OCULAR ISCHEMIC SYNDROME
The ocular ischemic syndrome is the conglomeration of ocular symptoms and signs attributable to severe carotid artery obstructive disease.1 Rarely, it can be caused by obstruction of the innominate artery or chronic ophthalmic artery obstruction. Fluorescein angiographic signs of the ocular ischemic syndrome are listed in Table 1.
Table 1. Fluorescein Angiographic Signs of the Ocular Ischemic Syndrome
| Sign | Incidence (%) |
| Delay, patchy choroidal filling | 60 |
| Increased arteriovenous transit time | 95 |
| Retinal vascular staining | 85 |
| Macular edema | 17 |
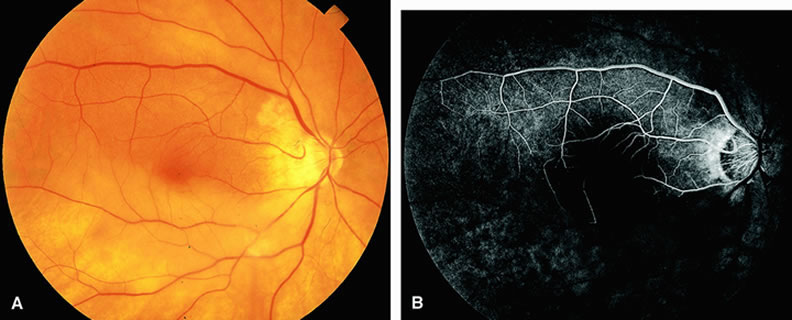
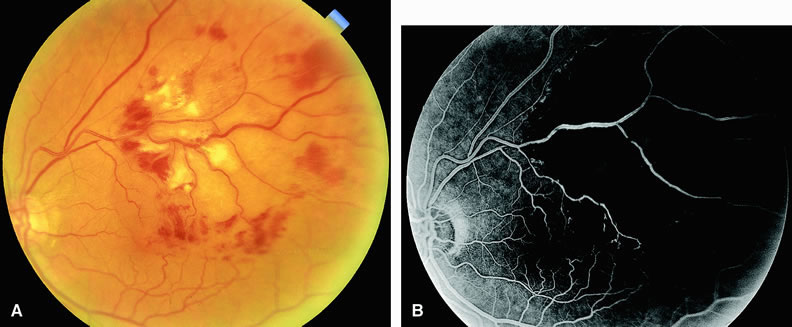
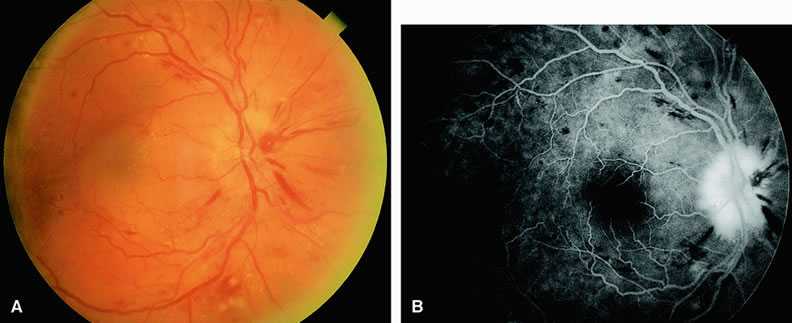
Normally, the choriocapillaris is completely filled with fluorescein dye within 5 seconds after the first appearance of dye within it. In eyes with the ocular ischemic syndrome, this filling can be delayed in extreme cases for 1 minute or longer. The posterior choroid is supplied by the temporal and nasal posterior ciliary arteries.2 In some normal eyes, but particularly in eyes with the ocular ischemic syndrome, delayed, asymmetric filling of the areas supplied by these vessels can be seen (Fig. 1).
Filling of the retinal arteries is often also delayed in eyes with the ocular ischemic syndrome, and this manifests as a delayed arm-to-retina circulation time. Although the retinal arteries usually start to fill within 15 seconds after an antecubital intravenous injection of sodium fluorescein dye, this time range varies according to several factors, including the site of injection, the rate of injection, and body circulation. A visible leading edge of dye (see Fig. 1) within a retinal artery is almost always abnormal after an intravenous injection and indicates diminished flow. In the Retina Vascular Unit at Wills Eye Hospital, Philadelphia, the upper-normal limit for retinal arteriovenous transit time (time from the first appearance of dye in the temporal retinal arteries of the arcades to the time when the corresponding veins are completely filled) is considered to be 10 to 11 seconds. The retinal arteriovenous transit time is usually prolonged in eyes with the ocular ischemic syndrome; in fact, this prolongation of time is the most common fluorescein angiographic feature in eyes with the ocular ischemic syndrome.
Leakage of fluorescein dye from the retinal vessels, particularly the arteries, occurs in 85% of eyes with the ocular ischemic syndrome (Fig. 2). Presumably, hypoxia and subsequent endothelial cell damage cause this leakage of dye. Leakage of fluorescein dye from the retinal vessels can be seen in the posterior pole and periphery. This hyperpermeability, combined with leakage of serum from microaneurysmal abnormalities, appears to account for the macular edema observed in some eyes with the ocular ischemic syndrome (see Fig. 2).
Leakage of fluorescein dye from neovascularization of the disc is seen in approximately one third of eyes with the ocular ischemic syndrome (see Fig. 2). Hyperfluorescence resulting from leaking neovascularization of the retina is less common. Retinal capillary nonperfusion is visible on fluorescein angiography in some cases. Ischemic optic neuropathy is rarely observed. Iris neovascularization is found in approximately two thirds of cases at the time the diagnosis is made.
Fluorescein angiography is helpful in differentiating the ocular ischemic syndrome from conditions that can mimic it, including central retinal artery obstruction, mild central retinal vein obstruction, and diabetic retinopathy. Of these conditions, only the ocular ischemic syndrome has delayed choroidal filling present. Moreover, late staining of the retinal arteries is unusual with the other conditions. An increased arteriovenous transit time is usually present in eyes with the ocular ischemic syndrome but can also be seen in eyes with central retinal artery or vein obstruction and eyes with diabetic retinopathy and nonperfusion of the retinal capillary bed.
OPHTHALMIC ARTERY OBSTRUCTION
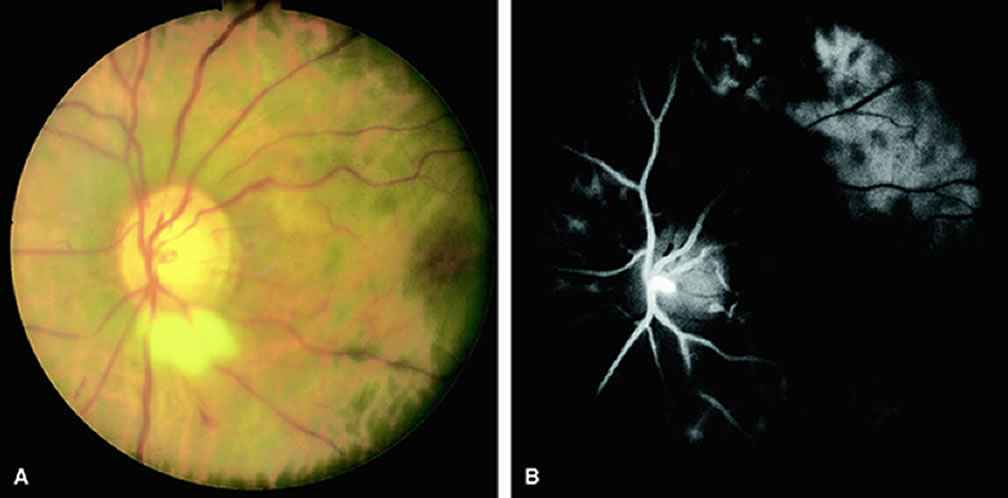
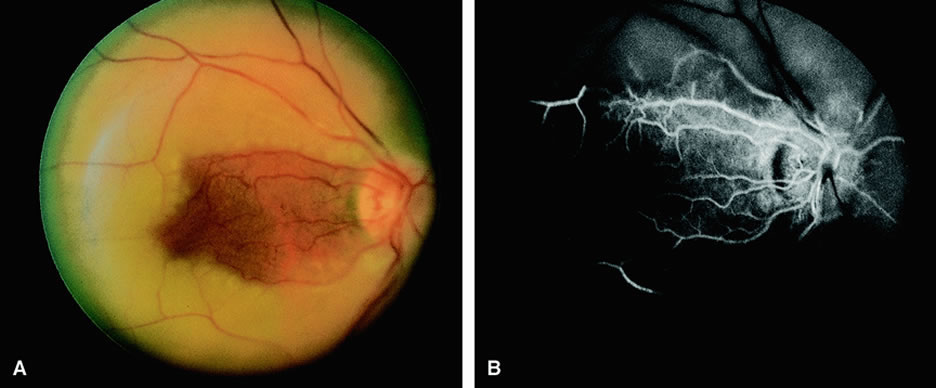
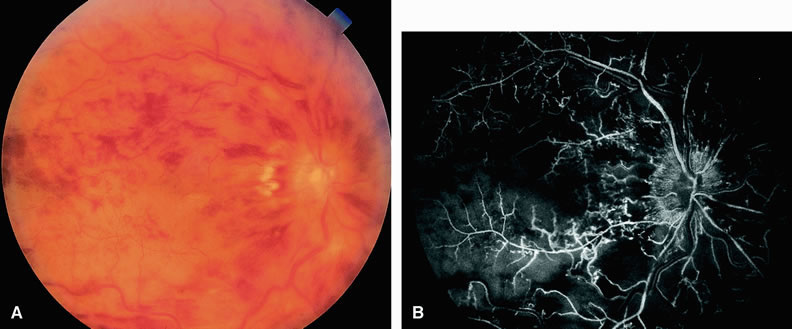
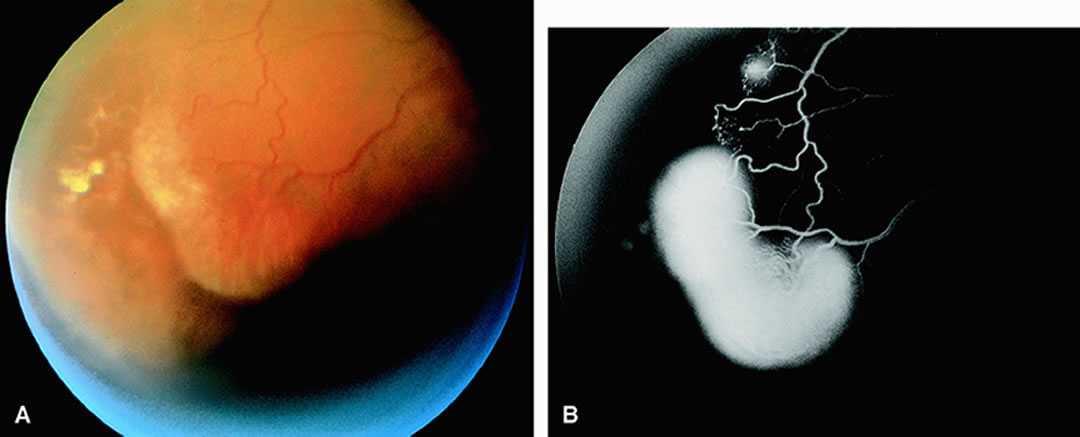
Clinically, acute ophthalmic artery obstructions differ from acute central retinal artery obstructions in that persons with the former often have no light perception and the retinal whitening appears more intense on examination.3 A cherry-red spot is often absent in eyes with acute ophthalmic artery obstruction, but its presence does not rule out the diagnosis (Fig. 3A). Electroretinography often reveals diminished amplitudes of both b- and a-waves that are caused by inner and outer retinal ischemia, respectively.3 In contrast, with a central retinal artery obstruction alone, the a-wave amplitude is usually normal and the b-wave amplitude is often diminished because of inner retinal ischemia.4
Fluorescein angiography of eyes with acute ophthalmic artery obstruction shows delayed filling of the retinal vessels and usually the choroidal vessels as well (see Fig. 3B and C). Focal, pinpoint areas of staining resulting from leakage of dye at the level of the retinal pigment epithelium can be seen in some instances. Diffuse staining is also occasionally observed. Prominent staining of the retinal vessels is usually absent with acute ophthalmic artery obstruction, although it can be seen with chronic ophthalmic artery obstruction.
RETINAL ARTERY OBSTRUCTION
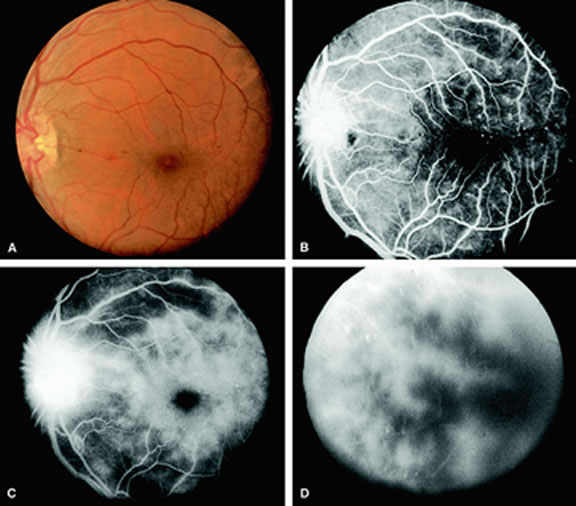
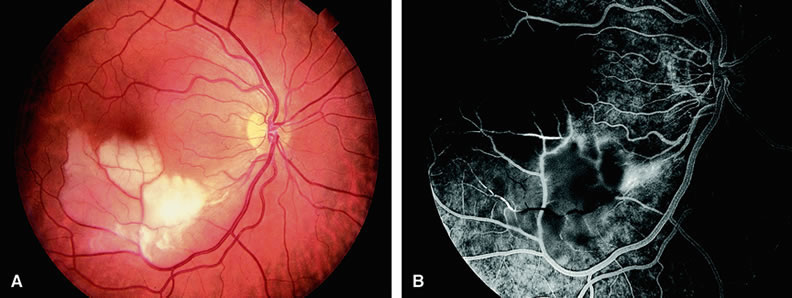
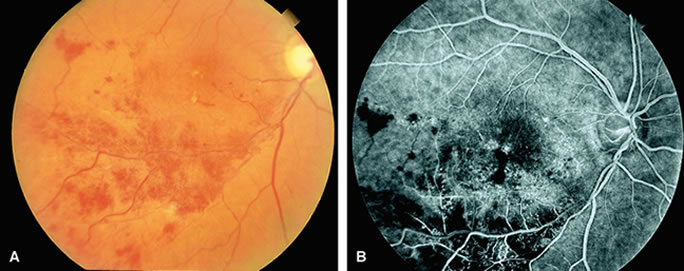
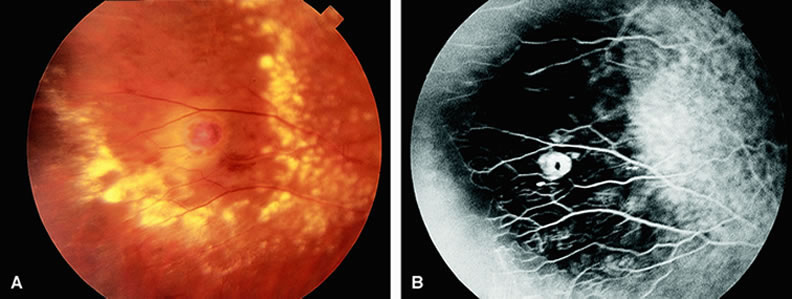
With acute central retinal artery obstruction, filling of the choroid is usually normal. Filling of the retinal arteries is often delayed, and in severe cases, a leading edge of dye can be seen (Fig. 4). A delay in retinal arteriovenous transit time is often noted.5 Box-carring or segmentation of the dye column can be seen in both the retinal arteries and veins when the obstruction is marked. In some cases, the flow appears normal because reperfusion of the blocked artery can occur fairly rapidly.6 Intraretinal leakage of dye in the late phases of the study, in a pattern consistent with macular edema, is generally not seen in eyes with acute central retinal artery obstruction. Fluorescein angiography can help identify eyes with acute central retinal artery obstruction in instances when the retinal whitening is subtle and the diagnosis is in question.
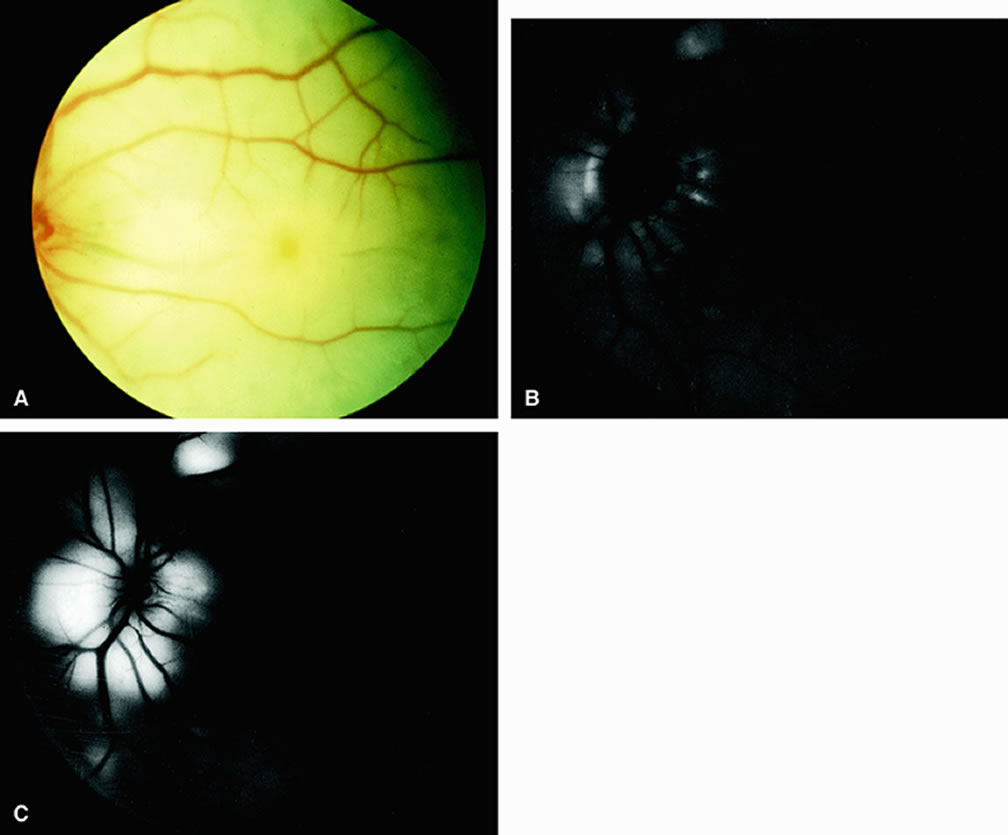
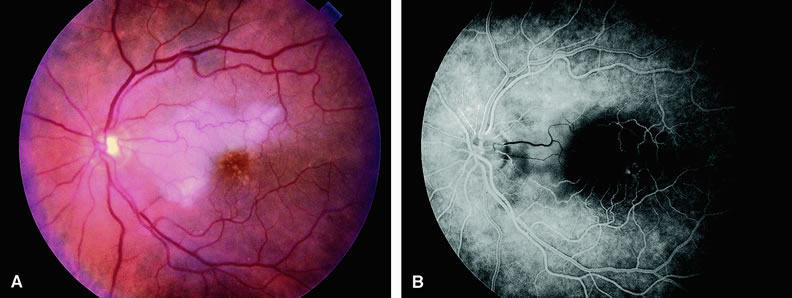
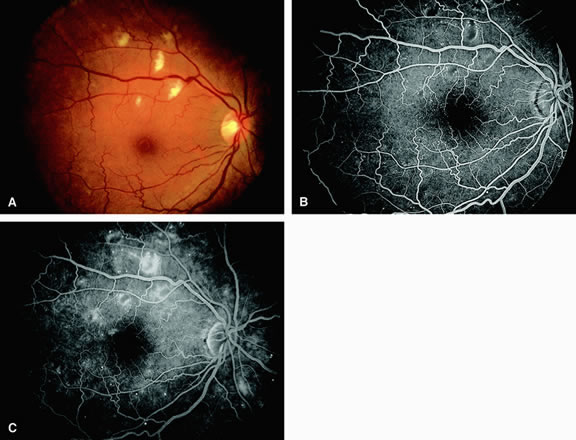
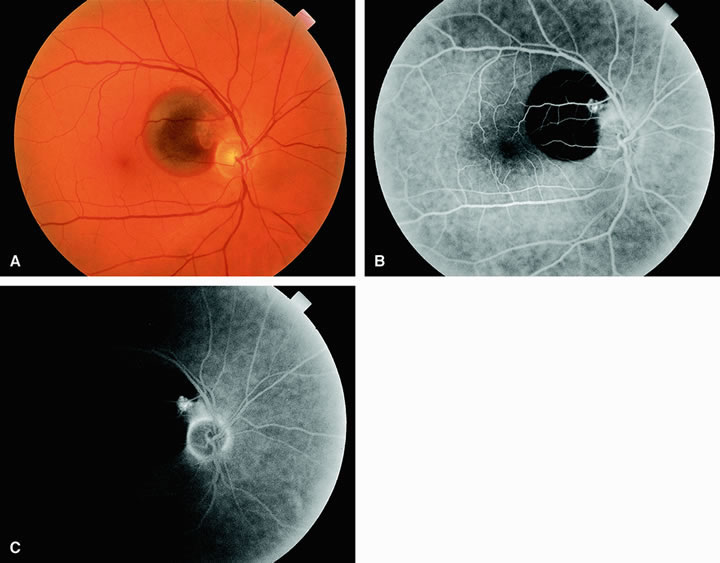
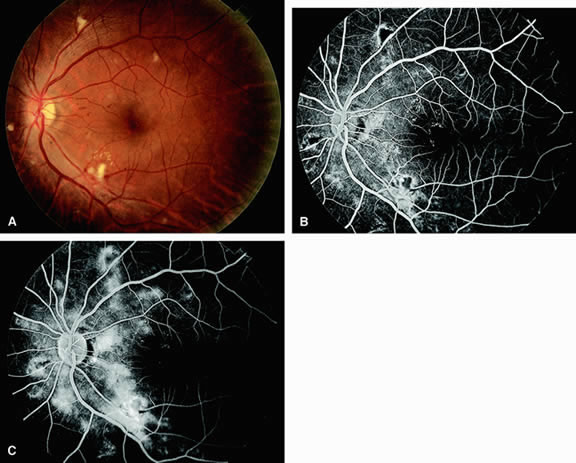
Approximately 10% of eyes with acute central retinal artery obstruction have a cilioretinal artery that supplies the retina in the papillomacular bundle and extends into the foveola.7 In more than 80% of these eyes, the visual acuity eventually improves to 20/50 or better. Fluorescein angiography typically shows earlier filling within the patent cilioretinal artery and the veins draining the area that it supplies compared with the filling of the remainder of the retina, which is supplied by the central retinal artery (Figs. 5 and 6).
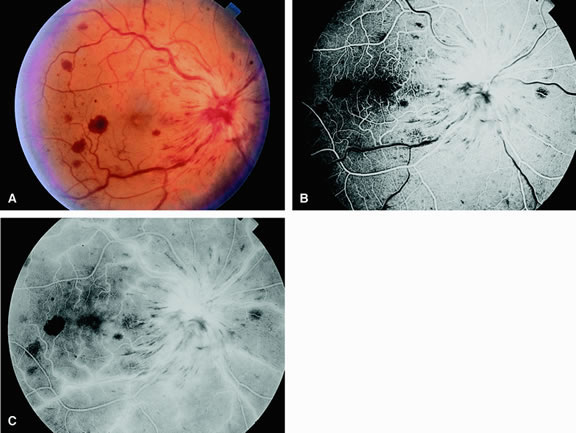
Fluorescein angiography of eyes with marked, acute branch retinal artery obstruction reveals a lack of filling of the retinal capillary bed within the distribution of the involved vessel. Retinal veins that normally drain the damaged area also demonstrate a delay in filling. With severe blockage, retrograde filling can be seen in the distal aspect of an obstructed branch retinal artery (Fig. 7).
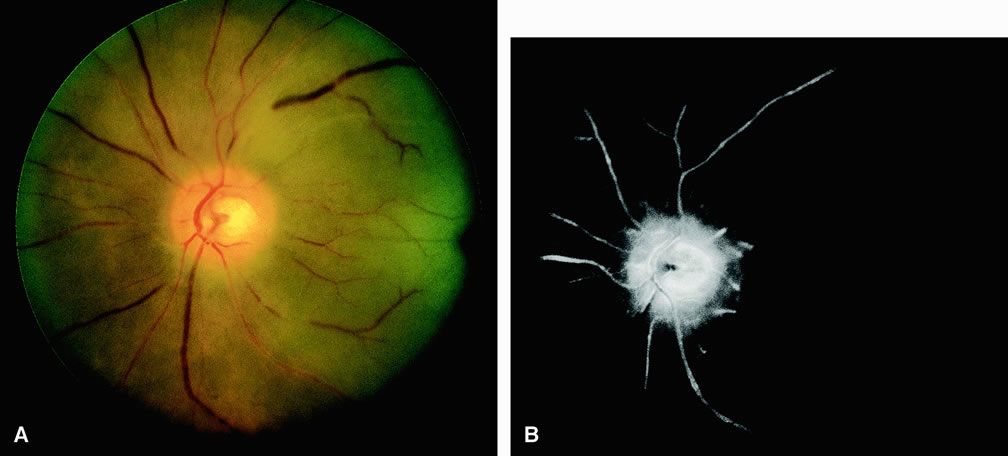
Cilioretinal artery obstruction is similar to branch retinal artery obstruction except that the former vessel usually emanates from the edge of the optic disc (Fig. 8). Cilioretinal artery obstructions can be seen as isolated fundus abnormalities or in association with central retinal vein obstruction or acute anterior ischemic optic neuropathy.8
Cotton-wool spots are small areas of superficial retinal whitening that usually develop secondary to obstruction of axoplasmic flow caused by areas of focal retinal ischemia.9 Fluorescein angiography in these cases usually demonstrates relative hypofluorescence in the early and middle phase of the study (Fig. 9). Late staining of the cotton-wool spot can occur. As shown in Table 2, the differential diagnosis of cotton-wool spots is extensive; however, cotton-wool spots are most commonly observed in the setting of diabetic retinopathy, hypertensive retinopathy, collagen vascular disease, or hematologic abnormalities.
Table 2. Abnormalities Associated with Cotton-Wool Spots in the Fundus
- Diabetic retinopathy
- Systemic arterial hypertension
- Collagen-vascular disease
- Systemic lupus erythematosus
- Dermatomyositis
- Polyarteritis nodosa
- Scleroderma
- Giant cell arteritis
- Systemic lupus erythematosus
- Cardiac valvular disease
- Mitral valve prolapse
- Rheumatic heart disease
- Endocarditis
- Mitral valve prolapse
- Acquired immunodeficiency syndrome (AIDS)
- Central and branch retinal vein obstruction
- Partial central retinal artery obstruction
- Leukemia
- Trauma
- Radiation retinopathy
- Metastatic carcinoma
- Leptospirosis
- Rocky Mountain spotted fever
- High-altitude retinopathy
- Severe anemia
- Acute blood loss
- Papilledema
- Papillitis
- Carotid artery atherosclerosis
- Dysproteinemias
- Septicemia
- Aortic arch syndrome (pulseless disease)
- Intravenous drug abuse
- Acute pancreatitis
- Onchocerciasis
- Systemic alpha interferon administration