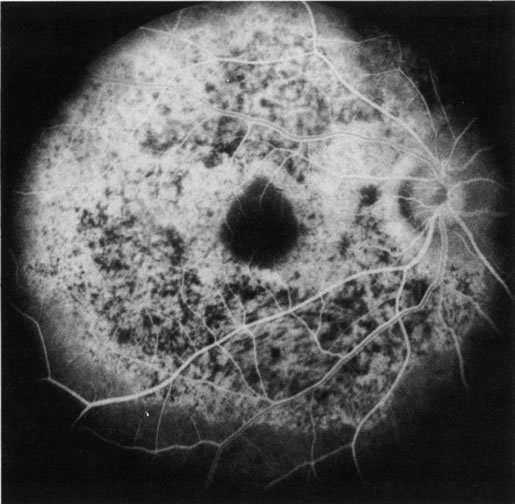
1. Schatz H: Fluorescein angiography: Basic principles and interpretation. In Ryan SJ (ed): Retina, Vol 2. Medical Retina, pp 3–80. St. Louis, CV Mosby, 1989 2. Richard G: Fluorescein Angiography: Textbook and Atlas. New York, Thieme Medical Publishers, 1990. 3. Green WR, McDonnell PJ, Yeo JH: Pathologic features of senile macular degeneration. Ophthalmology 92:615, 1985. 4. Elman MJ, Fine SL, Murphy RP et al: The natural history of serous retinal pigment epithelium detachment in
patients with age-related macular degeneration. Ophthalmology 93:224, 1986. 5. Folk JC: Aging macular degeneration: Clinical features of treatable disease. Ophthalmology 92:594, 1985. 6. Bressler NM, Bressler SB, Gragoudas ES: Clinical characteristics of choroidal neovascular membranes. Arch Ophthalmol 105:209, 1987. 7. Bressler SB, Bressler NM, Fine SL et al: Natural course of choroidal neovascular membranes within the foveal avascular
zone in senile macular degeneration. Am J Ophthalmol 93:157, 1982. 8. Yannuzzi LA, Negrão S, Iida T et al: Retinal angiomatous proliferation in age-related macular degeneration. Retina 21:416–434, 2001. 9. Fernandez LH, Freund KB, Yannuzzi LA et al: The nature of focal areas of hyperfluorescence or hot spots imaged with
indocyanine green angiography. Retina 22:557–568, 2002. 10. Ghazi NG. Retinal angiomatous proliferation in age-related macular degeneration. Retina 22:509–511, 2002. 11. Axer-Siegel R, Bourla D, Priel E et al: Angiographic and flow patterns of retinal choroidal anastomoses in age-related
macular degeneration with occult choroidal neovascularization. Ophthalmology 109:1726–1736, 2002. 12. Lafaut BA, Aisenbrey S, Vanden Broecke C et al: Clinicopathological correlation of deep retinal vascular anomalous complex
in age related macular degeneration. Br J Ophthalmol 84:1269–1274, 2000. 13. Slakter JS, Yannuzzi LA, Schneider U: Retinal choroidal anastomoses and occult choroidal neovascularization in
age-related macular degeneration. Ophthalmology 107:742–753, 2000. 14. Hartnett ME, Weiter JJ, Staurenghi G et al: Deep retinal vascular anomalous complexes in advanced age-related
macular degeneration. Ophthalmology. 103:2042–2053, 1996. 15. Kuhn D, Meunier I, Soubrane G et al: Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium
detachments. Arch Ophthalmol 113:1392–1398, 1995. 16. Schneider U, Gelisken F, Kreissig I: Retinal choroidal anastomosis in classic choroidal neovascularization demonstrated
by indocyanine green angiography. Acta Ophthalmol Scand 73:450–452, 1995. 17. Yannuzzi LA, Ciardella A, Spaide RF et al: The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 115:478–485, 1997. 18. Yannuzzi LA, Freund KB, Goldbaum M et al: Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology 107:767–777, 2000. 19. Yannuzzi LA, Noguiera FB, Spaide RF et al: Idiopathic Polypoidal choroidal vasculopathy: A peripheral lesion. Arch Ophthalmol 116:382–383, 1998. 20. Yannuzzi LA, Sorenson J, Spaide RF et al: Idiopathic polypoidal choroidal vasculopathy. Retina 10:1–8, 1990. 21. Yannuzzi LA, Wong DW, Sforzolini BS et al: Polypoidal choroidal vasculopathy and neovascularized age-related
macular degeneration. Arch Ophthalmol 117:1503–1510, 1999. 22. Spaide RF, Yannuzzi LA, Slakter JS et al: Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 15:100–110, 1995. 23. Kleiner RC, Bruckner AJ, Johnson RL: Posterior uveal bleeding syndrome. Ophthalmology 91:110, 1984. 24. Stern RM, Zakov N, Zegarra H et al: Multiple recurrent serous sanguineous retinal pigment epithelial detachments
in black women. Am J Ophthalmol 100:560–569, 1985. 25. Kleiner RC, Bruckner AJ, Johnson RL: The posterior uveal bleeding syndrome. Retina 10:9–17, 1990. 26. Perkovich BT, Zakov N, Berlin LA et al: An update on multiple recurrent serosanguineous retinal pigment epithelial
detachments in black women. Retina 10:18–26, 1990. 27. MacCumber MW, Dastgheib K, Bressler NM et al: Clinicopathological correlation of the multiple recurrent serosanguineous
retinal pigment epithelial detachment syndrome. Retina 15:11–110, 1995. 28. Auhja RM, Stanga PE, Vingerling JR et al: Polypoidal choroidal vasculopathy in exudative and hemorrhagic pigment
epithelial detachments. Br J Ophthalmol 84:479–484, 2000. 29. Escano MF, Fujii S, Ishibashi K et al: Indocyanine green videoangiography in macular variant of idiopathic polypoidal
choroidal vasculopathy. Jap J Ophthalmol 44:313–316, 2000. 30. Uyama M, Wada M, Nagai Y et al: Polypoidal choroidal vasculopathy: Natural history. Am J Ophthalmol 133:639–648, 2002. 31. Uyama M, Matsubara T, Fukushima I et al: Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 117:1035–1042, 1999. 32. Kwok AKH, Lai TYY, Chan CWN et al: Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol 86:892–897, 2002. 33. Lafaut BA, Aisenbrey S, van den Broecke C et al: Polypoidal choroidal vasculopathy pattern in age-related macular
degeneration. Retina 20:650–654, 2000. 34. Lafaut BA, Leyes AM, Snyers B et al: Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 238:752–759, 2000. 35. Lip PL, Hope-Ross MW, Gibson JM: Idiopathic polypoidal choroidal vasculopathy: A disease with diverse clinical
spectrum and systemic associations. Eye 5:695–700, 2000. 36. Lois N: Idiopathic polypoidal choroidal vasculopathy in a patient with atrophic
age-related macular degeneration. Br J Ophthalmol 85:1011–1012, 2001. 37. Moorthy RS, Lyon AT, Rabb MF et al: Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology 105:1380–1385, 1998. 38. Rosa RH Jr, Davis JL, Eifrig CW: Clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 120:502–508, 2002. 39. Ross RD, Gitter KA, Cohen C et al: Idiopathic polypoidal choroidal vasculopathy associated with retinal arterial
macroaneurysm and hypertensive retinopathy. Retina 16:105–111, 1996. 40. Schneider U, Gelisken F, Inhoffen W: Clinical characteristics of idiopathic polypoidal choroid vasculopathy. Ophthalmologe 98:1186–1191, 2001. 41. Imaizumi H, Takeda M: Knobby-like choroidal neovascularization accompanied with retinal
pigment epithelial detachment. Nippon Ganka Gakkai Zasshi 103:527–537, 1999. 42. Okubo A, Sameshima M, Uemara A et al: Clinicopathological correlation of polypoidal choroidal vasculopathy revealed
by ultrastructural study. Br J Ophthalmol 86:1093–1098, 2002. 43. Scasellati-Sforzolini B, Mariotti C, Bryan R et al: Polypoidal choroidal vasculopathy in Italy. Retina 21:121–125, 2001. 44. Giovannini A, Amato GP, D'Altobrando E et al: Optical coherence tomography (OCT) in idiopathic polypoidal choroidal
vasculopathy (IPCV). Doc Ophthalmol 97:367–371, 1999. 45. Iijima H, Iida T, Imai M et al: Optical coherence tomography of orange-red subretinal lesions in
eyes with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 129:21–26, 2000. 46. Iijima H, Imai M, Gohodo T et al: Optical coherence tomography of idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 127:301–305, 1999. 47. Otsuji T, Takahahashi K, Fukushima I et al: Optical coherence tomographic findings in idiopathic polypoidal choroidal
vasculopathy. Ophthalmic Surg Lasers 31:210–214, 2000. 48. Quaranta M, Maget-Faysse M, Coscas G: Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic
therapy with verteporfin. Am J Ophthalmol 134:277–280, 2002. 49. Shiraga F, Matsuo T, Yokoe S et al: Surgical treatment of submacular hemorrhage associated with idiopathic
polypoidal choroidal vasculopathy. Am J Ophthalmol 128:147–154, 1999. 50. Terasaki H, Miyake Y, Suzuki T et al: Polypoidal choroidal vasculopathy treated with macular translocation: Clinical
pathological correlation. Br J Ophthalmol 86:321–327, 2002. 51. Macular Photocoagulation Study Group: Argon laser photocoagulation for
neovascular maculopathy: Three-year results from randomized clinical
trials. Arch Ophthalmol 104:694, 1986 52. Macular Photocoagulation Study Group: Persistent and recurrent neovascularization
after krypton laser photocoagulation for neovascular lesions
of age-related macular degeneration. Arch Ophthalmol 108:825, 1990. 53. Moorfields Macular Study Group: Treatment of senile disciform macular degeneration: A
single-blind randomized trial by argon laser photocoagulation. Br J Ophthalmol 66:745, 1982. 54. Sorenson JA, Yannuzzi LA, Shakin JL: Recurrent subretinal neovascularization. Ophthalmology 92:1059, 1985. 55. Gass JDM: Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. St. Louis, CV Mosby, 1987 56. Miller JW, Walsh AW, Kramer M et al: Photodynamic therapy of experimental choroidal neovascularization using
lipoprotein-delivered benzoporphyrin. Arch Ophthalmol 113:8108, 1995. 57. Schmidt-Erfurth U, Miller JW, Sickenberg M et al: Photodynamic therapy with verteporfin for choroidal neovascularization
caused by age-related macular degeneration: Results of retreatments
in a phase 1 and 2 study. Arch Ophthalmol 117:1177–1187, 1999. 58. Miller JW, Schmidt-Erfurth U, Sickenberg M et al: Photodynamic therapy with verteporfin for choroidal neovascularization
caused by age-related macular degeneration: Results of a single
treatment in a phase 1 and 2 study. Arch Ophthalmol. 117:1161–1173, 1999. 59. Schmidt-Erfurth U, Miller J, Sickenberg M et al: Photodynamic therapy of subfoveal choroidal neovascularization: Clinical
and angiographic examples. Graefes Arch Clin Exp Ophthalmol 236:365–374, 1998. 60. Treatment of Age-Related Macular Degeneration with Photodynamic
Therapy (TAP) Study Group: Photodynamic therapy of subfoveal
choroidal neovascularization in age-related macular degeneration
with verteporfin: One-year results of 2 randomized clinical
trials—TAP report. Arch Ophthalmol 117:1329–1345, 1999. 61. Bressler NM: Photodynamic therapy of subfoveal choroidal neovascularization in age-related
macular degeneration with verteporfin: Two-year results
of 2 randomized clinical trials—TAP report 2. Arch Ophthalmol. 119:198–207, 2001. 62. Bressler NM, Arnold J, Benchaboune M et al: Verteporfin therapy of subfoveal choroidal neovascularization in patients
with age-related macular degeneration: Additional information
regarding baseline lesion composition's impact on vision outcomes—TAP
report No. 3. Arch Ophthalmol 120:1443–1454, 2002. 63. Blumenkranz MS, Bressler NM, Bressler SB et al: Verteporfin therapy for subfoveal choroidal neovascularization in age-related
macular degeneration: Three-year results of an open-label
extension of 2 randomized clinical trials—TAP Report
No. 5. Arch Ophthalmol 120:1307–1314, 2002. 64. Lim JI: Photodynamic therapy for choroidal neovascular disease: Photosensitizers
and clinical trials. Ophthalmol Clin North Am 15:473–478, 2002. 65. Spaide RF, Donsoff I, Lam DL et al: Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina 22:529–535, 2002. 66. Jampol LM, Scott L: Treatment of juxtafoveal and extrafoveal choroidal neovascularization in
the era of photodynamic therapy with verteporfin. Am J Ophthalmol 134:99–101, 2002. 67. Spaide RF, Martin ML, Slakter J et al: Treatment of idiopathic subfoveal choroidal neovascular lesions using photodynamic
therapy with verteporfin. Am J Ophthalmol 134:6–68, 2002. 68. Guidelines for using verteporfin (visudyne) in photodynamic therapy
to treat choroidal neovascularization due to age-related
macular degeneration and other causes. Retina . 2002 Feb;22(1):6–18. Review. 69. Dantas MA, Slakter JS, Negrao S, Fonseca RA, Kaga T, Yannuzzi LA. Photodynamic therapy with verteporfin in malatia levantinese. Ophthalmology . 2002 Feb;109(2):296–301. 70. Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age-related
macular degeneration: two-year results of a randomized
clinical trial including lesions with occult with no classic choroidal
neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol . 2002 Jan;133(1):168–9 71. Verteporfin in Photodynamic Therapy Report 2: Verteporfin therapy of subfoveal
choroidal neovascularization in age-related macular degeneration: Two-year
results of a randomized clinical trial including
lesions with occult with no classic choroidal neovascularization— Am J Ophthalmol 131:541–560, 2001. 72. American Academy of Ophthalmology: Photodynamic therapy with verteporfin
for age-related macular degeneration. Ophthalmology 107:2314–2317, 2000. 73. Schmidt-Erfurth U, Hasan T: Mechanisms of action of photodynamic therapy with verteporfin for the treatment
of age-related macular degeneration. Surv Ophthalmol 45:195–214, 2000. 74. Gass JDM: Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 63:587, 1967. 75. Yannuzzi LA: Type-A behavior and central serous chorioretinopathy. Retina 7:111, 1987 76. Benson WE, Shields JA, Annesley WH et al: Idiopathic central serous chorioretinopathy with bullous retinal detachment. Ann Ophthalmol 12:920, 1980. 77. Yannuzzi LA, Freund KB, Goldbaum M et al: Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology 107:767–777, 2000. 78. Gilbert CM, Owens SL, Smith PD et al: Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 68:815, 1984. 79. Spitznas M: Central serous retinopathy. In Ryan SJ (ed): Retina, Vol 2. Medical Retina, pp 217–227. St. Louis, CV Mosby, 1989. 80. Gass JDM: Photocoagulation treatment of idiopathic central serous choroidopathy. Trans Am Acad Ophthalmol Otolaryngol 83:456, 1977. 81. Annesley WH, Augsburger JJ, Shakin JL: Ten year follow-up of photocoagulated central serous choroidopathy. Trans Am Ophthalmol Soc 79:335, 1981. 82. Roseman RL, Olk RJ: Grid laser photocoagulation for atypic central serous chorioretinopathy. Ophthalmic Surg 19:786, 1988. 83. Yannuzzi LA, Kaufman SR, Slakter J et al: Grid laser photocoagulation treatment of diffuse retinal pigment epitheliopathy (in
press). 84. Klein ML, Van Buskirk M, Friedman E et al: Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol 91:247, 1974. 85. Yannuzzi LA, Shakin JL, Fisher YL et al: Peripheral retinal detachments and retinal pigment epithelial atrophic
tracts secondary to central serous pigment epitheliopathy. Ophthalmology 91:1554, 1984. 86. Zweng HC, Little, HL, Vassiliadis A:Argon Laser Photocoagulation, pp 117–126. St. Louis, CV Mosby, 1977. 87. Gass JDM: Bullous retinal detachment; An unusual manifestation of idiopathic central
serous choroidopathy. Am J Ophthalmol 75:810–821, 1973. 88. Straatsma BR, Allen RA, Petit TH: Central serous retinopathy, Trans Pacific Coast Oto-ophthalmol Soc 47:107–127, 1966. 89. Nade, AJ, Turan, MI Coles RS: Central serous retinopathy; A generalized disease of the pigment epithelium. Mod Probl Ophthalmol 20:76–88, 1979. 90. Giovannini A: L'epitheliopathie atrophique “en trace.” Bull Mem Soc Fr Ophtalmol 94:402–406, 1982. 91. Cohen D, Gaudric A, Coscas G et al: Epitheliopathie retinienne diffuse et chorioretinopathie sereuse centrale. J Fr Ophtalmol 6:339–349, 1983. 92. von Winning CHOM, Oostheruis JA, Renger-van Dijk , AH et al: Diffuse retinal pigment epitheliopathy. Ophthalmologica, 185:7–14, 1982. 93. von Graefe, A: Ueber centrale recidivierende Retinitis. Graefes Arch Clin Exp Ophthalmol 12:211–215, 1866. 94. Bennet G: Central serous retinopathy. Br J Ophthalmol 39:605–618, 1955. 95. Maumenee AE: Symposium: Macular diseases, clinical manifestations. Trans Pacific Coast Oto-ophthalmol Soc 40:139–160, 1959. 96. Gelber GS, Schatz H: Loss of vision due to central serous chorioretinopathy following psychological
stress. Am J Psychiatry 144:46–50, 1987. 97. Cordes FC: A type of foveomacular retinitis observed in the US Navy. Am J Ophthalmol 27:803, 1944. 98. Horniker, E: Su di una forma retinite centrale di origine vasoneurotica (retinite
central capillaro-spastica). Ann Ottal 55:578–600, 1927. 99. Cordes, FC: A type of foveo-macular retinitis observed in the U.S. Navy. Am J Ophthalmol 27:803–816, 1944. 100. Harrington DO: The autonomic nervous system in ocular diseases. Am J Ophthalmol 29:1405–1425, 1946. 101. Harrington, DO: Psychosomatic interrelationships in ophthalmology. Am J Opthalmol 31:1241–1251, 1948. 102. Horniker E: Uber eine Form von zentraler Retinitis auf angio-neurotischer Grundlage (Retinitis
centralis angio-neurotica). Graefes Arch Clin Exp Ophthalmol 123:286–360, 1929. 103. Huke J: Retinopathia centralis serosa (dissertation). Bonn, 1982. 104. Werry H, Arends C: Untersuchung zur Objektivierung von Personlichkeitsmerkmalen bei Patienten
mit Retinopathia centralis serosa. Klin Monatsbl Augenheilkd 172:363–370, 1978. 105. Garg SP, Dada T, Talwar D et al: Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol 81:962–964, 1997. 106. Bouzas EA, Scott MH, Mastorakos G et al: Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol 111:1929–1932, 1993. 107. Jain IS, Singh K: Maculopathy, a corticosteroid side effect. J All India Ophthalmol Soc 14:250–252, 1966. 108. Eckstein MB, Spalton DJ, Holde, G: Visual loss from central serous retinopathy in systemic lupus erythematosus, Br J Ophthalmol 77:607–609, 1993. 109. Wakakura M, Ishikawa S: An evaluation of corticosteroid treatment for central serous chorioretinopathy. Rinsho Ganka 34:123–129, 1980. 110. Wakakura M, Ishikawa S: Central serous retinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol 68:329–331, 1984. 111. Friberg TR, Eller, AW: Serous retinal detachment resembling central serous chorioretinopathy following
organ transplantation. Graefes Arch Clin Exp Ophthalmol 288:305–309, 1990. 112. Gass JDM, Little HL: Bilateral bullous exudative retinal detachment complicating idiopathic
central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology 102:737–747, 1995. 113. Gass JDM, Slamovits TL, Fuller DG et al: Posterior chorioretinopathy and retinal detachment after organ transplantation. Arch Ophthalmol 110:1717–1722, 1992. 114. Haimoivici R, Gragoudas ES, Duker JS et al: Central serous chorioretinopathy associated with inhaled or intranasal
corticosteroids. Ophthalmology 104:1653–1660, 1997. 115. Polack BCP, Baarsma GS, Snyer B: Diffuse retinal epitheliopathy complicating systemic corticosteroid treatment. Br J Ophthalmol 79:922–925, 1995. 116. Spraul CW, Lang CE, Lang GK: Central serous chorioretinopathy in systemic therapy with corticosteroids. Ophthalmologe 94:392–396, 1997. 117. Wakakura M, Song E, Ishikawa S: Corticosteroid induced central serous chorioretinopathy. Jpn J Ophthalmol 41:180–185, 1997. 118. Iida T, Spaide RF, Negrao SG et al: Central serous chorioretinopathy after epidural corticosteroid injection. Am J Ophthalmol 132:423–425, 2001. 119. Gass JD: Central serous chorioretinopathy and white subretinal exudation during
pregnancy. Arch Ophthalmol 109:677–681, 1991. 120. Fastenburg, DM, Ober, RR: Central serous choroidopathy in pregnancy. Arch Ophthalmol 1055–1058, 1983. 121. Quillen, DA, Gass, JDM, Brod Rd et al: Central serous chorioretinopathy in women. Ophthalmology 103:72–79, 1996. 122. Chumbley LC, Frank RN: Central serous retinopathy and pregnancy. Am J Ophthalmol 77:15–160, 1974. 123. Bedrossian RH: Central serous retinopathy and pregnancy. Am J Ophthalmol 78:152, 1974. 124. Cruysberg JRM, Deutman AF: Visual disturbances during pregnancy caused by central serous choroidopathy. Br J Ophthalmol 66:240–241, 1982. 125. Fastenberg DM, Ober RR: Central serous choroidopathy in pregnancy. Arch Ophthalmol 101:1055–1088, 1983. 126. Sunness JS, Haller JA, Fine SL: Central serous chorioretinopathy and pregnancy. Arch Ophthalmol 111:360–364, 1993. 127. Nagayosky K: Experimental study of chorioretinopathy by intravenous injection of adrenaline, Acta Soc Ophthalmol Jpn 75:1720–1727, 1971. 128. Yoshioka H, Katsume Y, Akume H: Experimental central serous chorioretinopathy in monkey eyes: Fluorescein
angiography findings. Ophthalmologica 185:168–178, 1982. 129. Yoshioka H, Sugita T, Nagayoski K: Fluorescein angiography findings in experimental retinopathy produced by
intravenous adrenaline injection. Folia Ophthalmol Jpn 21:648–652, 1970 130. Yoshioka H, Katsume Y: Experimental central serous chorioretinopathy. III. Ultrastuctural findings. Jpn J Ophthalmol 26:397–409, 1982. 131. Miki T, Sunada I, Higaki T; Studies in chorioretinitis induced in stress (repeated administration
of epinephrine). Acta Soc Ophthalmol Jpn 75:1037–1045, 1972. 132. Yasuzumi T, Miki T, Sugimoto K: Electron microscopic studies of epinephrine choroiditis in rabbits. I. Pigment
epithelium and Bruch's membrane in the healed stage. Acta Soc Ophthalmol Jpn 78:588–598, 1974. 133. Hilton AF, Harrison JD, Lamb AM et al: Ocular complications in haemodialysis and renal transplant patients. Aust N Z J Ophthalmol 10:247–253, 1982. 134. Gass JDM: Bullous retinal detachment and multiple retinal pigment epithelium detachments
in patients receiving haemodialysis. Graefes Arch Clin Exp Ophthalmol 230:454–458, 1992. 135. Cohen SM, Kokame GT, Gass JDM: Paraproteinemias associated with serous detachments of the retinal pigment
epithelium and neurosensory retina. Retina 16:467–473, 1996. 136. Cunningham ET Jr, Alfred PR, Irvine AR: Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology 103:2081–2090, 1996. 137. Semon HC, Wolff E: Acute lupus erythematosus with fundus lesions. Proc Royal Soc Med 27:153–157, 1933. 138. Clifton F, Greer CH: Ocular changes in acute systemic lupus erythematosus. Br J Ophthalmol 39:1–10, 1955. 139. Burniana A: Retinal detachment in collagen disease. Ann Oculist 202:1123–1130, 1969. 140. Jabs DA, Hanneken AM, Schachat AP et al: Choroidopathy in systemic lupus erythematosus. Arch Ophthalmol 106:230–234, 1988. 141. Matsuo T, Nakayama T, Koyama T et al: Multifocal pigment epithelial damages with serous retinal detachment in
systemic lupus erythematosus. Ophthalmologica 195:97–102, 1987. 142. Diddie KR, Aronson AJ, Ernest JT: Chorioretinopathy in a case of systemic lupus erythematosus. Trans Am Ophthalmol Soc 75:122–131, 1977. 143. Gold, DH, Morris, DA, Henkind, P: Ocular findings in systemic lupus erythematosus. Br J Ophthalmol 56:800–804, 1972. 144. Googe JM Jr, Brady SE, Argyle JC et al: Choroiditis in infantile periarteritis nodosa. Arch Ophthalmol 103:81–83, 1985. 145. Stefani FH, Brandt F, Pielsticker K: Periarteritis nodosa and thrombotic thrombocytopenic purpura and serous
retinal detachment in siblings. Br J Ophthalmol 62:402–407, 1978. 146. Jampol LM, Lahav M, Albert DM et al: Ocular clinical findings and basement membrane changes in Goodpasture's
syndrome. Am J Ophthalmol 79:452–463, 1975. 147. Kihyoun JL, Kalina RE, Klein ML: Choroidal involvement in systemic necrotizing vasculitis. Arch Ophthalmol 105:939–942, 1987. 148. Hassan L, Carvalho C, Yannuzzi LA et al: Central serous chorioretinopathy in a patient using methylenedioxymethamphetamine (MDMA) or “ecstasy.” Retina 21:559–561, 2001. 149. Klien BA: Ischemic infarcts of the choroid (Elshing's spots): A cause
of retinal separation in hypertensive disease with renal insufficiency: A
clinical an histopathological study. Am J Ophthalmol 66:1069–1088, 1968. 150. Stropes LL, Luft FC: Hypertensive crisis with bilateral bullous retinal detachment. JAMA 238:1948–1949, 1977. 151. Venecia G, Jampol LM: The eye in accelerated hypertension. II. Localized serous detachments of
the retina in patients. Arch Ophthalmol 102:68–73, 1984. 152. Gitter KA, Houser BP, Sarin LK et al: Toxemia of pregnancy: An angiographic interpretation of fundus changes. Arch Ophthalmol 80:449–454, 1968. 153. Gass JDM, Pautler SE: Toxemia of pregnancy pigment epitheliopathy masquerading as heredomacular
dystrophy. Trans Am Ophthalmol Soc 83:114–130, 1985. 154. Menchini U, Lanzetta P, Virgili G et al: Retinal pigment epithelium tear following toxemia of pregnancy. Eur J Ophthalmol 5:139–141, 1995. 155. Yamaguchi K, Abe S, Shiono T et al: Macular choroidal occlusion in dysplasmogenemia. Retina 11:423–425, 1991 156. Cogan DG: Fibrin clots in the choriocapillaris and serous detachment of the retina. Ophthalmologica 172:298–307, 1976. 157. Cogan DG: Ocular involvement in disseminated intravascular coagulopathy. Arch Ophthalmol 93:1–8, 1975. 158. Shimizu K, Tobari I: Central serous retinopathy dynamics of subretinal fluid, Mod Probl Ophthalmol 9:152–157, 1971. 159. Goldstein BG, Pavan PR: “Blow outs” in the retinal pigment epithelium. Br J Ophthalmol 71:676–681, 1987. 160. Spitznas M, Hogan MJ: Outer segments of photoreceptors and the retinal pigment epithelium: Interrelationship
in the human eye. Arch Ophthalmol 84:810–819, 1970. 161. Spitznas M, Huke J: Number, shape and topography of leakage points in acute type I central
serous retinopathy, Graefes Arch Clin Exp Ophthalmol 225:437–440, 1987 162. Faurschou S, Rosenberg T, Nielsen N: Central serous retinopathy and presenile disciform macular degeneration. Acta Ophthalmol 55:515–524, 1977. 163. Schatz H, Madera D, Johnson RN et al: Central serous chorioretinopathy occurring in patients 60 years of age
or older. Ophthalmology, 99:63–67, 1992. 164. Berger AL, Olk RJ, Burgess, D: Central serous choroidopathy in patients over 50 years of age. Ophthalmic Surg 22:583–590, 1991. 165. Scheide, A, Nasemann JE, Lund OE: Fluorescein and indocyanine angiographies of central serous chorioretinopathy
by scanning laser ophthalmoscope. Am J Ophthalmol 115:50–56, 1993. 166. Hayashi K, Hasegawa Y, Tokoro T: Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol 9:37–41, 1986. 167. Scheider A, Hintschich C, Dimitriou S: Central serous chorioretinopathy. Studies of the site of the lesion with
indocyanine green. Ophthalmologe 91:745–751, 1994. 168. Piccolino FC, Borgia L: Central serous chorioretinopathy and indocyanine green angiography. Retina 14:231–242, 1994. 169. Guyer DR, Yannuzzi, LA, Slakter JS et al: Digital indocyanine green videoangiography of central serous chorioretinopathy, Arch Ophthalmol 112:1057–1062, 1994. 170. Spaide RF, Campeas L, Haas, A et al: Central serous chorioretinopathy in younger and older adults, Ophthalmology 103:2070–2080, 1996. 171. Piccolino FC, Borgia L, Zinicola E et al: Indocyanine green angiographic findings in central serous chorioretinopathy. Eye 9:324–332, 1995. 172. Prunte C, Flammer J: Circulatory disorders of the choroid in patients with central serous chorioretinopathy. Klin Monatsbl Augenheilkd 208:337–339, 1996. 173. Lafaut BA, De Laey JJ: Indocyanine green angiography in central serous chorioretinopathy. Bull Soc Belge Ophtalmol 262:55–61, 1996. 174. Prunte C, Flammer J: Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 121:26–34, 1996. 175. Iida T, Hagimura N, Otani T et al: Choroidal vascular lesions in serous retinal detachment viewed with indocyanine
green angiography. Nippon Ganka Gakkai Zasshi 100:817–824, 1996. 176. Menchini U, Virgili G, Lanzetta P et al: Indocyanine green angiography in central serous chorioretinopathy. Int Ophthalmol 21:57–69, 1997. 177. Giovannini A, Scasellati Sforzolini B, D'Altobrando E: Choroidal findings in the course of idiopathic serous pigment epithelium
detachment detected by indocyanine green videoangiography. Retina 17:286–293, 1997. 178. Okushiba U, Takeda M: Study of choroidal vascular lesions in central serous chorioretinopathy
using indocyanine green angiography. Nippon Ganka Gakkai Zasshi 101:74–82, 1997. 179. Spaide, RF, Orlock D, Herrmann-Delemazure B et al: Wide-angle indocyanine green angiography. Retina 18:44–49, 1998. 180. Guyer DR: Central serous chorioretinopathy. In Yannuzzi LA, Flower RW, Slakter JS (eds): Indocyanine Green Angiography, pp 297–304. St. Louis, Mosby –Year Book, 1997. 181. Spaide RF, Hall L, Haas A: Indocyanine green videoangiography of central serous chorioretinopathy
in older adults. Retina 16:78–80, 1996. 182. Nanjian, M: Long-term follow-up of central serous retinopathy, Trans Ophthalmol Soc UK 97:656–661, 1977. 183. Dellaporta A: Central serous retinopathy. Trans Am Ophthalmol Soc 74:144–153, 1976. 184. Watzke RC, Burton TC, Woolson RF: Direct and indirect laser photocoagulation of central serous choroidopathy, Am J Ophthalmol 88:914–918, 1979. 185. Wessing A: Zur Pathogenese und Therapie der sogenannten Retinitis centralis serosa. Ophthalmologica 153:259–276, 1967. 186. Wessing A: Die Therapie der Retinitis centralis serosa mit Lichtcoagulation. Berl Dtsch Ophthalmol Ges 68:429–431, 1968. 187. Wessing A: Changing concepts of central serous retinopathy and its treatment. Trans Am Acad Ophthalmol Otolaryngol 77:275–280, 1973. 188. Watzke RC, Burton TC, Leaverton PA: Ruby laser photocoagulation therapy of central serous retinopathy. Trans Am Acad Ophthalmol Otolaryngol 78:205–211, 1974. 189. L'Esperance FA Jr: Argon and ruby laser photocoagulation of disciform macular disease. Trans Am Acad Ophthalmol Otolaryngol 75:609–625, 1971. 190. Spalter HF: Photocoagulation of central serous retinopathy: A preliminary report. Arch Ophthalmol 79:247–253, 1968. 191. Annesley WH Jr, Augsburger JJ, Shakin JL: Ten-year follow-up of photocoagulated central serous choroidopathy. Trans Am Ophthalmol Soc 79:335–346, 1981. 192. Leaver P, Williams C: Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 63:674–677, 1979. 193. Ficker L, Vafadis G, While A et al: Long term results of treatment of central serous retinopathy. Trans Ophthalmol Soc UK 105:473–475, 1986. 194. Ficker L, Vafadis G, While A et al: Long term follow-up of a prospective trial of argon laser photocoagulation
in the treatment of central serous retinopathy. Br J Ophthalmol 72:829–834, 1988. 195. Yannuzzi LA, Slakter JS, Kaufman SR et al: Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol, 2:103–114, 1992. 196. Piccolino FC: Laser treatment of eccentric leaks in central serous chorioretinopathy
resulting in disappearance of untreated juxtafoveal leaks. Retina 12:96–102, 1992. 197. L'Esperance, FA: Ocular Photocoagulation, pp 190–197. St. Louis, Mosby–Year Book, 1975. 198. Robertson DM, Ilstrup D: Direct, indirect and sham laser photocoagulation in the management of sham
central serous chorioretinopathy. Am J Ophthalmol 94:457–466, 1983. 199. Eng-Yiat Y, Robertson DM: The long term outcome of central serous chorioretinopathy. Arch Ophthalmol 114:689–692, 1996. 200. Brancato R, Scialdone A, Pece A et al: Eight-year follow-up of central serous chorioretinopathy
with and without laser treatment. Graefes Arch Clin Exp Ophthalmol 225:166–168, 1987. 201. Annesley VH Jr, Augsburger JJ, Shakin JL: Ten-year follow-up of photocoagulated central serous choroidopathy. Trans Am Ophthalmol 79:335–346, 1981. 202. Castro-Correia J, Coutinho MF, Rosas V: Long term follow up of central serous chorioretinopathy in 150 patients. Doc Ophthalmol 81:379–386, 1992. 203. Schatz H, Yannuzzi LA, Gitter KA: Subretinal neovascularization following argon laser photocoagulation treatment
for central serous chorioretinopathy; complication or misdiagnosis? Trans Am Acad Ophthalmol Otolaryngol 83:893–906, 1977. 204. Schatz, H, McDonald HR, Johnson RN et al: Subretinal fibrosis in central serous chorioretinopathy, Ophthalmology, 102:1077–1088, 1995. 205. Schatz H, D'Oesterloh M, McDonald RH et al: Development of retinal vascular leakage and cystoid macular edema secondary
to central serous chorioretinopathy. Br J Ophthalmol 77:744–746, 1993. 206. Weiler W, Foerester MH, Wessing A: Exudative retinal detachment, pigment epithelium tear and subretinal exudate
in a case of central serous chorioretinopathy. Klin Monatsbl Augenheilkd 199:450–453, 1991. 207. McDonald HR, Schatz H: Introduction to epiretinal membranes. In Ryan SJ (ed): Retina, Vol 2. Medical Retina. St. Louis, CV Mosby, 1989. 208. Margherio RR, Cox MS, Trese MT et al: Removal of epimacular membranes. Ophthalmology 92:1075, 1985. 209. Rice TA, de Bustros S, Michels RG et al: Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology 93:602, 1986. 210. Green RL, Byrne SF: Diagnostic ophthalmic ultrasound. In Ryan SJ (ed): Retina, Vol 1. Basic Science and Inherited Retina Disease, pp 191–273. St. Louis, CV Mosby, 1989. 211. Sidd RJ, Fine SL, Owens SL et al: Idiopathic preretinal gliosis. Am J Ophthalmol 94:44, 1982. 212. Foos RY: Vitreoretinal juncture: Epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci 16:416, 1977. 213. McDonnell PJ, Fine SL, Hillis Al: Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol 93:777, 1982, 214. Morgan CM, Schatz H: Idiopathic macular holes. Am J Ophthalmol 99:437, 1985, 215. Hee MR, Puliafito CA, Wong C et al: Optical coherence tomography of macular holes. Ophthalmology 102:748–756, 1995. 216. Dai H, Li Y, Long L et al: The observation on detachment of posterior hyaloid membrane in idiopathic
macular holes by optical coherence tomography. Chung Hua Yen Ko Tsa Chih 38:667–669, 2002. 217. Apostolopoulos MN, Koutsandrea CN, Moschos MN et al: Evaluation of successful macular hole surgery by optical coherence tomography
and multifocal electroretinography. Am J Ophthalmol 134:667–674, 2002. 218. Uemoto R, Yamamoto S, Aoki T et al: Macular configuration determined by optical coherence tomography after
idiopathic macular hole surgery with or without internal limiting membrane
peeling. Br J Ophthalmo. 86:1240–1242, 2002. 219. Ullrich S, Haritoglou C, Gass C et al: Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol 86:390–393, 2002. 220. Ip MS, Baker BJ, Duker JS et al: Anatomical outcomes of surgery for idiopathic macular hole as determined
by optical coherence tomography. Arch Ophthalmol 120:29–35, 2002. 221. Tanner V, Chauhan DS, Jackson TL et al: Optical coherence tomography of the vitreoretinal interface in macular
hole formation. Br J Ophthalmol 85:1092–1097, 2001. 222. Haouchine B, Massin P, Gaudric A: Foveal pseudocyst as the first step in macular hole formation: A prospective
study by optical coherence tomography. Ophthalmology 108:15–22, 2001. 223. Jumper JM, Gallemore RP, McCuen BW 2nd et al: Features of macular hole closure in the early postoperative period using
optical coherence tomography. Retina 20:232–237, 2000. 224. Spiritus A, Dralands L, Stalmans P et al: OCT study of fellow eyes of macular holes. Bull Soc Belge Ophtalmol 275:81–84, 2000. 225. Gallemore RP, Jumper JM, McCuen BW 2nd et al: Diagnosis of vitreoretinal adhesions in macular disease with optical coherence
tomography. Retina 20:115–120, 2000. 226. Akasaka Y, Nishikawa S, Tamai M: Analysis of the retinal edema of full-thickness macular holes by
scanning laser ophthalmoscopy and optical coherence tomography. Tohoku J Exp Med 189:233–238, 1999. 227. Ripandelli G, Coppe AM, Bonini S et al: Morphological evaluation of full-thickness idiopathic macular holes
by optical coherence tomography. Eur J Ophthalmol 9:212–216, 1999. 228. Stalmans P, Spileers W, Dralands L: The use of optical coherence tomography in macular diseases. Bull Soc Belge Ophtalmol 272:15–30, 1999. 229. Mori K, Abe T, Yoneya S: Vitreoretinal tomography and foveolar traction in macular hole development
and macular pseudohole. Nippon Ganka Gakkai Zasshi 103:371–378, 1999. 230. Pal E, Givort G, Laroche A et al: Macular imaging with optical coherence tomography. J Fr Ophtalmol 21:484–494, 1998. 231. Smiddy WE, Michels RG, Glaser BM et al: Vitrectomy for impending idiopathic macular holes. Am J Ophthalmol 105:371, 1988. 232. Jost BF, Hutton WL, Fuller DG et al: Vitrectomy in eyes at risk for macular hole formation. Ophthalmology 97:843, 1990. 233. Akiba J, Yoshida A, Trempe CL: Risk of developing a macular hole. Arch Ophthalmol 108:1088, 1990. 234. Margherio RR, Schepens CL: Macular breaks: I. Diagnosis, etiology, and observations. Am J Ophthalmol 74:219, 1972. 235. Morgan CM, Schatz H: Involutional macular thinning. Ophthalmology 93:153, 1986. 236. Guyer DR, Green WR, de Bustros S et al: Histopathologic features of idiopathic macular holes and cysts. Ophthalmology 97:1045, 1990. 237. Avila MP, Jalkh AE, Murakami K et al: Biomicroscopic study of the vitreous in macular breaks. Ophthalmology 90:1277, 1983. 238. Johnson RN, Gass JDM: Idiopathic macular holes: Observations, stages of formation, and implications
of surgical intervention. Ophthalmology 95:917, 1988. 239. Gass JDM, Anderson DR, Davis EB: A clinical, fluorescein angiographic, and electron microscopic correlation
of cystoid macular edema. Am J Ophthalmol 100:82, 1985. 240. Flach AJ, Dolan BJ, Irvine AR: Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution
for chronic aphakic and pseudophakic cystoid macular edema. Am J Ophthalmol 103:479, 1987. 241. Cox SN, Hay E, Bird AC: Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol 106:1190, 1988. 242. Fishman GA, Gilbert LD, Fiscella RG et al: Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol 107:1445, 1989. 243. Zierhut M, Thiel HJ, Schlote T: Treatment of uveitic macular edema with acetazolamide. Doc Ophthalmol 97:409–413, 1999. 244. Wolfensberger TJ: The role of carbonic anhydrase inhibitors in the management of macular
edema. Doc Ophthalmol 97:387–397, 1999. 245. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following
cataract surgery. Trans Am Ophthalmol Soc 96:557–634, 1998. 246. Moldow B, Sander B, Larsen M et al: The effect of acetazolamide on passive and active transport of fluorescein
across the blood-retina barrier in retinitis pigmentosa complicated
by macular oedema. Graefes Arch Clin Exp Ophthalmol 236:881–889, 1998. 247. Grover S, Fishman GA, Fiscella RG et al: Efficacy of dorzolamide hydrochloride in the management of chronic cystoid
macular edema in patients with retinitis pigmentosa. Retina 17:222–231, 1997. 248. Rho D: Acetazolamide treatment of CME in patients with uveitis. Ophthalmology 103:1717, 1996. Flach A. Acetazolamide treatment of CME in patients with uveitis. Ophthalmology 103:1715–1716, 1996. 249. Whitcup SM, Csaky KG, Podgor MJ et al: A randomized, masked, cross-over trial of acetazolamide for cystoid
macular edema in patients with uveitis. Ophthalmology 103:1054–1062, 1996. 250. Horgan SE, Fraser SG, Ferrante PF et al: Cystoid macular oedema in chronic myeloid leukaemia: treatment with acetazolamide
and response to bone marrow transplantation. Eye 10:394–6, 1996. 251. Avila MP, Weiter JJ, Jalkh AE et al: Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 91:1573, 1984. 252. Brancato R, Pece A, Avanza P, Radrizzani E: Photo-coagulation scar expansion after laser therapy for choroidal
neovascularization in degenerative myopia. Retina 10:239, 1990. 253. Soubrane G, Pison J, Bornert P et al: Néovaisseaux sous-rétiniens de la myopic dégénérative: Résultats de la photo-coagulation. Bull Soc Ophtalmol Fr 86:269, 1986. 254. Rabb MF, Garoon I, LaFranco FP: Myopic macular degeneration. Int Ophthalmol Clin 21:51, 1981. 255. Avetisov ES, Savitskaya NF: Some features of ocular microcirculation in myopia. Ann Ophthalmol 9:1261, 1977. 256. VIP Report No. 1: Photodynamic therapy of subfoveal choroidal neovascularization
in pathologic myopia with verteporfin. 1-year results
of a randomized clinical trial.— Ophthalmology 108:841–852, 2001. 257. Dean Hart JCD, Frank HJ: Retinal opacification after blunt non-perforating concussional injuries
to the globe. Trans Ophthalmol Soc UK 95:94, 1975. 258. Burton TC: Unilateral Purtscher's retinopathy. Ophthalmology 87:1096, 1980. 259. Grant WM: Toxicology of the Eye, 3rd ed. Springfield, IL, Charles C Thomas, 1986. 260. Cruess AF, Schachat AP, Nicholl J et al: Chloroquine retinopathy: Is fluorescein angiography necessary? Ophthalmology 92:1127, 1985. 261. Easterbrook M: The use of Amsler grids in early chloroquine retinopathy. Ophthalmology 91:1368, 1984. 262. Bullock JD, Albert DM: Flecked retina: Appearance secondary to oxalate crystals from methoxyflurane
anesthesia. Arch Ophthalmol 93:26, 1975. 263. Meredith TA, Wright JD, Gammon JA et al: Ocular involvement in primary oxalosis. Arch Ophthalmol 102:584, 1984. 264. Fielder AR, Garner A, Chambers TL: Ophthalmic manifestations of primary oxalosis. Br J Ophthalmol 64:782, 1980. 265. Mackool RJ, Muldoon T, Fortier A et al: Epinephrine-induced cystoid macular edema in aphakic eyes. Arch Ophthalmol 95:791, 1977. 266. McKeown CA, Swartz M, Blom J et al;: Tamoxifen retinopathy. Br J Ophthalmol 65:177, 1981. 267. Craythorn JM, Swartz M, Creel DJ: Clofazimine-induced bull's-eye retinopathy. Retina 6:50, 1986. 268. Cunningham CA, Friedberg DN, Carr RE: Clofazimine-induced generalized retinal degeneration. Retina 10:131, 1990. 269. Balian JV: Accidental intraocular tobramycin injection: A case report. Ophthalmic Surg 14:353, 1983. 270. McDonald HR, Schatz H, Allen AW et al: Retinal toxicity secondary to intraocular gentamicin injection. Ophthalmology 93:871, 1986. 271. Swartz M: Other diseases: Drug toxicity and metabolic and nutritional conditions. In Ryan SJ (ed): Retina, Vol 2. Medical Retina, pp 737–748. St. Louis, CV Mosby, 1989. 272. Tso MOM, La Piana FG: The human fovea after sungazing. Trans Am Acad Ophthalmol Otolaryngol 79:788, 1975. 273. Penner R, McNair JN: Eclipse blindness: Report of an epidemic in the military population of
Hawaii. Am J Ophthalmol 61:1452, 1966. 274. Freedman J, Gombos GM: Fluorescein fundus angiography in self-induced solar retinopathy: A
case report. Can J Ophthalmol 6:124, 1971. 275. McDonald HR, Irvine AR: Light-induced maculopathy from the operating microscope in extracapsular
cataract extraction and intraocular lens implantation. Ophthalmology 90:945, 1983. 276. Khwarg SG, Geoghegan M, Hanscom TA: Light-induced maculopathy from the operating microscope. Am J Ophthalmol 98:628, 1984. 277. Calkins JL, Hochheimer BF: Retinal light exposure from operating microscopes. Arch Ophthalmol 97:2363, 1979. 278. Mannis MJ, Becker B: Retinal light exposure and cystoid macular edema. Arch Ophthalmol 98:1133, 1980. |