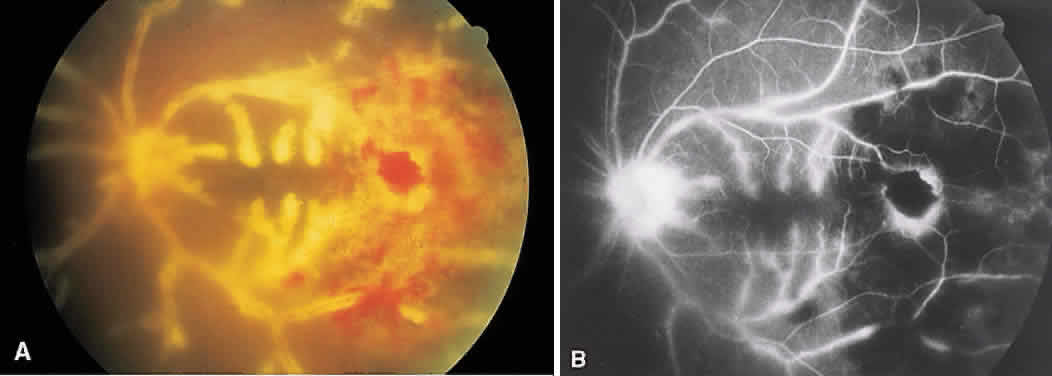
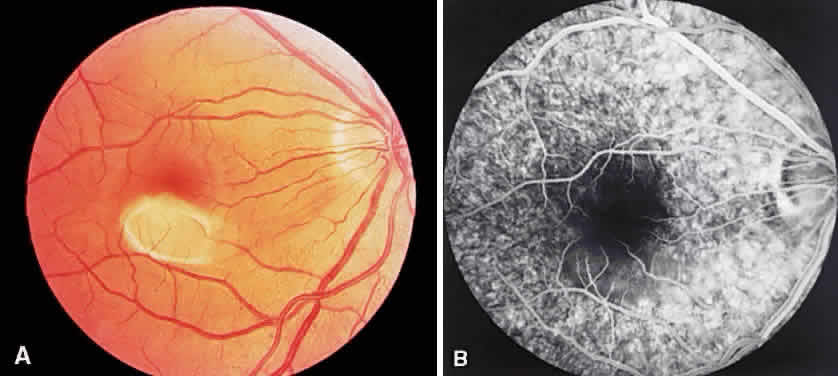
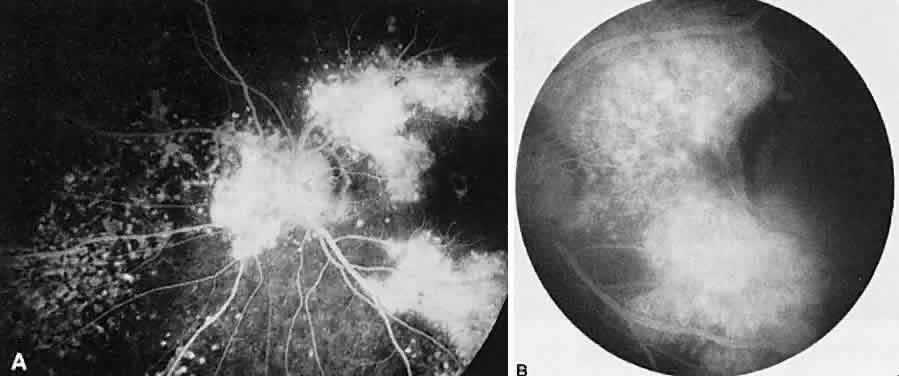
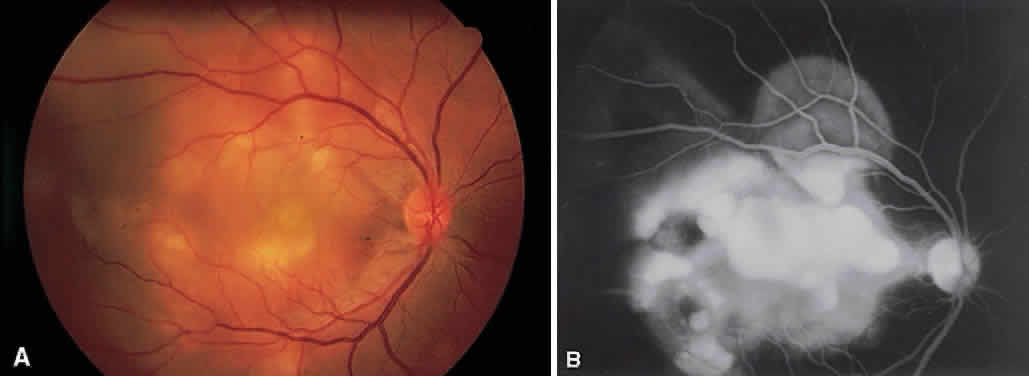
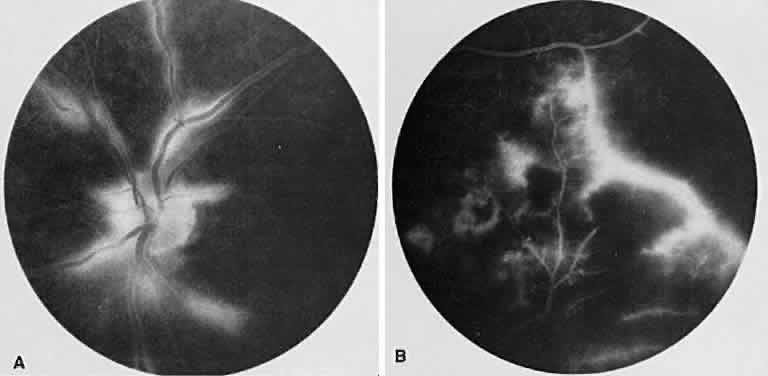
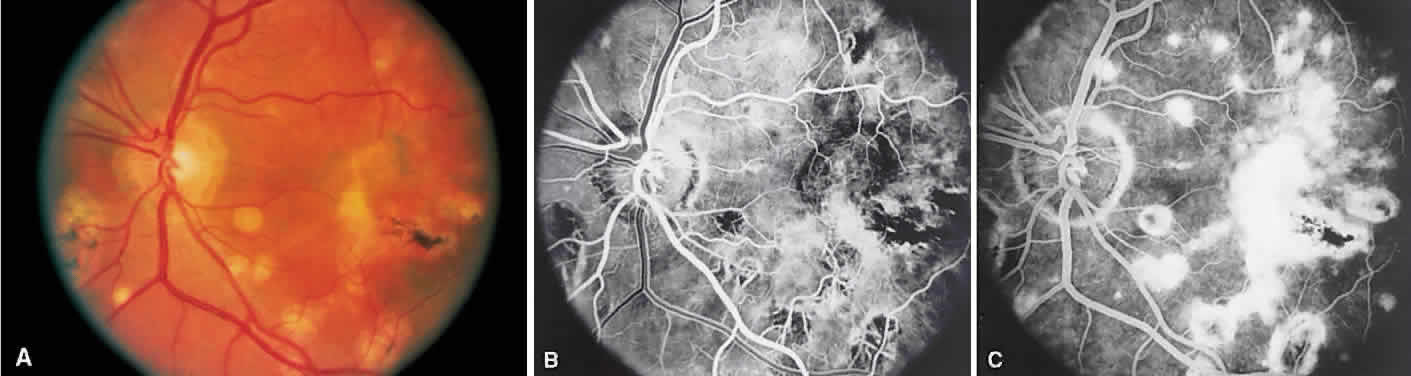
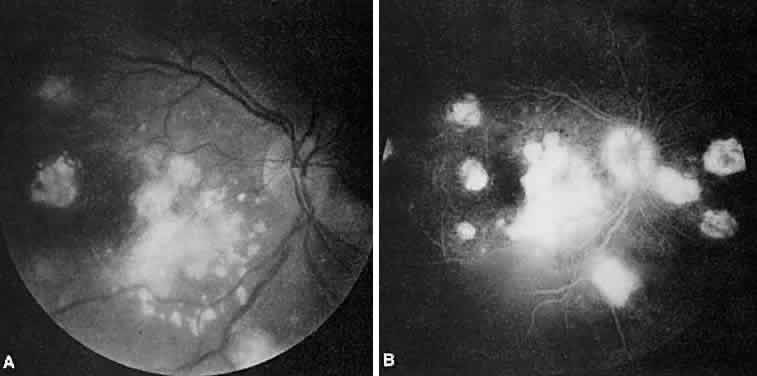
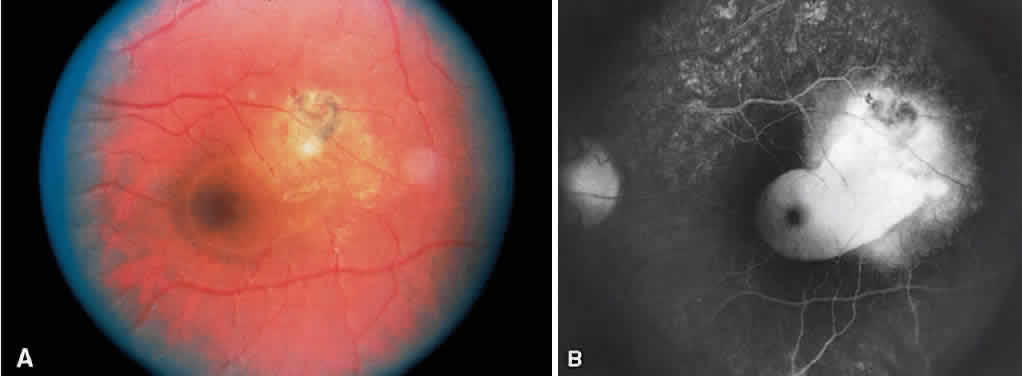
1. Archer D, Krill AF, Newell FW: Fluorescein studies of normal choroidal circulation. Am J Ophthalmol 69:543, 1970 2. Rabb MF, Burton TC, Schatz H et al: Fluorescein angiography of the fundus: A schematic approach to interpretation. Surv Ophthalmol 22:387, 1978 3. Schatz H, Burton TC, Yannuzzi LA et al: Interpretation of Fundus Fluorescein
Angiography. St Louis: CV Mosby, 1978 4. Freund KB, Yannuzzi LA, Schneider U et al: A schematic approach to clinical
interpretation. In Yannuzzi LA, Flower RW, Slakter JS (eds): Indocyanine
Angiography. St Louis: Mosby, 1997:129 5. Guyer DR, Yannuzzi LA, Slakter JS et al: Digital indocyanine green videoangiography of occult choroidal neovascularization. Ophthalmology 101:1727, 1994 6. Yaannuzzi LA, Hope-Ross M, Slakter JS et al: Analysis of vascularized pigment epithelial detachments using indocyanine
green videoangiography. Retina 14:99, 1994 7. Shimizu K: Harada's, Behçet's, Vogt-Koyanagi syndromes: Are they
clinical entities? Trans Am Acad Ophthalmol Otolaryngol 77:281, 1973 8. Saari M: Vogt-Koyanagi-Harada syndrome. Ophthalmologica 169:326, 1974 9. Ober RR, Smith RE, Ryan SJ: Subretinal neovascularization in the Vogt-Koyanagi-Harada syndrome. Int Ophthalmol 6:225, 1983 10. Freund KB, Yannuzzi LA: Vogt-Koyanagi-Harada syndrome. In Yannuzzi LA, Flower
RW, Slakter JS (eds): Indocyanine Angiography. St Louis: Mosby, 1997:261 11. Dreyer WB, Zegarra H, Zakov ZN et al: Sympathetic ophthalmia. Am J Ophthalmol 92:816, 1981 12. Sharp DC, Bell RA, Patterson E et al: Sympathetic ophthalmia: Histologic and fluorescein angiographic correlation. Arch Ophthalmol 102:232, 1984 13. Bernasconi O, Auer C, Zografos L et al: Indocyanine green angiographic findings in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol 236:635, 1998 14. Benson WE, Shields JA, Tasman W et al: Posterior scleritis. Arch Ophthalmol 97:1482, 1979 15. Margargal LE, Donoso LA, Goldberg RE et al: Ocular manifestations of relapsing polychondritis. Retina 1:96, 1981 16. Cleary PE, Watson PG, McGill JI et al: Visual loss due to posterior segment disease in scleritis. Trans Ophthalmol Soc UK 95:297, 1975 17. Auer C, Herbort CP: Indocyanine green angiographic features in posterior scleritis. Am J Ophthalmol 126:471, 1998 18. Gass JDM, Sever RJ, Grizzard WS et al: Multifocal pigment epithelial detachments by reticulum cell sarcoma. Retina 4:135, 1984 19. Shimuzu K: Fluorescein fundus angiography. Mod Probl Ophthalmol 10:224, 1972 20. Michelson JB, Michelson PE, Chisari FV: Subretinal neovascular membrane and disciform scar in Behçet's
disease. Am J Ophthalmol 90:182, 1980 21. Atmaca LS: Fundus changes associated with Behçet's disease. Graefes Arch Clin Exp Ophthalmol 227:340, 1989 22. Matsu T, Sato Y, Shiraga F et al: Choroidal abnormalities in Behçet's disease observed by simultaneous
indocyanine green and fluorescein angiography with scanning
laser ophthalmoscopy. Ophthalmology 106:295, 1999 23. Klaeger A, Tran VT, Hiroz C et al: Indocyanine green angiography in Behçet's uveitis. Retina 20:309, 2000 24. Atmaca LS, Batioglu F: Indocyanine green videoangiography and color Doppler imaging in Behçet's
disease. Acta Ophthalmol Scand 77:444, 1999 25. Algvere P: Fluorescein studies of retinal vasculitis in sarcoidosis. Acta Ophthalmol Copenh 48:1129, 1970 26. Chumbley L, Kearns TP: Retinopathy of sarcoidosis. Am J Ophthalmol 73:123, 1972 27. Sanders MD, Shilling JS: Retinal, choroidal, and optic disc involvement in sarcoidosis. Trans Ophthalmol Soc UK 96:140, 1976 28. Asdourain GK, Goldberg MF, Busse BJ: Peripheral retinal neovascularization in sarcoidosis. Arch Ophthalmol 93:787, 1975 29. Wolfensberger TJ, Herbort CP: Indocyanine green angiographic features in ocular sarcoidosis. Ophthalmology 106:285, 1999 30. Pruett RC, Brockhurst RJ, Letts NF: Fluorescein angiography of peripheral uveitis. Am J Ophthalmol 77:448, 1974 31. Zenker HJ: Fluorescein angiography in inflammation of the peripheral fundus. III. Involvement
of the pars plana corporis ciliaris. Ophthalmologica 190:205, 1985 32. Felder KS, Brockhurst RJ: Neovascular fundus abnormalities in peripheral uveitis. Arch Ophthalmol 100:750, 1982 33. Shorb SR, Irvine AR, Kimura SJ et al: Optic disk neovascularization associated with chronic uveitis. Am J Ophthalmol 82:175, 1976 34. Arkfeld DF, Brockhurst RJ: Peripapillary subretinal neovascularization in peripheral uveitis. Retina 5:157, 1985 35. Gass JDM: Vitiliginous chorioretinitis. Arch Ophthalmol 99:1778, 1981 36. Fuerst DJ, Tessler HT, Fishman GA et al: Birdshot retinochoroidopathy. Arch Ophthalmol 102:214, 1984 37. Brucker AJ, Deglin EA, Bene C et al: Subretinal choroidal neovascularization in birdshot retinochoroidopathy. Am J Ophthalmol 99:40, 1985 38. Chang B, Lumbroso L, Rabb MF et al: Birdshot chorioretinopathy. In Yannuzzi
LA, Flower RW, Slakter JS (eds): Indocyanine Angiography. St Louis: Mosby, 1997:231 39. Dreyer RF, Gass JDM: Multifocal choroiditis and panuveitis. Arch Ophthalmol 102:1776, 1984 40. Cantrill HL, Folk JC: Multifocal choroiditis associated with progressive subretinal fibrosis. Am J Ophthalmol 101:170, 1986 41. Slakter JS, Giovannini A: Multifocal choroiditis and the presumed ocular
histoplasmosis syndrome. In Yannuzzi LA, Flower RW, Slakter JS (eds): Indocyanine
Angiography. St Louis: Mosby, 1997:271 42. Deutman AF, Lion F: Choriocapillaris nonperfusion in acute multifocal placoid pigment epitheliopathy. Am J Ophthalmol 84:652, 1977 43. Hedges TR, Sinclair S, Gragoudas ES: Evidence for vasculitis in acute posterior multifocal placoid pigment epitheliopathy. Ann Ophthalmol 10:539, 1978 44. Gass JDM: Stereo Atlas of Macular Disease. St Louis: CV Mosby, 1987:508 45. Schatz H, Park D, McDonald HR et al: Acute multifocal posterior placoid
pigment epitheliopathy. In Yannuzzi LA, Flower RW, Slakter JS (eds): Indocyanine
Angiography. St Louis: Mosby, 1997:239 46. Chisholm H, Gass JDM, Hutton WL: The late stage of serpiginous (geographic) choroiditis. Am J Ophthalmol 82:343, 1976 47. Schatz H, Maumenee AE, Patz A: Geographic helicoid peripapillary choroidopathy. Trans Am Acad Ophthalmol Otolaryngol 78:747, 1974 48. Hamilton AM, Bird AC: Geographical choroidopathy. Br J Ophthalmol 58:784, 1974 49. Laatikainen L, Erkkila H: Subretinal and disc neovascularization in serpiginous choroiditis. Br J Ophthalmol 66:326, 1982 50. Giovannini A, Ripa E, Scassellati-Sforzolini B et al: Indocyanine green angiographic findings in serpiginous choroidopathy. Eur J Ophthalmol 9:299, 1996 51. Mones JM, Slakter JS: Serpiginous choroidopathy. In Yannuzzi LA, Flower
RW, Slakter JS (eds): Indocyanine Angiography. St Louis: Mosby, 1997:247 52. Mamalis N, Daily MJ: Multiple evanescent white dot syndrome: A report of eight cases. Ophthalmology 94:1209, 1987 53. Jampol LM, Sieving PA, Pugh D et al: Multiple evanescent white dot syndrome. Arch Ophthalmol 102:671, 1984 54. Aaberg TM, Campo RV, Joffe L: Recurrences and bilaterality in the multiple evanescent white dot syndrome. Am J Ophthalmol 100:29, 1985 55. Ie D, Glaser BM, Murphy RP et al: Indocyanine green angiography in multifocal evanescent white dot syndrome. Am J Ophthalmol 117:7, 1994 56. Krill AE, Deutman AF: Acute retinal pigment epitheliitis. Am J Ophthalmol 74:193, 1972 57. Chittum M, Kalina RE: Acute retinal pigment epitheliitis. Ophthalmology 94:1114, 1987 58. Deutman AF: Acute retinal pigment epitheliitis. Am J Ophthalmol 78:571, 1974 59. Bos PJM, Deutman AF: Acute macular neuroretinopathy. Am J Ophthalmol 80:573, 1975 60. Rush JA: Acute macular neuroretinopathy. Am J Ophthalmol 83:490, 1977 61. Priluck IA, Buettner H, Robertson DM: Acute macular neuroretinopathy. Am J Ophthalmol 86:775, 1978 62. Sieving PA, Fishman GA, Salzano T et al: Acute macular neuroretinopathy: Early receptor potential changes suggest
photoreceptor pathology. Br J Ophthalmol 68:229, 1984 63. Price FW, Schlaegel TF: Bilateral acute retinal necrosis. Am J Ophthalmol 89:419, 1980 64. Hayreh SS: So-called acute retinal necrosis syndrome: An acute ocular panvasculitis
syndrome. Dev Ophthalmol 10:40, 1985 65. Rabb MF, Jampol LM, Fish RH et al: Retinal periphlebitis in patients with acquired immunodeficiency syndrome
with cytomegalovirus retinitis mimics acute frosted retinalperiphlebitis. Arch Ophthalmol 110:1257, 1992 66. Augsburger JJ, Henry RY: Retinal aneurysms in adult cytomegalovirus retinitis. Am J Ophthalmol 86:794, 1978 67. Digre KB, Blodi CF, Bale JF: Cytomegalovirus infection in a healthy adult associated with recurrent
branch retinal artery occlusion. Retina 7:230, 1987 68. Newsome DA, Green WR, Miller ED et al: Microvascular aspects of acquired immune deficiency syndrome retinopathy. Am J Ophthalmol 98:590, 1984 69. Brown RM, Mendis U: Retinal arteritis complicating herpes zoster ophthalmicus. Br J Ophthalmol 57:344, 1973 70. Marsh RJ, Easty DL, Jones BR: Iritis and iris atrophy in herpes zoster ophthalmicus. Am J Ophthalmol 78:255, 1974 71. Kovacs B, Vastag O: Fluoroangiographic picture of the acute stage of the retinal lesion in
subacute sclerosing panencephalitis. Ophthalmologica 177:264, 1978 72. Morgan CM, Webb RM, O'Connor GR: Atypical syphilitic chorioretinitis and vasculitis. Retina 4:225, 1984 73. Tait IA: Uveitis due to secondary syphilis. British Journal of Venereal Diseases 59:397, 1983 74. Folk JC, Weingeist TA, Corbett JJ et al: Syphilitic neuro-retinitis. Am J Ophthalmol 95:480, 1983 75. Crouch ER, Goldberg MF: Retinal periarteritis secondary to syphilis. Arch Ophthalmol 93:384, 1975 76. Saari M: Disciform detachment of the macula. Acta Ophthalmol Copenh 56:510, 1978 77. de Sousze EC, Jalkh AC, Trempe CL et al: Unusual central chorioretinitis as the first manifestation of early secondary
syphilis. Am J Ophthalmol 105:271, 1988 78. Bellmann C, Holz FG, Breitbart A et al: Bilateral acute syphilitic posterior placoid chorioretinopathy: Angiographic
and autofluorescence characteristics. Ophthalmologe 96:522, 1999 79. Cangemi FE, Friedman AH, Josephberg R: Tuberculoma of the choroid. Ophthalmology 87:252, 1980 80. Goldberg MF: Presumed tuberculous maculopathy. Retina 2:47, 1982 81. Wolfensberger TJ, Piguet B, Herbort CP: Indocyanine green angiographic features in tuberculous chorioretinitis. Am J Ophthalmol 127:350, 1999 82. Macular Photocoagulation Study: Argon laser photocoagulation for ocular
histoplasmosis. Arch Ophthalmol 101:1347, 1983 83. Gass JDM: Stereo Atlas of Macular Disease. St Louis: CV Mosby, 1987:118 84. Slakter JS, Giovannini A: Multifocal choroiditis and the presumed ocular
histoplasmosis syndrome. In Yannuzzi LA, Flower RW, Slakter JS (eds): Indocyanine
Angiography. St Louis: Mosby, 1997:271 85. Robertson DM, Riley FC, Hermana PE: Endogenous Candida oculomycosis. Arch Ophthalmol 91:33, 1974 86. Schwartz PL: Segmental retinal periarteritis as a complication of toxoplasmosis. Ann Ophthalmol 9:157, 1977 87. Willerson D, Aaberg TM, Reeser F et al: Unusual ocular presentation of acute toxoplasmosis. Br J Ophthalmol 61:693, 1977 88. Gilbert H: Unusual presentation of acute ocular toxoplasmosis. Graefes Arch Clin Exp Ophthalmol 215:53, 1980 89. Braunstein RA, Gass JDM: Branch artery obstruction caused by acute toxoplasmosis. Arch Ophthalmol 98:512, 1980 90. Luxenburg MN: Branch retinal artery occlusion associated with recurrent toxoplasmic retinochoroiditis. Arch Ophthalmol 105:130, 1987 91. Saari M, Miettinen R, Nieminen H et al: Retinochoroidal vascular anastomosis in toxoplasmic chorioretinitis. Acta Ophthalmol Copenh 53:44, 1975 92. Owens PL, Goldberg MF, Busse BJ: Prospective observation of vascular anastomosis between the retina and
choroid in recurrent toxoplasmosis. Am J Ophthalmol 88:402, 1979 93. Kennedy JE, Wise GN: Retinochoroidal vascular anastomosis in uveitis. Am J Ophthalmol 71:1221, 1971 94. Gaynon MW, Boldrey EE, Strahlman ER et al: Retinal neovascularization and ocular toxoplasmosis. Am J Ophthalmol 98:585, 1984 95. Fine SL, Owens SL, Haller JA et al: Choroidal neovascularization as a late complication of ocular toxoplasmosis. Am J Ophthalmol 91:318, 1981 96. Folk JC, Lobes LA: Presumed toxoplasmic papillitis. Ophthalmology 91:64, 1984 97. Schlaegel TF, Weber JC: The macula in toxoplasmosis. Arch Ophthalmol 102:697, 1984 98. Doft BH, Gass JDM: Punctuate outer retinal toxoplasmosis. Arch Ophthalmol 103:1332, 1985 99. Auer C, Bernasconi O, Herbort CP: Toxoplasmic retinochoroiditis: New insights provided by indocyanine green
angiography. Am J Ophthalmol 123:131, 1997 100. Guex-Crosier Y, Auer C, Bernasconi O et al: Toxoplasmic retinochoroiditis: Resolution without treatment of the perilesional
satellite dark dots seen by indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol 236:476, 1998 101. Auer C, Bernasconi O, Herbort CP: Indocyanine green angiography features in toxoplasmic retinochoroiditis. Retina 19:22, 1999 102. Tanikawa H, Akimoto S, Honda M et al: Indocyanine green video angiographic findings of frosted branch angitis
in a child. Nippon Ganka Gakkai Zasshi 102:399, 1998 103. Barsante CF: Cysticercus sub-retinalis. In Shimizu K (ed): Fluorescein
Angiography. Tokyo: Igaku Shoin, 1974:193 104. Gass JDM, Gildberg WR, Guerry RK et al: Diffuse unilateral subacute neuroretinitis. Ophthalmology 85:521, 1978 |