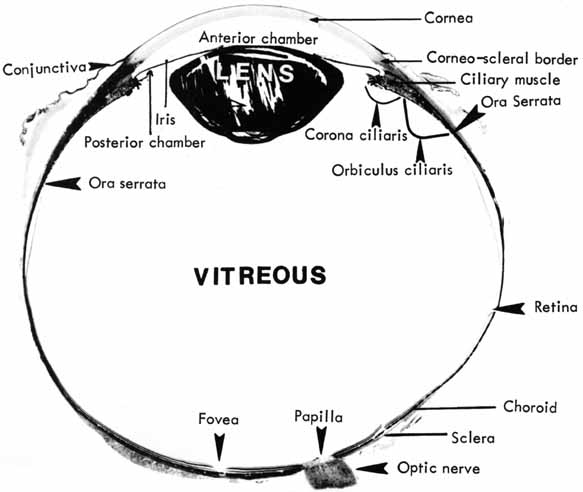
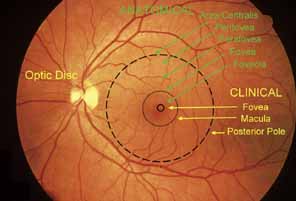
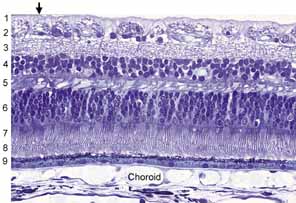
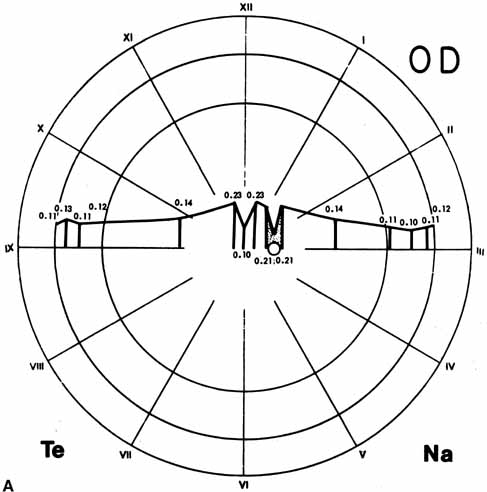
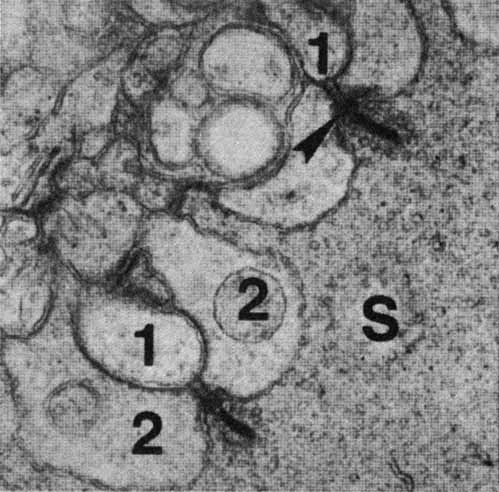
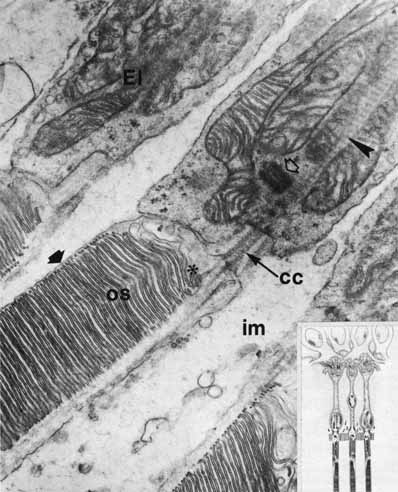
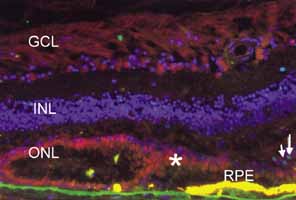
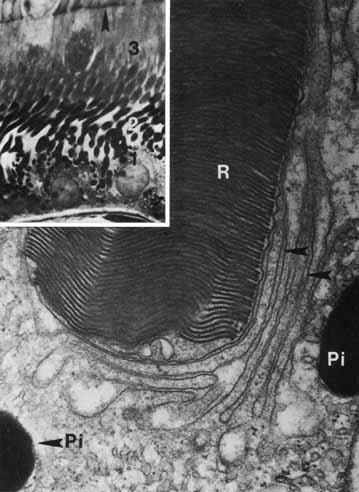
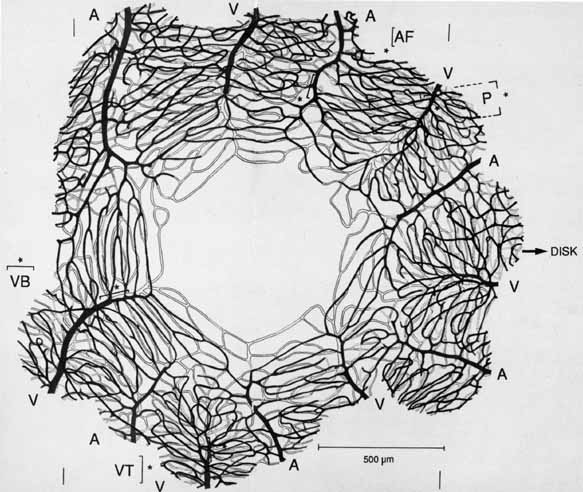
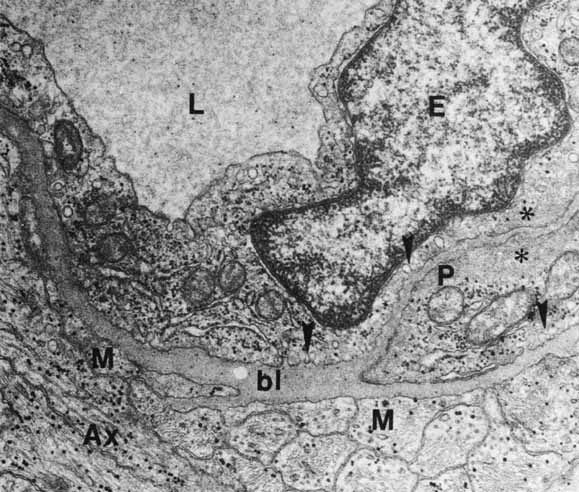
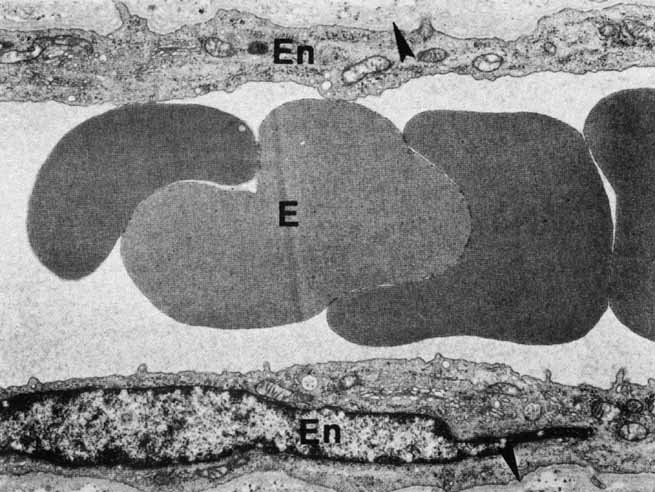
1. McDonnell JM: Ocular embryology and anatomy. In Ryan SJ, Ogden TE (eds): Retina, Vol 1. St. Louis: CV Mosby, 1989:11–15 2. Nussbaum JJ, Pruett RC, Delori FC: Historic perspectives—Macular yellow pigment: The first 200 years. Retina 1:296, 1981 3. Straatsma BR, Foos RY, Spencer LM: The retina—Topography and clinical correlations. In the New Orleans
Academy of Ophthalmology Symposium on Retina and Retinal Surgery. St. Louis: CV Mosby, 1969 4. Straatsma BR, Landers MB, Kreiger AE: The ora serrata in the adult human eye. Arch Ophthalmol 80:3, 1968 5. White MH, Lambert HM, Kincaid MC, et al: The ora serrata and the spiral of Tillaux—Anatomic relationship and
clinical correlation. Ophthalmology 96:608, 1989 6. Smiddy WE, Michels RG, Green WR: Lens and peripheral retinal relationships during vitrectomy. Retina 11:199, 1991 7. Hogan MJ: The vitreous, its structure, and relation to the ciliary body and retina. Invest Ophthalmol Vis Sci 2:418, 1963 8. Teng CC, Chi HH: Vitreous changes and the mechanism of retinal detachment. Am J Ophthalmol 44:335, 1957 9. Schepens CL: Vitreous changes in retinal detachment. In Schepens CL, Nutens A (eds): The Vitreous and Vitreoretinal Interface. New York: Springer-Verlag, 1987:85–107 10. Neubauer AS, Priglinger S, Ullrich S, et al: Comparison of foveal thickness measured with the retinal thickness analyzer
and optical coherence tomography. Retina 21:596–601, 2001 11. Blanks JC: Morphology of the retina. In Ryan SJ, Ogden TE (eds): Retina, Vol 1 St. Louis: CV Mosby, 1989:37–52 12. Osterberg G: Topography of the layer of rods and cones in the human retina. Acta Ophthalmol (suppl) 6:1, 1935 13. Yamada E: Some structural features of the fovea centralis in the human retina. Arch Ophthalmol 82:151, 1969 14. Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human Eye—An Atlas and Textbook. Philadelphia: WB Saunders, 1971 15. Handelman GJ, Snodderly DM, Krinsky NI, et al: Biological control of primate macular pigment—Biochemical and densitometric
studies. Invest Ophthalmol Vis Sci 32:257, 1991 16. Bone RA, Landrum JT, Fernandez L, Tarsis SL: Analysis of the macular pigment by HPLC—Retinal distribution and
age study. Invest Ophthalmol Vis Sci 29:843, 1988 17. Snodderly DM, Handelman GJ, Adler AJ: Distribution of individual macular pigment carotenoids in central retina
of macaque and squirrel monkeys. Invest Ophthalmol Vis Sci 32:268, 1991 18. Bone RA, Landrum JT, Mayne ST, et al: Macular pigment in donor eyes with and without AMD: A case-control
study. Invest Ophthalmol Vis Sci 42:235–240, 2001 19. Thomson LR, Toyoda Y, Langner A, et al: Elevated retinal zeaxanthin and prevention of light-induced photoreceptor
cell death in quail. Invest Ophthalmol Vis Sci 43:3538–3549, 2002 20. Bec P, Ravault M, Arne JL, Trepsat C: In the Fundus Periphery. New York: Masson Publishing, 1985:5 21. Byer NE: Cystic retinal tuft and miscellaneous peripheral retinal findings. In Jakobiec FA, Albert DM (eds): The Principles and Practice of Ophthalmology—Clinical Practice. Philadelphia: WB Saunders, 1993:1064–1070 22. Straatsma BR, Foos RY, Feman SS: Degenerative diseases of the peripheral retina. In Duane TD (ed): Clinical Ophthalmology, Vol 3, p 1. Hagerstown, MD:. Harper & Row, 1976 23. Spencer LM, Foos RY, Straatsma BR: Enclosed bays of the ora serrata—Relationship to retinal tears. Arch Ophthalmol 83:421, 1970 24. Spencer LM, Foos RY, Straatsma BR: Meridional folds, meridional complexes, and associated abnormalities of
the peripheral retina. Am J Ophthalmol 70:697, 1970 25. Sebag J: Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol 225:89, 1987 26. Sebag J: Aging of the vitreous. Eye 1:254, 1987 27. Pei YF, Smelser GK: Some fine structural features of the ora serrata region in primate eyes. Invest Ophthalmol Vis Sci 7:672, 1968 28. Wise GN, Dollery CT, Henkind P: The Retinal Circulation. New York: Harper & Row, 1971 29. Duke-Elder S, Cook C: System of Ophthalmology, Vol III, Part I, Embryology. St. Louis: CV Mosby, 1963 30. Mann I: The Development of the Human Eye. New York: Grune & Stratton, 1964 31. Edward DP, Kaufman LM: Anatomy, development, and physiology of the visual system. Pediatr Clin N Am 50:1–23, 2003 32. Cepko CL, Austin CP, Yang X, et al: Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 93:589–595, 1996 33. Lopashov CV, Stroeva OG: Development of the Eye—Experimental Studies. New York: Daniel Davey & Co, 1964 34. Vetter ML, Moore KB: Becoming glial in the neural retina. Developmental Dynamics 221:146–153, 2001 35. Hollenberg MJ, Spira AW: Early development of the human retina. Can J Ophthalmol 7:472, 1972 36. Spira AW, Hollenberg MJ: Human retinal development—Ultrastructure of the inner retinal layers. Dev Biol 31:1, 1973. Leventhal AG: Evidence that retinal ganglion cell density affects foveal development. Perspect Dev Neurobiol 3:203–211, 1996 37. Galli-Resta L: Putting neurons in the right places: local interactions in the genesis
of retinal architecture. Trends in Neuroscience 25:638–643, 2002 38. Rapaport DH, Rakic P, Lavail MM: Spatiotemporal gradients of cell genesis in the primate retina. Prospectives on Developmental Neurobiology 3:147–159, 1996 39. Shatz CJ: Emergence of order in visual system development. Proc Natl Acad Sci USA 93:602–608, 1996 40. Wong RO, Chernjavsky A, Smith SJ, et al: Early functional neural networks in the developing retina. Nature 374:716–718, 1995 41. Nag TC, Wadhwa S: Expression of GABA in the fetal, postnatal, and adult human retina: an
immunohistochemical study. Vis Neurosci 14:425–432, 1997 42. Catsicas M, Mobbs P: GABAb receptors regulate chick retinal calcium waves. J Neurosci 21:897–910, 2001 43. Leventhal AG: Evidence that retinal ganglion cell density affects foveal development. Perspect Dev Neurobiol 3:203–211, 1996 44. Hendrickson A, Kupfer C: The histogenesis of the fovea in the macaque monkey. Am J Med Sci 272:746, 1976 45. Peters A, Palay SL, Webster H deF: The Fine Structure of the Nervous System: The Neurons and Supporting Cells. Philadelphia: WB Saunders, 1976 46. Farquhar MG, Palade GE: Junctional complexes in various epithelia. J Cell Biol 17:375, 1963 47. Bennett MUL: A comparison of electrically mediated transmission. In Pappas GD, Purpura DP (eds): Structure and Function of Synapses. New York: Raven Press, 1972:221–256 48. Friend DS, Gilula NB: Variations in tight and gap junctions in mammalian tissues. J Cell Biol 53:758, 1972 49. Becker D, Bonness V, Mobbs P: Cell coupling in the retina: patterns and purpose. Cell Biology International 22:781–792, 1998 50. Dowling JE, Boycott BB: Organization of the primate retina—Electron microscopy. Proc R Soc Ser B 166:80, 1966 51. Raviola E, Gilula NB: Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci USA 70:1677, 1973 52. Kolb H, Famiglietti EV: Rod and cone pathways in the inner plexiform layer of cat retina. Science 186:47, 1974 53. Witkovsky P, Owen WG, Woodworth M: Gap junctions among the perikarya, dendrites, and axon terminals of the
luminosity-type horizontal cell of the turtle retina. J Comp Neurol 216:359, 1983 54. Naka KI, Christensen BN: Direct electrical connections between transient amacrine cells in the catfish
retina. Science 214:464, 1981 55. Cook JE, Becker DL: Gap junctions in the vertebrate retina. Microsc Res Tech 31:408–419, 1995 56. Witkovsky P, Stell WK: Retinal structure in the smooth dogfish Mustelus canis—Electron microscopy of serially sectioned bipolar cell synaptic
terminals. J Comp Neurol 150:147, 1973 57. Famiglietti EV, Kolb H: A bistratified amacrine cell and synaptic circuitry in the inner plexiform
layer of the retina. Brain Res 84:293, 1975 58. Massey SC, Mills SL: Gap junctions between AII amacrine cells and calbindin-positive
bipolar cells in the rabbit retina. Vis Neurosci 16:1181–1189, 1999 59. Mills SL, Massey SC: A series of biotinylated tracers distinguishes three types of gap junctions
in retina. J Neurosci 20:8629–8636, 2000 60. Iuvone PM: Neurotransmitters and neuromodulators in the retina--Regulation, interaction, and
cellular effects. In Adler R, Farber D (eds): The Retina—A Model for Cell Biology Studies, Part II. Orlando: Academic Press, 1986:1–72 61. Weiler R, Ball AK, Ammermuller J: Neurotransmitter systems in the turtle retina. In Osborne N, Chader G (eds): Progress in Retinal Research, Vol 10. Oxford: Pergamon Press, 1991:1–26 62. Crooks J, Kolb H: Localization of GABA, glycine, glutamate, and tyrosine hydroxylase in the
human retina. J Comp Neurol 315:287, 1992 63. Zucker CL, Ehinger B: Heterogeneity of receptor immunoreactivity at synapses of glycine-utilizing
neurons. Proc R Soc Lond 249:89, 1992 64. Sun H, Crossland WJ: Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine, and
GABA immunoreactivity in the chick retina. Anat Rec 260:158–179, 2000 65. Johnson J, Rickman DW, Brecha NC: Somatostatin and somatostatin subtype 2A expression in the mammalian retina. Microsc Res Tech 50:103–111, 2000 66. Dowling JE: The Retina—An Approachable Part of the Brain. Cambridge: Harvard University Press, 1987 67. Gray EG: Electron microscopy of presynaptic organelles of the spinal cord. J Anat 97:101, 1963 68. Whittaker VP: The use of synaptosomes in the study of synaptic and neural membrane function. In Pappas GD, Purpura DP (eds): Structure and Function of Synapses. New York: Raven Press, 1972:87–100 69. Vollrath L, Spiwoks-Becker I: Plasticity of retinal ribbon synapses. Microsc Res Tech 35:472–487, 1996 70. Wagner HJ: Presynaptic bodies (“ribbons”): from ultrastructural
observations to molecular perspectives. Cell Tissue Res 287:434–446, 1997 71. Yazulla S, Kleinschmidt J: Carrier-mediated release of GABA from retinal horizontal cells. Brain Res 263:63, 1983 72. Pfenninger KH: The cytochemistry of synaptic densities: II. Proteinaceous components and
the mechanism of synaptic connectivity. J Ultrastruct Res 35:451, 1971 73. Fawcett DW: A Textbook of Histology, 11th ed. Philadelphia: WB Saunders, 1986 74. Kuffler SW, Nicholls JG: From Neuron to Brain—A Cellular Approach to the Function of the Nervous
System. Sutherland: MA, Sinauer Associates Publishers, 1976 75. Morigiwa K, Vardi N: Differential expression of ionotropic glutamate receptor subunit in the
outer retina. J Comp Neurol 405:173–184, 1999 76. Lin B, Martin PR, Solomon SG, et al: Distribution of glycine receptor subunits on primate retinal ganglion cells: a
quantitative analysis. Eur J Neurosci 12:4155–4170, 2000 77. Koulen P, Sassoe-Pognetto M, Grunert U, et al: Selective clustering of GABA(A) and glycine receptors in the
mammalian retina. J Neurosci 16:2127–2140, 1996 78. Grunert U: Distribution of GABA and glycine receptors on bipolar and ganglion cells
in the mammalian retina. Microsc Res Tech 50:130–140, 2000 79. Pattnaik B, Jellali A, Sahel J, et al: GABAC receptors are localized with microtubule-associated protein 1B
in mammalian cone photoreceptors. J Neurosci 20:6789–6796, 2000 80. Shields CR, Tran MN, Wong RO, et al: Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic
inhibition in retinal bipolar cells. J Neurosci 20:2673–2682, 2000 81. Keyser KT, MacNeil MA, Dmitrieva N, et al: Amacrine, ganglion and displaced amacrine cells in the rabbit retina express
nicotinic acetylcholine receptors. Vis Neurosci 17:743–752, 2000 82. Wheeler-Schilling TH, Marquordt K, Kohler K, et al: Expression of purinergic receptors in bipolar cells of the rat retina. Brain Res Mol Brain Res 76:415–418, 2000 83. Nguyen-Legros J, Versaux-Botteri C, Vernier P: Dopamine receptor localization in the mammalian retina. Mol Neurobiol 19:181–204, 1999 84. Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M: Retinal photoreceptor density decreases with age. Ophthalmology 102:1853–1859, 1995 85. Mohand-Said S, Hicks D, Leveillard T, et al: Rod-cone interactions: developmental and clinical significance. Progress in Retinal and Eye Res 20:451–467, 2001 86. Dunaief JL, Dentchev T, Ying G, et al: The role of apoptosis in age-related macular degeneration. Arch Ophthalmol 120:1435–1442, 2002 87. Missotten L: The ultrastructure of the human retina. Editions Arscia SA. Bruxelles: Uitgaven N V Presses academiques europeennes S C, 1965 88. Missotten L: L'ultrastructure des cones de la retine humaine. Bull Soc Belge Ophtalmol 132:472, 1963 89. Cohen AI: Rods and cones and the problem of visual excitation. In Straatsma BR, Hall MO, Allen RA, Crescitelli F (eds): The Retina—Morphology, Function and Clinical Characteristics. Berkeley, Los Angeles: University of California Press, 1969:31–62 90. Hargrave PA: Molecular dynamics of the rod cell. In Adler R, Farber D (eds): The Retina—A Model for Cell Biology Studies, Part I. Orlando: Academic Press, 1986:207–237 91. Wald G, Brown PK: Human rhodopsin. Science 127:222, 1958 92. Young RW: The renewal of photoreceptor cell outer segments. J Cell Biol 33:61, 1967 93. Hogan MJ, Wood I, Steinberg RH: Phagocytosis by pigment epithelium of human retinal cones. Nature 252:305, 1974 94. Besharse JC: Photosensitive membrane turnover in differentiated membrane domains and
cell-cell interaction. In Adler R, Farber D (eds): The Retina—A Model for Cell Biology Studies, Part I. Orlando: Academic Press, 1986:297–352 95. Young RW: Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci 15:700, 1976 96. Mullen RJ, LaVail MM: Inherited retinal dystrophy—Primary defect in pigment epithelium
determined with experimental rat chimeras. Science 192:799, 1976 97. Anderson DH, Fisher SK: The photoreceptors of diurnal squirrels—Outer segment structure, disc
shedding, and protein renewal. J Ultrastruct Res 55:119, 1976 98. Hogan MJ, Wood I, Steinberg RH: Phagocytosis by pigment epithelium of human retinal cones. Nature 252:305, 1974 99. Adler AJ, Severin KM: Proteins of the bovine interphotoreceptor matrix—Tissues of origin. Exp Eye Res 2:755, 1981 100. Hewitt AT, Adler R: The retinal pigment epithelium and interphotoreceptor matrix—Structure
and specialized functions. In Ryan SJ, Ogden TE (eds): Retina, Vol 1. St. Louis: CV Mosby, 1989:57–64 101. Webster H deF, Ames A: Reversible and irreversible changes in the fine structure of nervous tissue
during oxygen and glucose deprivation. J Cell Biol 26:885, 1965 102. Lessell S, Kuwabara T: Phosphatase histochemistry of the eye. Arch Ophthalmol 71:851, 1964 103. Matsusaka T: ATPase activity in the ciliary rootlet of the human retinal rods. J Cell Biol 33:203, 1967 104. Fine BS, Zimmerman LE: Observation on the rod and cone layer of the human retina—A light
and electron microscopic study. Invest Ophthalmol Vis Sci 2:446, 1963 105. Uga S, Nakao F, Mimura M, Ikui H: Some new findings on the fine structure of the human photoreceptors cells. J Electron Microse (Tokyo) 19:71, 1970 106. Kuwabara T: Microtubules in the retina. In Rohen JW (ed): The Structure of the Eye, II Symp. Stuttgart: Schattauer-Verlay, 1965:69–84 107. Spitznas M: Zur Feinstruktur der sog. Membrana limitans externa der menschilichen Retina. Graefes Arch Clin Exp Ophthalmol 180:44, 1970 108. Kolb H, Famiglietti EV: Rod and cone pathways in retina of cat. Invest Ophthalmol Vis Sci 15:935, 1976 109. Apple DJ, Rabb MF: Ocular Pathology—Clinical Applications and Self-Assessment, 3rd
ed. St. Louis: CV Mosby, 1985 110. Fine BS, Zimmerman LE: Müller's cells and the “middle limiting membrane” of
the human retina—An electron microscopic study. Invest Ophthalmol Vis Sci 1:304, 1962 111. Boycott BB, Kolb H: The connections between bipolar cells and photoreceptors in the retina
of the domestic cat. J Comp Neurol 148:91, 1973 112. Villegas GM: Ultrastructure of the human retina. J Anat 98:501, 1964 113. Kolb H, Linberg KA, Fisher SK: Neurons of the human retina—A Golgi study. J Comp Neurol 318:147, 1992 114. Mariani AP: Giant bistratified bipolar cells in the monkey retina. Anat Rec 206:215, 1983 115. Rodieck RW: The primate retina. Comp Primate Biol 4(Neurosciences) 203, 1988 116. Allen RA: The retinal bipolar cells and their synapses in the inner plexiform layer. In Straatsma BR, Hall MO, Allen RA, Crescitelli F (eds): The Retina--Morphology, Function and Clinical Characteristics. Berkeley, Los Angeles: University of California Press 1969:101–143 117. Fine BS: Synaptic lamellae in the human retina—An electron microscopic study. J Neuropathol Exp Neurol 22:255, 1963 118. Wu SM, Gao F, Maple BR: Integration and segregation of visual signals by bipolar cells in the tiger
salamander retina. In Kolb H, Ripps H, Wu S (eds): Progress in Brain Research, Vol 131. Amsterdam: Elsevier Science BV, 2001:125–143 119. Kolb H: Organization of the outer plexiform layer of the primate retina: Electron
microscopy of Golgi-impregnated cells. Philos Trans R Soc Lond (Biol) 258:261, 1970 120. Kolb H, Nelson R, Ahnelt P, et al: Cellular organization of the vertebrate retina. In Kolb H, Ripps H, Wu S (eds): Progress in Brain Research, Vol 131. Amsterdam: Elsevier Science BV, 2001:3–26 121. Boycott BB, Hopkins JM, Sperling HG: Cone connections of the horizontal cells of the rhesus monkey's retina. Proc R Soc Lond (Biol) 299:345, 1987 122. Yamada E: Some observations on the fine structure of the human retina. Fukuoka Acta Med 57:163, 1966 123. Kolmer W: Uber Krystalloide in Nervenzellen der menschlichen Netzhaut. Anat Anz 51:314, 1918 124. Yoshida M: The fine structure of the so-called crystalloid body of the human
retina as observed with the electron microscope. J Electron Microsc (Tokyo) 14:285, 1966 125. Uga S, Ikui H: Some observations on the Kolmer's crystalloid of the human retina. J Electron Microsc (Tokyo) 18:153, 1969 126. Dietrich CE: Feinstructurelle Untersuchungen and den Horizontalzellen der menschlichen
Netzhaut. Z Zellforsch 98:277, 1969 127. Yamada E, Ishikawa T: The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol 30:383, 1965 128. Kolb H, Ahnelt P, Fisher SK, et al: Chromatic connectivity of the three horizontal cell types in the human
retina. Invest Ophthalmol Vis Sci (suppl) 30:348, 1989 129. Dacey DM, Lee BB, Stafford DK, et al: Horizontal cells of the primate retina: cone specificity without spectral
opponency. Science 271:656–659, 1996 130. Wassle H, Dacey DM, Haun T, et al: The mosaic of horizontal cells in the macaque monkey retina: with a comment
on biplexiform ganglion cells. Vis Neurosci 17:591–608, 2000 131. Polyak SL: The Retina. Chicago: University of Chicago Press, 1941 132. Weiler R, He S, Vaney DI: Retinoic acid modulates gap junctional permeability between horizontal
cells of the mammalian retina. Eur J Neurosci 11:3346–3350, 1999 133. Xin D, Bloomfield SA: Dark- and light-induced changes in coupling between horizontal
cells in mammalian retina. J Comp Neurol 405:75–87, 1999 134. Dowling JE, Boycott BB: Neural connections of the retina: Fine structure of the inner plexiform
layer. Cold Spring Harb Symp Quant Biol 30:393, 1965 135. Hughes A, Wieniawa-Narkiewicz E: A newly identified population of presumptive microneurons in the cat retinal
ganglion cell layer. Nature 284:468, 1980 136. Wong ROL, Hughes A: The morphology, number, and distribution of a large population of confirmed
displaced amacrine cells in the adult cat retina. J Comp Neurol 255:159, 1987 137. Yoshida K, Watanabe D, Ishikane H, et al: A key role of starburst amacrine cells in originating retinal directional
selectivity and optokinetic eye movement. Neuron 30:771–780, 2001 138. Zucker CL, Ehinger B: Synaptic connections involving immunoreactive glycine receptors in the
turtle retina. Vis Neurosci 10:907, 1993 139. Gallego A: Horizontal and amacrine cells in the mammalian's retina. Vision Res (suppl) 3:33, 1971 140. Curio CA, Allen KA: Topography of ganglion cells in human retina. J Comp Neurol 300:5, 1990 141. Schein SJ: Anatomy of macaque fovea and spatial densities of neurons in foveal representation. J Comp Neurol 269:479, 1988 142. Kolb H, Marshak D: The midget pathways of the primate retina. Documenta Ophthalmologica 106:67–81, 2003 143. Polyak S: The vertebrate visual system. In Kluver H (ed): The Vertebrate Visual System. Chicago: University of Chicago Press, 1957 144. Bunt A, Minckler D: Displaced ganglion cells in the retina of the monkey. Invest Ophthalmol Vis Sci 16:95, 1977 145. Dowling JE, Boycott BB: Retinal ganglion cells: A correlation of anatomical and physiological approaches. In Straatsma BR, Hall MO, Allen RA, Crescitelli F (eds): The Retina--Morphology, Function and Clinical Characteristics. Berkeley, Los Angeles: University of California Press, 1969:145–161 146. Kenyon GT, Marshak DW: Gap junctions with amacrine cells provide a feedback pathway in ganglion
cells within the retina. Proc R Soc Lond B Biol Sci 265:919–925, 1998 147. Wagner HG, MacNichol EFJr , Wolbarsht ML: The response properties of single ganglion cells in the goldfish retina. J Gen Physiol 43:45, 1960 148. Wiesel TN, Hubel DH: Spatial and chromatic intersections in the lateral geniculate body of the
rhesus monkey. J Neurophysiol 29:1115, 1966 149. Gallego A: Connections transversales au niveau des couches plexiformes de la retine. Actual Neurophysiol 6:5, 1965 150. Brown JE, Major D: Cat retinal ganglion cell dendritic fields. Exp Neurol 15:70, 1966 151. Shapley R, Perry VH: Cat and monkey retinal ganglion cells and their visual functional roles. Trends Neurosci 9:229, 1986 152. Boycott GG, Wassle H: The morphological types of ganglion cells of the domestic cat's retina. J Physiol (Lond) 240:397, 1974 153. Berson E: Electrical phenomena in the retina. In Moses R, Hart W (eds): Adler's Physiology of the Eye. St. Louis: CV Mosby, 1987:506–567 154. Abe H, Iwata K: Checkerboard pattern reversal VER in the assessment of glaucomatous field
defects. Acta Soc Ophthalmol Jpn 80:829, 1976 155. Quigley HA, Sanchez RM, Dunkelberger GR, et al: Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci 28:913, 1987 156. Provencio I, Rodriguez IR, Jiang G, et al: A novel human opsin in the inner retina. J Neurosci 20:600–605, 2000 157. Hattar S, Liao HW, Takao M, et al: Melanopsin-containing retinal ganglion cells: architecture, projections, and
intrinsic photosensitivity. Science 295:1065–1070, 2002 158. Barinaga M: How the brain's clock gets daily enlightenment. Science 295:955–957, 2002 159. Provencio I, Rollag MD, Castrucci AM: Photoreceptive net in the mammalian retina. Nature 415:493, 2002 160. Lucas RJ, Freedman MS, Munoz M, et al: Regulation of the mammalian pineal by non-rod, non-cone, ocular
photoreceptors. Science 284:505–507, 1999 161. Harman A, Abrahams B, Moore S, et al: Neuronal density in the human retinal ganglion cell layer from 1 to 77 years. Anat Rec 260:124–131, 2000 162. Jonas JG, Dichtl A: Evaluation of the retinal nerve fiber layer. Surv Ophthalmol 40:369–378, 1996 163. Pollock SC, Miller NR: The retinal nerve fiber layer. Int Ophthalmol Clin 26:201, 1986 164. Vrabec F: The temporal raphe of the human retina. Am J Ophthalmol 62:926, 1966 165. Quigley HA, Addicks EM: Quantitative studies of the retinal nerve fiber layer defects. Arch Ophthalmol 100:807, 1982 166. Radius RL: Thickness of the retinal nerve fiber layer in primate eyes. Arch Ophthalmol 98:1625, 1980 167. Varma R, Skaf M, Barron E: Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology 103:2114–2119, 1996 168. Takamoto T, Schwartz B: Differences by quadrant of retinal nerve fiber layer thickness in healthy
eyes. J Glaucoma 11:359–364, 2002 169. Bowd C, Weinreb RN, Williams JM, et al: The retinal nerve fiber layer thickness in ocular hypertensive, normal, and
glaucomatous eyes with optical coherence tomography. Arch Ophthalmol 118:22–26, 2000 170. McLeod D, Marshall J, Kohner EM, et al: The role of axoplasmic transport in the pathogenesis of retinal cotton-wool
spots. Br J Ophthalmol 61:177, 1977 171. Ochs S: Rate of fast axoplasmic transport in mammalian nerve fibers. J Physiol 227:627, 1972 172. Edstrom A, Mattsson H: Fast axonal transport in vitro in the sciatic system of the frog. J Neurochem 19:205, 1972 173. Guedes V, Schuman JS, Hertzmark E, et al: Optical coherence tomography measurement of macular and nerve fiber layer
thickness in normal and glaucomatous human eyes. Ophthalmology 110:177–189, 2003 174. Tjon-Fo-Sang MJ, de Vries J, Lemij HG: Measurement by nerve fiber analyzer of retinal nerve fiber layer thickness
in normal subjects and patients with ocular hypertension. Am J Ophthalmol 122:220–227, 1996 175. Aydin A, Wollstein G, Price LL, et al: Optical coherence tomography assessment of retinal nerve fiber layer thickness
changes after glaucoma surgery. Ophthalmology 110:1506–1511, 2003 176. Uga S: Some structural features of the retina müllerian cells in the juxtaoptic
nerve region. Exp Eye Res 19:105, 1974 177. Jean-Louis G, Katz BJ, Digre KB, et al: Acquired and progressive retinal nerve fiber layer myelination in an adolescent. Am J Ophthalmol 130:361–362, 2000 178. Rosen B, Barry C, Constable IJ: Progression of myelinated retinal nerve fibers. Am J Ophthalmol 127:4710473, 1999 179. Leys AM, Leys MJ, Hooymans JM, et al: Myelinated nerve fibers and retinal vascular abnormalities. Retina 16:89–96, 1996 180. Makarov FN, Hollender H, Stone J: The structure of interrelationships between neuroglial and ganglion cells
in the retina. Neuroscience and Bahavioral Physiology 30:707–711, 2000 181. Boulder Committee: Embryonic vertebrate central nervous system. Revised
terminology. Anat Rec 166:257, 1970 182. Weigert F: Beitrage zur Kenntnis der normalen menschlichen Neuroglia. Frankfurt am Main, Weisbrod, 1895 183. Palay SL, Chan-Palay V: Cerebellar Cortex, Cytology and Organization. New York: Springer-Verlag, 1974 184. Mori S, Leblond CP: Electron microscopic features and proliferation of astrocytes in the corpus
callosum of the rat. J Comp Neurol 137:197, 1969 185. Vaughn JE, Hinds PL, Skoff RP: Electron microscopic studies of wallerian degeneration in rat optic nerves. I. The
multipotential glia. J Comp Neurol 140:175, 1970 186. De Robertis D, Gerschenfeld HM: Submicroscopic morphology and function of glial cells. In Rev Neurobiol 3:1, 1961 187. Kuffler SW, Nicholls JG: The physiology of neuroglial cells. Ergeb Physiol 57:1, 1966 188. Palay SL: The role of neuroglia in the organization of the central nervous system. In Rodahl K, Issekutz B Jr (eds): Nerve as a Tissue. New York: Hoeber, 1966:3–10 189. Cammermeyer J: The life history of the microglial cell: A light microscopic study. In Ehrenpreis S, Solnitzky OC (eds): Neurosciences Research, Vol 3. New York: Academic Press, 1970:43–129 190. Matthews MA, Kruger L: Electron microscopy of nonneuronal cellular changes accompanying neural
degeneration in thalamic nuclei of the rabbit. II. Reactive elements
within the neuropil. J Comp Neurol 148:313, 1973 191. Stenwig AE: The origin of brain macrophages in traumatic lesions, wallerian degeneration, and
retrograde degeneration. J Neuropathol Exp Neurol 31:696, 1972 192. Blakemore WF: Microglial reaction following thermal necrosis of the rat cortex: An electron
microscope study. Acta Neuropathol 21:11, 1972 193. Inomata H: Electron microscopic observations on Müller's fiber in the human
retina. Acta Soc Ophthalmol Jpn 69:2133, 1965 194. Hogan MJ, Feeney L: Ultrastructure of Müller cells and perivascular glia. Invest Ophthalmol Vis Sci 2:101a, 1963 195. Ogden TE: The glia of the retina. In Ryan WSJ, Ogden TE (eds): Retina, Vol I. St. Louis: CV Mosby, 1989:53–56 196. Uga S, Nakao F: The structure and function of the Müller cells in the human retina. II. The
structure of the Müller cells in the normal retina. Acta Soc Ophthalmol Jpn 74:1018, 1970 197. Magalhaes MM: Functional cytoarchitecture of the retina Müller cell. In Yamada E, Mishima S (eds): The Structure of the Eye, III. Tokyo: Jpn J Ophthalmol, 1976:333–345 198. Magalhaes MM, Coimbra A: The rabbit retina Müller cell. A fine structural and cytochemical
study. J Ultrastruct Res 39:310, 1972 199. Edwards RB, Adler AJ, Dev S, Claycomb RC: Synthesis of retinoic acid from retinol by cultured rabbit Müller
cells. Exp Eye Res 54:481, 1992 200. Das SR, Bhardwaj N, Kjeldbye H, Gouras P: Müller cells of chicken retina synthesize 11-cis-retinol. Biochem J 285:907, 1992 201. Eisenfeld AJ, Bunt-Milam AH, Saari JC: Localization of retinoid-binding proteins in developing rat retina. Exp Eye Res 41:299, 1985 202. Milam AH, De Leeur AM, Gaur VP, Saari JC: Immunolocalization of cellular retinoic acid-binding protein to
Müller cells and/or a subpopulation of GABA-positive amacrine
cells. J Comp Neurol 296:123, 1990 203. Inomata H: Fine structure of cystoid degeneration in the peripheral retina. Acta Soc Ophthalmol Jpn 69:1357, 1965 204. Gass JD: Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses
concerning its role in the pathogenesis of macular hole
and foveomacular retinoschisis. Arch Ophthalmol 117:821–823, 1999 205. Hogan MJ, Feeney L: The ultrastructure of the retinal vessels. III. Vascular-glial relationships. J Ultrastruct Res 9:47, 1963 206. Wolter JR: The cells of Remak and the astroglia of the normal human retina. Arch Ophthalmol 53:832, 1955 207. Wolter JR: Glia of the human retina. Am J Ophthalmol 48:370, 1959 208. Wolter JR: The astroglia of the human retina and other glial elements of the retina
under normal and pathologic conditions. Am J Ophthalmol 40:88, 1955 209. Provis JM: Development of the primate retinal vasculature. Progress in Retinal and Eye Research 20:799–821, 2001 210. Ramirez JM, Ramirez AI, Salazar JJ, et al: Changes of astrocytes in retinal ageing and age-related macular
degeneration. Exp Eye Res 73:601–615, 2001 211. Vrabec F: Microglia in the monkey and rabbit retina. J Neuropathol Exp Neurol 29:217, 1970 212. Provis JM, Diaz CM, Penfold PL: Microglia in human retina: a heterogeneous population with distinct ontogenies. Perspect Dev Neurobiol 3:213–222, 1996 213. Roque RS, Rosales AA, Jingjing L, et al: Retina-derived microglial cells induce photoreceptor cell death
in vitro. Brain Res 836:110–119, 1999 214. Smelser GK, Ishikawa T, Pei YF: Electron microscopic studies of intra-retinal spaces—Diffusion
of particulate materials. In Rohen JW (ed): Structure of the Eye, II Symp. Stuttgart: Schattauer-Verlay, 1965:109–121 215. Iwasaki M, Myers KM, Rayborn ME, Hollyfield JG: Interphotoreceptor matrix in the human retina: Cone-like domains
surround a small population of rod photoreceptors. J Comp Neurol 319:277, 1992 216. Hageman GS, Johnson LV: Structure, composition and function of the retinal interphotoreceptor matrix. In Osborne N, Chader G (eds): Progress in Retinal Research, Vol 10. Oxford: Pergamon Press, 1991:207–250 217. Jerdan JA, Kao L, Glaser BM: The inner limiting membrane: A modified basement membrane? Invest Ophthalmol Vis Sci (suppl) 27:230a, 1986 218. Kohno T, Sorgente N, Ishibashi T, et al: Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci 28:506, 1987 219. Foos RY: Vitreoretinal juncture: Topographical variations. Invest Ophthalmol Vis Sci 11:801, 1972 220. Anderson DR: Ultrastructure of the optic nerve head. Arch Ophthalmol 83:63, 1970 221. Bargmann W: Histologie und mikroskopische Anatomie des Menschen, 6th ed. Stuttgart: Georg Thieme Verlag, 1967 222. Archer D, Krill AE, Newell FW: Fluorescein studies of normal choroidal circulation. Am J Ophthalmol 69:543, 1970 223. Ashton N: The mode of development of the retinal vessels in man. In Cant JS (ed): The William MacKenzie Symposium on the Ocular Circulation in Health and
Disease. St. Louis: CV Mosby, 1969:7–18 224. Cogan DG: Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc UK 83:465, 1963 225. Mintz Hittner HA, Knight Nanan DM, Satriano DR, et al: A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology 106:1409–1413, 1999 226. Mutlu F, Leopold IH: The structure of the human retinal vascular system. Arch Ophthalmol 71:93, 1964 227. Kuwabara T, Cogan DG: Studies of retinal vascular patterns. Part I. Normal architecture. Arch Ophthalmol 64:904, 1960 228. Justice J, Lehmann RP: Cilioretinal arteries. Arch Ophthalmol 94:1355, 1976 229. Maharajan VS, Dua HS, Scott R, et al: Unexpected visual improvement with a pinhole in central retinal artery
occlusion and cilioretinal artery sparing fovea. Eye 16:194–196, 2002 230. Ernest JT: Macrocirculation and microcirculation of the retina. In Ryan SJ, Ogden TE (eds): Retina, Vol I. St. Louis: CV Mosby, 1989:65–66 231. Harris A, Cuilla TA, Chung HS, et al: Regulation of retinal and optic nerve blood flow. Arch Ophthalmol 116:1491–1495, 1998 232. Laties AM: Central retinal artery innervation: Absence of adrenergic innervation to
the intra-ocular branches. Arch Ophthalmol 77:405, 1967 233. Laties AM: Neurovascular relationships in the retina. Anat Rec 163:216, 1969 234. Forster BA, Ferrari-Dileo G, Anderson DR: Adrenergic alpha 1 and alpha 2 binding sites are present in bovine retinal
blood vessels. Invest Ophthalmol Vis Sci 28:1741, 1987 235. Denis P, Elena PP: Recepteurs beta-adrenergiques vasculares retiniens chez I'homme. Ophthalmologie 3:62, 1989 236. Ferrari-Dileo G, Davis EB, Anderson DR: Response of retinal vasculature to phenylephrine. Invest Ophthalmol Vis Sci 31:1181, 1990 237. Shin DH, Tsai CS, Parrow KA, et al: Vasoconstrictive effect of topical timolol on human retinal arteries. Graefes Arch Clin Exp Ophthalmol 229:298, 1991 238. Hogan MJ, Feeney L: Ultrastructure of the retinal vessels. Part I. The larger vessels. J Ultrastruct Res 9:10, 1963 239. Shakib M, Cunha-Vaz JG: Studies on the permeability of the blood-retinal barrier. IV. Junctional
complexes of the retinal vessels and their role in the permeability
of the blood-retinal barrier. Exp Eye Res 5:229, 1966 240. Gardner TW, Antonetti DA, Barber AJ, et al: The molecular structure and function of the inner blood-retinal
barrier. Documenta Ophthalmologica 97:229–237, 1999 241. Ashton N: Studies on the retinal capillaries in relation to diabetic and other retinopathies. Br J Ophthalmol 47:521, 1963 242. Hogan MJ, Feeney L: The ultrastructure of the retinal blood vessels. II. The small vessels. J Ultrastruct Res 9:29, 1963 243. Green WR, Chan CC, Hutchins GM, Terry JM: Central retinal vein occlusion: A prospective histopathologic study of 29 eyes
in 28 cases. Retina 1:27, 1981 244. Feist RM, Ticho BH, Shapiro MJ, Farber M: Branch retinal vein occlusion and quadrant variation in arteriovenous crossings. Am J Ophthalmol 113:664, 1992 245. Wong TY, Klein R, Nieto FJ, et al: Retinal microvascular abnormalities and 10-year cardiovascular mortality—a
population-based case-control study. Ophthalmology 110:933–940, 2003 246. Weinberg DV, Egan KM, Seddon JM: Asymmetric distribution of arteriovenous crossings in the normal retina. Ophthalmology 100:31, 1993 247. Opremcak EM, Bruce RA: Surgical decompression of branch retinal vein occlusion via arteriovenous
crossing sheathotomy: a prospective review of 15 cases. Retina 19:1–5, 1999 248. Zhao J, Sastry SM, Sperduto RD, et al: The Eye Disease Case-Control Study Group: Arteriovenous crossing
patterns in branch retinal vein occlusion. Ophthalmology 100:423, 1993 249. Rassam SM, Patel V, Chen HC, et al: Regional retinal blood flow and vascular autoregulation. Eye 10:331–337, 1996 250. Rawji MH, Flanagan JG: Intraocular and interocular symmetry in normal retinal capillary perfusion. J Glaucoma 10:4–12, 2001 251. Toussaint D, Kuwabara T, Cogan DG: Retinal vascular patterns. II. Human retinal vessels studied in three dimensions. Arch Ophthalmol 65:575, 1961 252. Marquardt R: Contribution to topography and anatomy of the retinal vessels of the human
eye. Klin Monatsbl Augenheilkd 148:50, 1966 253. Michaelson IC: Retinal Circulation in Man and Animals. Springfield, IL:. Charles C Thomas, 1954 254. Duke-Elder S, Wybar KC: System of Ophthalmology, Vol II. The Anatomy of the Visual System. St. Louis: CV Mosby, 1961 255. Snodderly DM, Weinhaus RS, Choi JC: Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci 12:1169, 1992 256. Iwasaki M, Inomata H: Relation between superficial capillaries and foveal structures in the human
retina. Invest Ophthalmol Vis Sci 27:1698, 1986 257. Henkind P: New observations on the radial peripapillary capillaries. Invest Ophthalmol 6:103, 1967 258. Henkind P: Radial peripapillary capillaries: Past-present-future. In Shimizu K (ed): Fluorescein Angiography. Tokyo: Igaku Shoin, 1974:91–95 259. Kornzweig AL, Elasoph I, Feldstein M: Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol 80:696, 1968 260. Kuwabara T, Cogan DG: Retinal vascular patterns, Part VI. Mural cells of the retinal capillaries. Arch Ophthalmol 69:492, 1963 261. Martin AR, Bailie JR, Robson T, et al: Retinal pericytes control expression of nitric oxide synthase and endothelin–1 in
microvascular endothelial cells. Microvasc Res 59:131–139, 2000 262. Bandopadhyay R, Orte C, Lawrenson JG, et al: Contractile proteins in pericytes at the blood-brain and blood-retinal
barriers. J Neurocytology 30:35–44, 2001 263. Chen Q, Anderson DR: Effect of CO2 on intracellular pH and contraction of retinal capillary
pericytes. Invest Ophthalmol Vis Sci 38:643–651, 1997 264. Cogan DG, Toussaint D, Kuwabara T: Retinal vascular patterns, Part IV. Diabetic retinopathy. Arch Ophthalmol 66:366, 1961 265. Hammes H, Lin J, Renner O, et al: Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51:3107–3112, 2002 266. Circulating anitpericyte autoantibodies in diabetic retinopathy. Retina 19:390–400, 1999 267. Leuenberger PM: Ultrastructure of the aging retinal vascular system with special reference
to quantitative and qualitative changes of capillary basement membranes. Gerontologia 19:1, 1973 |