Due to the avascular nature of the cornea, much of its oxygen requirement for metabolic activities comes from atmospheric oxygen dissolved in the tear film. When the eyelids are closed, oxygen can also predominantly enter the tear film from the superficial conjunctival capillaries. Most nutrients such as carbohydrates, vitamins, amino acids, and other substrates are primarily delivered to the cornea through the leaky corneal endothelial barrier with a minor contribution from the vascular arcades of the limbus. Certain growth factors, immune constituents, and other substrates like retinol are also secreted by the lacrimal gland and delivered to the cornea by the tears. Carbon dioxide and other metabolic end products are removed across the corneal endothelium to the aqueous humor, by the tear film, or through the limbal capillaries.
EMBRYOLOGY AND DEVELOPMENT
Following lens vesicle formation, which occurs between 4 to 5 weeks of gestation (27 to 36 days), surface ectodermal cells cover the defect left by lens vesicle invagination and become the primitive corneal epithelium composed initially of two cell layers. Similar to the avian cornea, the primitive epithelium of the primate cornea appears to be responsible for forming a prominent primary acellular corneal stroma, also known as the Bowman's layer, which becomes detectable around 20 weeks of gestation. It should be noted, however, that the primate cornea's ectodermal phase (primary acellular stroma) is de-emphasized compared to the mesodermal phase (secondary cellular stroma) and is less well organized in ultrastructure compared to that of the avian cornea.
Sometime between when the eyelids fuse together (8 weeks gestation) and by the time the eyelids open (26 weeks gestation), the epithelium stratifies to four to five cell layers thick. The basal epithelial cells, which secrete an underlying basement membrane called the basal lamina, are cuboidal to columnar. Above this layer are interdigitating wing cells and, most superficially, a layer of flattened squamous epithelial cells. Early in gestation, adhesion complexes (hemidesmosomes, anchoring fibrils, and anchoring plaques) on the basal surface of the epithelium are absent. Rudimentary adhesion complexes only become detectable by 19 weeks of gestation. With further development in utero, the number of hemidesmosomes increases, anchoring fibril penetration into the Bowman's layer increases, and Bowman's layer thickens.
A first wave of neural crest-derived mesodermal cells begins to extend beneath the corneal epithelial cells from the limbus around 5 weeks of gestation (33 days); these cells form the primitive endothelium. The primitive endothelium is initially composed of two cell layers, which by 8 weeks of gestation become a monolayer that starts to produce Descemet's membrane. The epithelium and endothelium remain closely opposed until 7 weeks of gestation (49 days) when a second wave of mesoderm begins to grow centrally from the limbus between the epithelium and endothelium. This tissue forms the secondary cellular corneal stroma, and extracellular collagen is produced within a few days. During the third month of gestation, Descemet's membrane is secreted by the endothelial cells and can be recognized in histologic sections. By the seventh month of gestation, the cornea resembles that of the adult in most structural characteristics other than size.
At birth in the full-term infant, the horizontal diameter of the cornea is only around 9.8 mm (surface area 102 mm2), or approximately 75% to 80% of the size of the adult human cornea. (Note that the posterior segment is less than 50% of adult size at birth.) Additionally, at birth, the cross-sectional thickness of the epithelium averages 50 μm, the Bowman's layer averages 12 μm, the central cellular corneal stroma averages 450 μm, Descemet's membrane averages 4 μm, and the endothelium averages 5-μm thick.
During infancy, the cornea continues to grow over the first years of life, reaching adult size at 2 years old with a horizontal diameter of 11.7 mm (surface area 138 mm2) and changes very little in size, shape, and optical properties thereafter. The only significant structure in the cornea that thickens with age is the Descemet's membrane, which gradually increases an additional 6 to 11 μm from birth to senescence.
OPTICAL PROPERTIES
The average index of refraction of the cornea and tear film taken as a whole is about 1.376. The refractive power for the anterior surface of the cornea may be computed by the following formula:
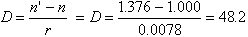
where D equals diopters of optical power, n is the index of refraction of air (1.000), n' is the index of refraction of the whole cornea, and r is the radius of curvature of the anterior corneal surface in meters.
The posterior surface of the cornea is bathed with aqueous humor. By applying the same formula, we arrive at the following value for the optical power of the posterior corneal surface, where n' is the refractive index of aqueous humor (1.336):
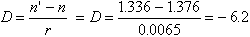
Therefore, the total optical power of the cornea is 48.2 − 6.2, or approximately 42.0 D, which is about two-thirds of the 60.0 D total optical power of the human eye. Because the cornea is thinner in the center than in the periphery, it should act as a minus lens but functions as a plus lens because the aqueous humor neutralizes most of the minus optical power on the posterior corneal surface. If we compute the power of the posterior corneal surface in air, we would find the following:
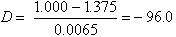
By subtracting the plus power of the anterior surface, the resulting net power for the cornea in air becomes −96.0 D + 42.0 D = −54.0 D, a powerful minus lens.
From the previous calculations, it is obvious that the most important refracting surface is that of the anterior cornea. However, if a large air bubble is placed in the anterior chamber so that it contacts the corneal endothelium or if the anterior surface of the cornea is submerged in water—thereby destroying the corneal-air interface—tremendous changes in the refractive power of the eye occur. When the eye is open underwater, the optical imagery is extremely blurred; the index of refraction of water is quite similar to that of the cornea and most of the optical power of the anterior corneal surface is lost. If the tear film–air interface is maintained by the use of a mask or goggles, then underwater mask vision is as sharp and clear as normal terrestrial vision.
A contact lens placed on the corneal surface is an efficient way of correcting optical errors in the dioptic system of the eye. This is particularly true for errors that are caused by abnormalities of corneal curvature. The contact lens placed upon the corneal surface has the same index of refraction as the cornea and becomes covered immediately with the normal corneal tear film. Therefore, the contact lens becomes, in effect, a new part of the cornea.
Several surgical procedures have also been developed to permanently alter the curvature of the cornea, thereby reducing nonpathologic refractive errors. The procedures most commonly performed today include laser-assisted in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), and astigmatic keratotomy (AK). LASIK and PRK are surgical techniques that use an excimer laser (193 nm) to ablate the anterior corneal stroma with submicron accuracy and create a new refractive surface. LASIK is different from PRK in that it involves a mechanical or laser created lamellar keratectomy step before the excimer ablation step, which is applied to the underlying residual mid-stromal bed. PRK ablation directly occurs on the surface of the cornea (Bowman's layer and anterior stroma). Both procedures have been used on myopic and hyperopic eyes with up to a mild degree of astigmatism and produce similar visual outcomes. AK is an incisional keratotomy technique that cuts up to 90% depth arcs in the peripheral cornea using a scalpel to reduce astigmatism by flattening the cornea in the visual axis over the area of the cut. AK is usually performed in association with cataract surgery.
GROSS ANATOMY
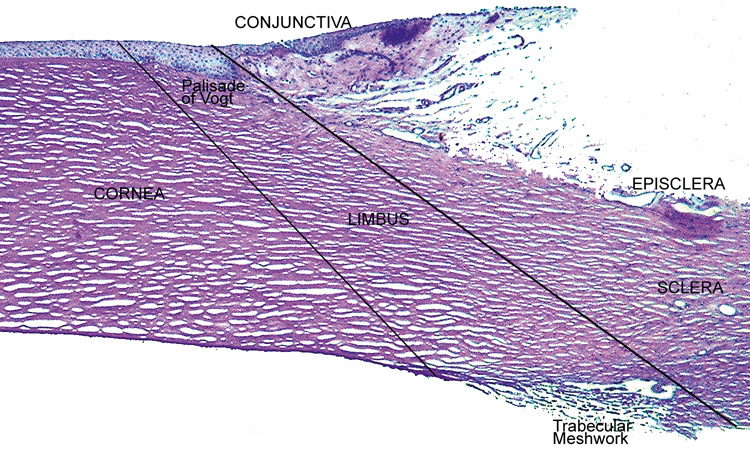
When viewed anteriorly in the living eye, the adult human cornea is somewhat elliptical; the largest measurement is typically in the horizontal meridian (mean 11.7 mm) and the smallest is in the vertical meridian (mean 10.6 mm). The elliptical configuration is brought about by an anterior extension of the opaque scleral structure superiorly and inferiorly. When viewed from the posterior surface of the dissected eye, the cornea is circular, with an average horizontal and vertical measurement of 11.7 mm. The average radius of curvature of the anterior corneal surface is 7.8 mm, which is significantly less than the 11.5 mm average radius of curvature of the sclera. This results in a small, 1.5- to 2-mm wide transition zone that forms an external and internal surface groove, or scleral sulci, where the steeper cornea meets that of the flatter sclera. These sulcus typically are not as obvious to see clinically because they are filled in by overlying episclera and conjuctiva or trabecular meshwork.
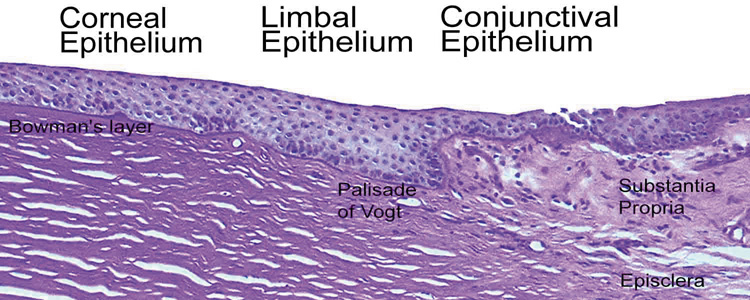
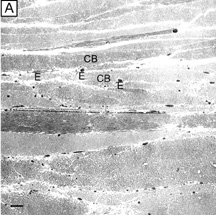
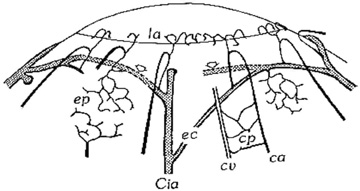
The tissue in this transition zone is known as the limbus and is important because it is used as surgical landmark for various anterior segment surgeries, contains corneal epithelial stem cells, contains the conventional outflow pathway for the aqueous humor, is the inciting site of pathology in a few immunologic or cancerous diseases, and possibly contains stem-like corneal endothelial cells (Fig. 2). The limbus is a definite anatomic landmark, or geographical reference, used for planning surgical entry into the anterior segment because it appears clinically as a blue transition zone. Therefore, an incision placed anterior to blue zone is anatomically in the peripheral cornea, safely inside the trabecular meshwork.
|
The cornea is thinner in the center, measuring on average 520 μm and increases in thickness to approximately 700 μm as it reaches the limbus.1–4 The central 4 mm of the cornea overlying the pupil contains the optical center of central vision. It typically has a regular surface and structure, and commonly is near exact in spherical configuration. The more peripheral portions of the cornea are often slightly irregular and torical in configuration rather than spherical, giving the whole cornea a hyperbolic shape. If the central cornea is not exactly spherical, the condition of astigmatism (stigma meaning a point) usually results. With astigmatism, an optical image without a single point focus forms within the eye resulting in two line foci.
The average radius of curvature for the posterior corneal surface is shorter than the anterior corneal surface at 6.5 mm. In adult humans, the conjunctival surface area has been measured at 17.65 cm2, and the corneal surface area measures 1.38 cm2, giving a c/c ratio of 12.8.5 Because topical lipophilic drug delivery to the anterior chamber occurs primarily through the cornea with the conjunctiva supplying a minor secondary delivery route, surface area ratios of the cornea and conjunctiva are important because a large conjunctiva-to-corneas (c/c) surface area ratio results in less drug delivery to the anterior chamber. Therefore, surface area ratios need to be considered when comparing drug delivery studies.
MICROSCOPIC ANATOMY, ULTRASTRUCTURE, AND PHYSIOLOGY OF THE EPITHELIUM
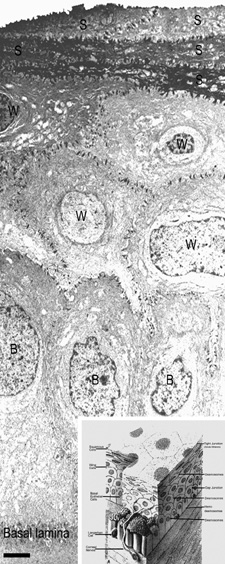
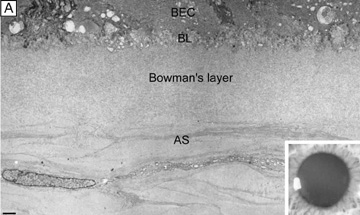
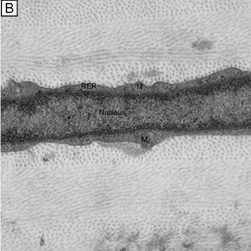
The anterior surface of the human cornea is covered by a transparent, nonkeratinized, stratified (five- to seven-cell layer) squamous epithelium uniformly around 50 μm in thickness that is continuous with the epithelium of the limbus and conjunctiva (Figs. 1, 2, and 3). The basal corneal epithelial cells actively secrete extracellular material (type IV collagen, laminin, heparin, and small amounts of fibronectin and fibrin) that forms an underlying 75-nm thick basement membrane called the basal lamina. On electron microscopy, the morphology of basal lamina appears to be composed of two distinct layers: a 25-nm thick lamina lucida and a 50 nm thick lamina densa (Fig. 3).
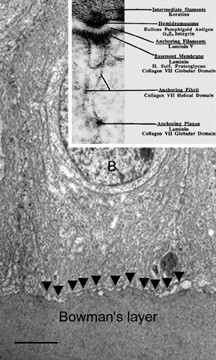
The cytoplasm of a corneal epithelial cell primarily contains cytoskeletal intermediate filaments and has sparse cytoplasmic organelles (i.e., mitochondria, endoplasmic reticulum, and golgi apparatus). The predominant cytoplasmic filament is keratin, whereas actin and microtubules are two other major types found in corneal epithelial cells. The epithelial cells are held together to one another by numerous anchoring junctions called desmosomes, whereas the basal surface of the epithelium adheres to the basal lamina and underlying Bowman's layer through aa adhesion complex composed of hemidesmosomes, anchoring fibrils (type VII collagen), and anchoring plaques (Fig. 4). The function of the corneal epithelium is twofold: (a) to form a barrier from the environment to the corneal stroma of the cornea, and (b) to form a smooth refractive surface on the cornea through interaction with the tear film.
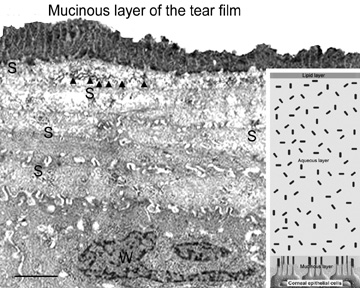
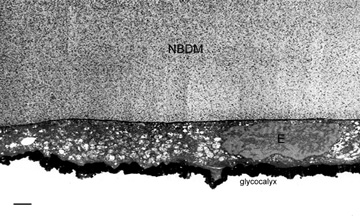
The epithelial cells differentiate from the basal layer to form two to three cell layers of wing cells and finally to form two to three cell layers of squamous cells (Fig. 3). The squamous cells form a barrier junction because they are surrounded by a continuous encircling band of zonula occludens tight junctions, which serve as a semipermeable, high-resistance (12–16 kΩ cm2) membrane6,7 by closing off the intercellular space. This barrier prevents the movement of fluid from the tears into the stroma and also protects the cornea and intraocular structures from infectious pathogens. The apical surface of the corneal epithelium is specialized to maintain the tear film as microplicae and microvilli on the surface of the most superficial epithelial cells is covered with a glycocalyx and membrane-spanning mucins (MUC 1 and possibly MUC 4); altogether these structures and substances form the 1.0 μm thick mucinous layer of the tear film (Fig. 5).8–10
The tear film typically measures 7 to 10 μm in thickness and contains three layers (mucinous, aqueous, and lipid layers). Recently, the aqueous layer of the tear film has been found to contain gel-forming mucins (MUC 5AC and 2) along with lysosyme, immunoglobulin A, transferring, defensin, and trefoil factor (Fig. 5, inset).10 Overall, the tear film functions in maintaining a healthy ocular surface by preventing ocular infections and forming a smooth optical surface required for clear vision. Deficiencies in the components that compose any of these layers potentially can cause ocular surface disease.
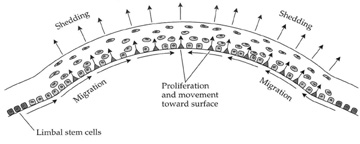
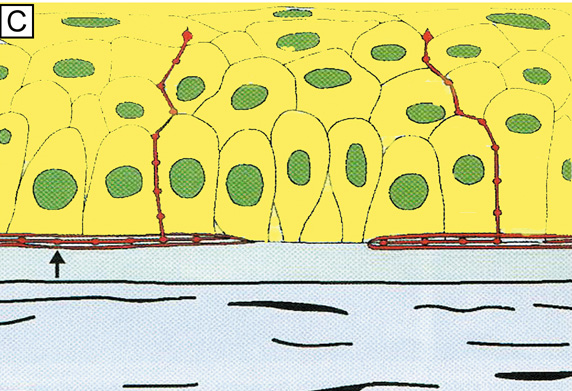
The corneal epithelium is in a state of constant healing as squamous cell are continuously shed into the tear film (Fig. 6). It is estimated that all the cell layers of the corneal epithelium completely turn over every 7 to 10 days. The epithelial surface is maintained by basal epithelial cells, which can undergo mitosis resulting in two daughter cells that are found anterior to the basal cell layer, thus forming two wing cells and eventually squamous cells.11–13 This delicate balance of shedding followed by proliferation is critical in maintaining a smooth and uniform epithelial surface around 50 μm in thickness. The shedding step is primarily induced by the friction that occurs from involuntary eyelid blinking, which happens on average every 7 seconds.
The signal for basal epithelial cell proliferation probably comes via the gap junctions, especially in the more basal layers, with connexin 43 being one of the common gap junction proteins present.7,14 Interestingly, gap junction communication between corneal epithelial cells is promoted by low concentrations of bicarbonate, which is abundant in the aqueous humor and tear film.15 The barrier function of the epithelium itself is due to zonula occludens tight junctions between the most superficial epithelial junction, which are made up of the tight junction proteins ZO-1, occludin, and claudin-1 as well as other claudin sub-types.16 Under normal conditions, this barrier is extremely tight, preventing the movement of ions between the superficial squamous cells. In some instances (e.g., trauma, contact lens wear, infection), however, this barrier can be compromised. Activation of protein kinase C (PKC) by the mitogen-activated protein kinase (MAP kinase) signaling pathway, which in itself is activated by external stimuli such as growth factors and tyrosine kinases, appears to cause disruption of the epithelial barrier and perhaps is an initiating factor for basal cells to proliferate.17
In addition to basal epithelial cell mitosis, the corneal epithelium is maintained by migration of new basal epithelial cells into the cornea from the limbus. The cells migrate centripetally at about 120 μm/week and originate from a subpopulation of limbal epithelial cells.18–20 Therefore, it appears that the corneal epithelium is maintained by a balance among the processes of limbal cell proliferation, basal corneal epithelial cell migration and proliferation, and shedding of superficial squamous corneal epithelial cells.21–22 This concept has been coined the X, Y, Z hypothesis by Thoft [X (basal corneal epithelial cell proliferation) + Y (limbal cell proliferation and basal corneal epithelial cell centripetal migration) = Z (epithelial cell loss from the surface)].22
When this equilibrium is disturbed, corneal epithelial cell wound healing typically begins. Interestingly, after injury, these processes typically return back to equilibrium with a certain degree of adaptability. For instance, after epithelial and stromal injury, if a stromal deficit occurs (e.g., corneal ulcers) then the epithelium can still maintain a smooth anterior surface either by developing elongated hypertrophic basal epithelial cells and/or by developing epithelial hyperplasia (> six cell layers).23
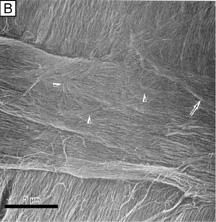
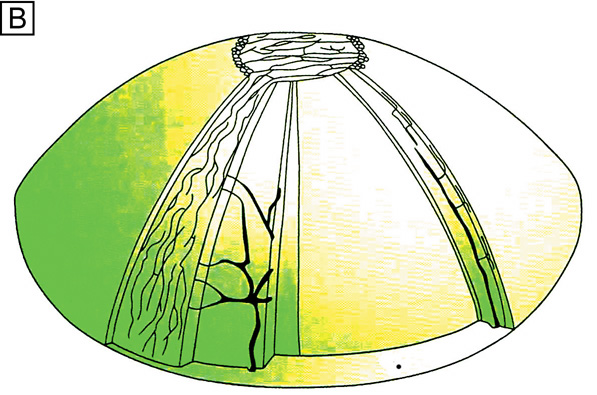
As first described in the early 1960s by Maumenee, and expanded upon in the early 1970s by the work of Davanger and Evensen and in the early 1980s by Shapiro, Friend and Thoft, the limbal epithelium contains cells that have the ability to resurface the cornea following loss of the corneal epithelium. 24–26 This concept was applied clinically by Thoft in 1977, who described the use of autologous limbal epithelial transplants to treat unilateral chemical injury.27 Subsequently, further study has determined that a portion of limbal basal epithelial cells are actually undifferentiated corneal epithelial cells, also known as corneal epithelial stem cells.28–29 These stem cells have the characteristics of being extremely long-lived, with very slow cell cycle times and a high proliferative potential. They anatomically are found within a well-defined, protective microenvironment called the palisades of Vogt (Fig. 7) and are controlled via delicate regulatory mechanisms.30–32 The progeny of stem cells are referred to as transient amplifying (TA) cells, which are cells that have more rapid cell cycles, yet have far less replicative potential than their parent stem cells. During terminal differentiation from the basalepithelial cell layer, the daughter cells migrate anteriorly in the epithelium becoming wing cells and, lastly, squamous cells.
|
When the mammalian cornea is mounted in an Ussing chamber, it generates a transepithelial potential of 25 to 40 mV. This relatively high voltage is consistent with the low ionic conductance of the apical epithelial cell membranes and the high resistance of the tight junctions of the paracellular pathway, which normally is in the range of 12 to 16 kΩ cm2.6 Almost 50% of the short-circuit current across the corneal epithelium is carried by chloride ions moving through apical membrane channels into the tears. This current is due to ionic gradients set up by epithelial transport of Na+ and Cl− ions. Na+ ions are pumped from the epithelial cells toward the stroma by the Na+/K+ adenosine triphosphatase (ATPase) in the lateral membranes of the cells, which, as in other cells, maintains an inward Na+ gradient. A Na+-chloride co-transporter, also located in the lateral membrane, facilitates the influx of Na+ down its electrochemical gradient, carrying along chloride ions. The chloride ions then diffuse through channels in the apical membranes. This chloride secretion is blocked when the Na+/K+ ATPase is inhibited by ouabain, demonstrating the coupling of chloride secretion to Na+ transport.33–36
In addition to the transport mechanisms described, the corneal epithelial cells also contain a Na+/H+ exchanger and a lactate-H+ co-transporter. These transport mechanisms serve to regulate intracellular pH by extrusion of lactate and H+ ions.37 The above discussion raises questions concerning the overall role of these transport mechanisms and second-messenger systems in corneal epithelial function. It has been demonstrated in vitro that ion transport can osmotically move water from the stroma to tears6; however, in vivo epithelial ion transport probably has a limited role, if any, in corneal deturgescence as compared to the endothelium.
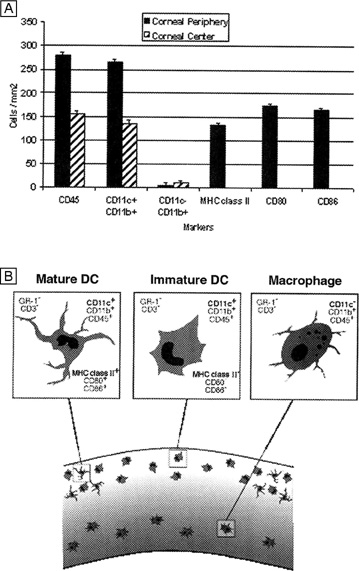
Finally, it has been shown in both animal and human specimens that the corneal epithelium, although devoid of melanocytes, does contain immune cells. The basal epithelial cell layer of the peripheral cornea, along with the limbus and the conjunctiva, appears to have a subpopulation of cells intermixed that are bone-marrow–derived immune surveillance cells with high constitutive expression of major histocompatibility complex (MHC) type II antigen and co-stimulatory molecules.38 This type of immune cell has previously been termed a Langerhans cell,39 which functions as a “professional” antigen-presenting cell with an extraordinary capacity to initiate T-cell lymphocyte-dependent responses.40 The functional steps of this cell type include the uptake and processing of antigens, the migration out of the cornea to lymph nodes where they stimulate naïve T-lymphocyte immune responses by presenting antigens and overexpressing co-stimulatory molecules. Recently, the central corneal epithelium also was found to have a similar subpopulation of immune cells that are apparently “immature” Langerhans cell types because their constitutive expression of MHC type II antigen and costimulatory molecules is low.41 Interestingly, these “immature” Langerhans cells under certain circumstances (e.g., inflammation or trauma) may develop the requisite signals for T-cell priming.41
MICROSCOPIC ANATOMY, ULTRASTRUCTURE AND PHYSIOLOGY OF THE STROMA
The corneal stroma accounts for 90% of the corneal thickness. It is predominantly composed of water (78% hydrated or 3.5 g H2O/g dry weight), which is stabilized by an organized structural network of insoluble and soluble cellular and extracellular proteins (Fig. 1).42 The dry weight of the adult human corneal stroma is made up of collagen (68%), keratocyte constituents (10%), proteoglycans (9%), and salts, glycoproteins, or other substances.43
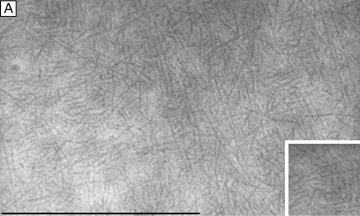
Collagen is a water-insoluble structural protein organized into an inextensible scaffold that forms the basic structural framework of connective tissues. In the human cornea, stromal collagens are poorly-ordered in the Bowman's layer (i.e., the primary acellular stroma that is presumably ectodermally-derived), whereas they form a highly-ordered lattice-like structure in the secondary cellular stroma (neural crest-derived).44 Overall, the corneal collagens are functionally important in establishing tissue transparency and in resisting tensile forces because collagen fibrils and filaments hold the cornea together (i.e., cohesive strength), ultimately defining the size of the tissue.
Keratocyte components make up the second major component of the cornea's dry weight. Interspersed between collagen lamellae throughout the secondary cellular stroma, keratocytes form a closed, highly-organized syncytium. Keratocytes are neural crest-derived cells that enter the cornea during the second mesenchymal wave of corneal embryologic development. They function as modified fibroblasts during neonatal life, forming the extracellular matrix of the secondary cellular stroma. Subsequently, they typically remain in the cellular stroma throughout life as modified fibrocytes where they maintain the extracellular matrix of the corneal stroma, usually in an inconspicuous, or relatively transparent, fashion.
Proteoglycans, which are water-soluble glycoproteins, are the third major component of the cornea's dry weight. Each proteoglycan molecule consists of a protein core with a covalently attached anionic polysaccharide side chain called a glycosaminoglycan (GAG). The proteoglycans are three-dimensionally ordered in the corneal stroma because the protein core of the molecule is noncovalently attached to collagen fibrils evenly throughout the tissue, whereas the GAG sidechain extends into the interfibrillar space where it acts as a pressure-exerting polyelectrolyte gel.45–47 After Hedbys showed that the cornea collapses to around 20% of its volume if the proteoglycans in the tissue are precipitated with cetylpyridinium,48 it has become quite apparent that the primary function of proteoglycans is to provide tissue volume, maintain spatial order of collagen fibrils, and resist compressive forces. Water, collagens, proteoglycans, and keratocytes work together to maintain and establish a transparent cornea, while also creating a tough and resistant structure that keeps ocular integrity intact and maintains a stable shape.
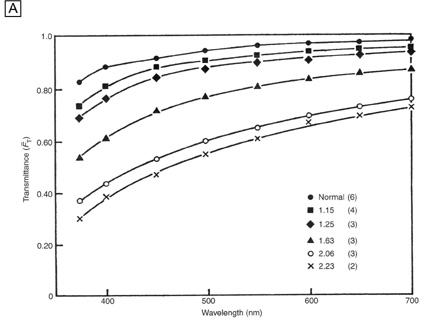
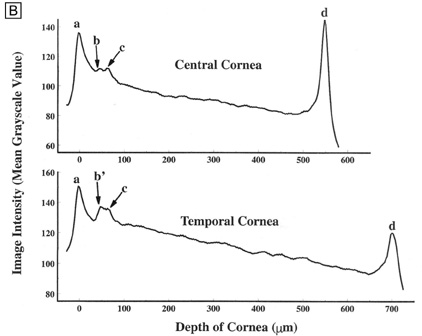
Interestingly, although the cornea primarily absorbs light between 200 to 295 nm (ultraviolet)49 and transmits variable amounts of light from around 300 nm in wavelength (ultraviolet) to 2,500 nm in wavelength (infrared)50, peak transmission (95% to 99% of the incident light) occurs along wavelengths where light is visible to humans (visible spectrum of light = 400 to 700 nm). The remaining portion that is not directly transmitted (1% to 5%) is scattered in all directions by the cornea in a wavelength dependent fashion or absorbed by the tissue (Fig. 8).51 Clinical slit-lamp examination and in vivo confocal microscopy suggests that most of the scattered light (i.e., reflected and scattered light) comes from cellular components in the cornea (endothelial cells > epithelial cells > nerve cells > keratocytes) as opposed to collagen fibrils (Figs. 1B and 8, bottom).52–53
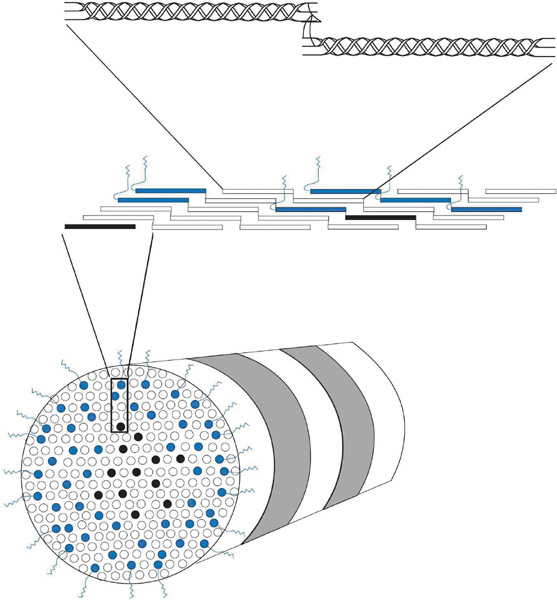
Collagen molecules measure 1.5 nm in width by 300 nm in length and are composed of a triple helix of three alpha chains (Fig. 9, top). There currently are 19 known collagen types as determined by the combination of alpha-chains that form the collagen molecule. The most common types usually aggregate into structural, banded (repeating pattern of polar amino acids seen on transmission electron microscopy [TEM]) fibrils by ordering themselves into a quarter-staggered parallel arrangement that is further stabilized in position by covalent intermolecular cross-links (Fig. 9, middle).
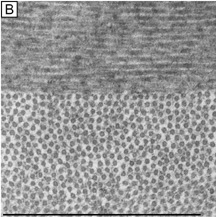
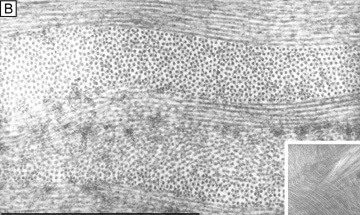
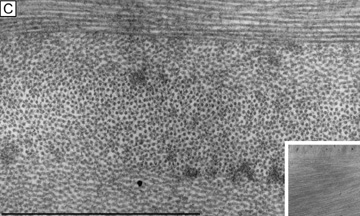
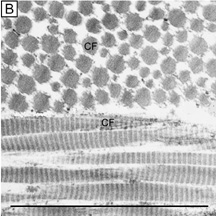
Collagen fibrils are generally heterotypic (composed of two or more types of collagen molecules) and reach certain diameters based on their composition of collagen types. The fibrils in the corneal stroma are a co-polymerization of collagen types I, III, and V molecules that form uniform 22-nm diameter fibrils in the acellular Bowman's layer and uniform 25-nm diameter fibrils in the cellular corneal stroma with only slight variability (Figs. 9, bottom and 10).54–55 Although the refractive index of collagen fibrils (1.47) is different from that of the extrafibrillar matrix (1.35), the highly uniform size (25 ± 2 SD nm) and interfibrillar spaces (40 ± 5 SD nm) along with the predominantly parallel directionality of these fibrils results in a highly-ordered lattice-like array of fibrils (i.e., not a true crystalline lattice, but one with short-range order) that allows transparency of the cornea due to destructive interference effects (Fig. 10B, C).56
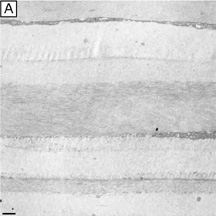
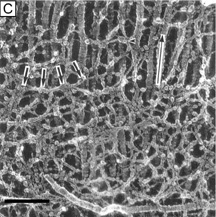
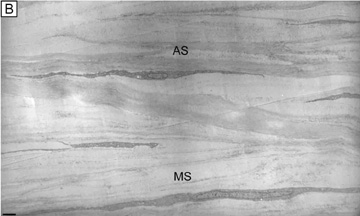
Collagen type VI is the third most common type of collagen in the corneal stroma, but is unique in that it is only able to aggregate into repeating tetramers of type VI molecules. Thus, it forms only 10 to 15 nm diameter, beaded (20 × 30 nm diameter ovals with a periodicity of 100 nm), nonbanded filaments (Fig. 11A). Functionally, it acts as a bridging filament that binds corneal lamellae together where they cross each other (Fig. 11B, C). Along with fibril-associated collagens with interrupted triple helices (FACIT collagens, type XII and XIV collagen molecules), it also bridges intralamellar fibrils together (Fig. 11D).57,58 Overall, this three-dimensional, supramolecular scaffold created by human corneal stromal collagens results in a one-dimensional ordered (∼22 nm diameter collagen fibrils; ∼ 40 nm interfibrillar spaces × random directionality) 12-μm thick acellular Bowman's layer (Figs. 12A and 13A) and a three-dimensionally-ordered (∼25 nm diameter collagen fibrils; ∼ 40 interfibrillar spaces × parallel directionality) series of successive stacks of lamellae in the cellular stroma measuring in the center of cornea around 450 μm in thickness.59–61
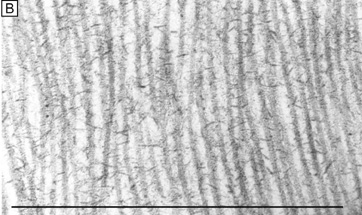
Although difficult to count exactly, the central cornea has been found to have approximately 300 corneal lamellae, whereas the peripheral cornea has approximately 500.61 Although these lamellae are generally described as running parallel to the corneal surface in orthogonal directions to one another, regional difference exist in their size, directionality, and amount of interweaving. The anterior third of the cellular corneal stroma has thinner (0.2- to 1.2-μm thick), narrower (0.5- to 30-μm wide), and mostly obliquely oriented lamellae with extensive vertical and horizontal interweaving (Figs. 12B and 13B), whereas the posterior two-thirds has thicker (1- to 2.5-μm thick), wider (100- to 200-μm wide), and mostly parallel oriented lamellae with only slight horizontal interweaving (Figs. 12C and 13C).60
Additionally, only lamellae in the posterior two-thirds of the cellular cornea stroma run in the classic orthogonal directions, whereas all of the lamellae of the anterior third and a minority of lamellae in the posterior two-thirds run in nonorthogonal directions. Because the lamellae in the anterior-most layers of cellular stroma branch so extensively, it is not surprising that they also interweave with (i.e., are attached to) the Bowman's layer in a polygonal fashion, creating a mosaic appearance that can be seen on the anterior corneal surface under certain circumstances (Fig. 12A, inset). Finally, all corneal lamellae appear to stretch across the cornea from limbus to limbus where they turn and form an annulus approximately 1.5- to 2.0-mm wide around the cornea. This maintains the curvature of the cornea, while blending with limbal collagen fibrils.61–63
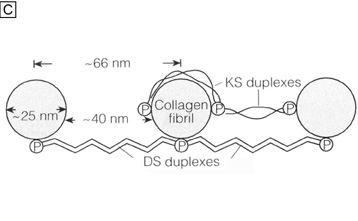
Corneal proteoglycans previously were referred to as extrafibrillar amorphous ground substance because their water soluble state made them difficult to fully delineate with light and electron microscopy (Fig. 14A). It was not until an electron-dense, cationic dye called cupromeronic blue and a critical-electrolyte-concentration of 0.1 M MgCl2 were used in combination to stain specifically for the sulfate-ester groups on corneal stromal proteoglycans that the shape, size, arrangement, and location of this material was observed with light and electron microscopy (Fig. 14B).64 Since that time, it has become quite apparent that corneal proteoglycans are not amorphous, but rather are tadpole-shaped structural molecules composed of a globular core protein (10 to 15 nm in diameter) with a covalently attached GAG sidechain or tail (7 nm wide × 45 to 70 nm in length). They are arranged in the cornea stroma orthogonal to collagen fibrils with a constant separation around 65 nm between each other along the fibrils. Their core proteins noncovalently bind to collagen fibrils in specific gap zones along the peripheral portions of the collagen fibril (core proteins with dermatan sulfate sidechains bind to d and e gap zones and those with keratan sulfate side chains bind to a and c gap zones).65 GAGs are highly negatively-charged, stiff polymers that extend into the interfibrillar space and form antiparallel duplexes with adjacent GAG sidechains, thereby crosslinking different collagen fibrils together by forming dumbbell-like structures (Fig. 14C).
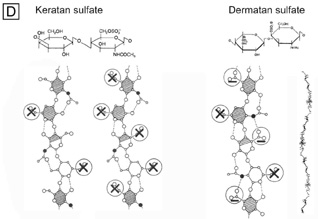
Because the genes that produce the core proteins have now been cloned, four types of corneal proteoglycan core proteins have been identified: decorin, lumican, keratocan, and mimecan.66 Subsequently, decorin was found to contain a single dermatan sulfate GAG sidechain, whereas lumican and mimecan had single keratan sulfate GAG sidechains and keratocan had three keratan sulfate GAG sidechains. Thus, there are four known types of proteoglycan core proteins and only two types of GAGs: keratan sulfate (60%) and dermatan sulfate (40%), found in the human cornea stroma. The GAGs are polymers of repeating disaccharide units of galactose and N-acetylglucosamine or iduronic acid and N-acetylgalactosamine, respectively (Fig. 14D).65 Because GAG sidechains are posttranslationally added to the core protein portions in the golgi apparatus, there seems to be some flexibility in how long or how sulfated they can become depending of the type and functional needs of the connective tissue producing them. The human cornea is unique in that the GAGs are fibril-associated, small in length (keratan sulfate ≅45 nm and dermatan sulfate ≅70 nm), and with a higher proportion are oversulfated.
Interestingly, a comparative study of corneas from 12 mammalian species suggests that dermatan sulfate is the preferred proteoglycan in oxygen-rich environments such as thin corneas (e.g., mice) or in the anterior portion of thicker corneas (e.g., humans or rabbits), whereas keratin sulfate is a functional substitute produced through an alternate metabolic pathway in thicker corneas, especially in the posterior portion where oxygen levels may be dramatically reduced (Fig. 15, inset).48,66 Functionally, this duality is quite useful because dermatan sulfate appears to be more efficient at holding water as it absorbs less total amounts of water, but most in a tightly-bound state (i.e., in a nonfreezable state) than does keratin sulfate.47,67 These explanations are consistent with the fact that dermatan sulfate is highest in amount in the anterior portion of the corneal stroma in humans (region of highest oxygen tension and most affected by evaporation), whereas keratin sulfate is highest in amount in the posterior portion of the corneal stroma (region with the lowest oxygen tension, least affected by evaporation, and area where the need for loosely-bound water is required for transport across the endothelium through the endothelial cell metabolic pump) (Fig. 15).
|
The corneal stroma is maintained by a closed, highly-organized syncytium of keratocytes communicating with each other through gap junctions present on their long dendritic processes.68 Keratocytes occupy 10% to 40% of the stromal volume (decreases from 40% in infancy to 10% in adulthood) that on two-dimensional, cross-sectional views appear as flattened (cell body = 20 μm in length × 1 μm in height), quiescent (i.e., scant intracytoplasmic organelles) cells lying between corneal lamella (Fig. 16A, B). In actuality, keratocytes are three-dimensional, stellate-shaped cells composed of a cell body (≅1 × 15 × 20 μm) with numerous dendritic-processes that extend up to 50 μm in length from the cell body. Two-dimensional tangential sections of the normal cornea suggest that these cells are more highly metabolically active in the resting state than initially presumed; in tangential sections (cell body view = 15 μm width × 20 μm), an abundance of cytoplasmic organelles is commonly seen (Fig. 16C, D).69
|
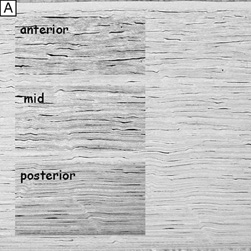
Tangential sections also have shown more clearly that the anterior stromal keratocytes contain twice the number of mitochondria than the posterior two-thirds of the stroma, which correlates with the oxygen tension levels in the cornea. It also has demonstrated that a higher density or volume of cells reside in the anterior stroma than in the mid or posterior stroma (Fig. 17). Moreover, these views make it easy to appreciate that, in all levels, the keratocytes are highly spatially-ordered as they turn in a clockwise direction like a corkscrew.
|
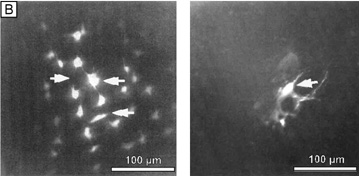
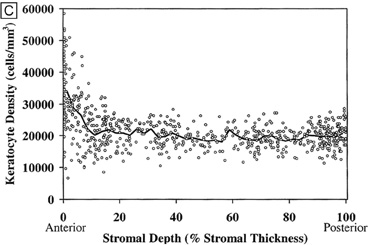
In vivo confocal microscopy of normal human corneas has shown keratocyte densities average around 20,000 keratocytes/mm3 (or an area of 328 cells/mm2). The focal zone of increased cell density directly under the epithelial surface averages 35,000 keratocytes/mm3 in the anterior-most 9-μm layer and gradually tapers to 20,000 keratocytes/mm3 over the initial 60 μm in depth (Fig. 17C).70 Confocal microscopy also concurs with previous studies as it shows that stromal cellular density decreases with age at approximately 0.45% per year of life. Interestingly, studies using immunohistochemistry on animal corneas71,72 and ultrastructural studies on human corneas69,72 suggest that not all the cells in the corneal stroma are keratocytes (i.e., fibrocytes), but some are one of three types of bone marrow-derived immune cells (“professional” dendritic cells, “nonprofessional” dendritic cells, and histiocytes) (Fig. 18). Thus, although the majority of cells in the stroma are keratocytes, which maintain the corneal stroma by synthesizing and secreting collagen type I, III, V, VI, XII, XIV, keratan sulfate, dermatan sulfate, and matrix metalloproteinases, a minority of cells in the stroma are also “professional” stromal dendritic cells, “nonprofessional” stromal dendritic cells, or stromal histiocytes. They appear to play a pivotal role in the induction of immune tolerance versus immune initiation into cell-mediated immune pathways. Additionally, the stromal histiocytes play a role in innate immunity as phagocytic effectors cells.
|
Two ion channels similar to those found in excitable cells have been characterized in keratocytes isolated from corneal stroma.73 These channels include a delayed rectifer K+ channel and a tetrodotoxin-sensitive Na+ channel. The physiologic function of these channels has not been determined, but they may function in maintaining the ionic balance of the cells in their inactive state. In addition, because these channels allow the keratocytes to elicit action potentials when injected with a clamping current, it is possible they may act as intercellular signalers via the gap junctions between cells. This could be a route for information exchange between cells in different regions of the cornea.
Corneal Edema
The Donnan effect states that the swelling pressure in a charged gel, like the corneal stroma, results from ionic imbalances. The fixed negative charges on corneal proteoglycan GAG sidechains (one carboxylic acid and one sulfate ester sidechain per disaccharide repeat on a dermatan sulfate GAG polymer, and one or two sulfate ester sidechains per disaccharide repeat on a keratin sulfate GAG polymer) have a central role in this effect as the antiparallel GAG duplexes (tertiary structure) produce long-range electrostatic repulsive forces that induce an expansive force termed swelling pressure (SP). Because the corneal stroma also has a cohesive tensile strength that resists this expansion, the normal SP of the nonedematous corneal stroma is around 55 mm Hg.74,75 If the stroma is further compressed (e.g., increasing intraocular pressure [IOP] or mechanical applanation) or expanded (e.g., corneal edema), the SP will correspondingly increase or decrease, respectively.
Conversely, the negatively-charged GAG sidechains also form a twofold helix in aqueous solution (secondary structure) that attracts and binds Na+ counterions, which results in an osmotic effect leading to the diffusion and subsequent absorption of water to proteoglycans via the Na+ cations. Thus, the central corneal thickness is maintained around its average value of 520 μm because the fixed negatively-charged proteoglycans induce a swelling pressure through anionic repulsive forces. Additionally, the hydration level of corneal stroma is maintained around 78% water because these proteoglycans also imbibe water through cationic attractive forces.45
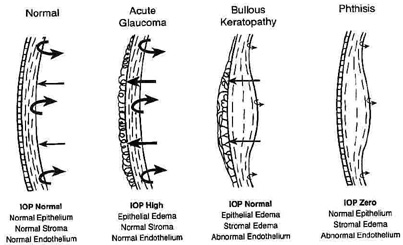
Under normal circumstances, the negative pressure drawing fluid into the cornea, called the imbibition pressure (IP) of the corneal stroma, is around −40 mm Hg.76 This implies that the negative charges on corneal proteoglycans are only about one-quarter (≅27 %) saturated, or bound, with water with the remaining unbound proportion wanting to bind more Na+ and absorb more water if given the opportunity. Fortunately, the highly impermeable epithelium and mildly impermeable endothelium keep the diffusion of electrolytes and fluid flow in the stroma to such a low level [resistance to diffusion of electrolytes and fluid flow = epithelium (2000) >> endothelium (10) > stroma (1)] that the continuously working endothelial cell metabolic pump can maintain stromal hydration in the normal range. Although IP = SP when corneas are in the ex vivo state, IP actually is lower than SP in the in vivo state because the hydrostatic pressure induced by the IOP must now be accounted for. This is best represented by the equation IP = IOP − SP76 and explains why the hydration level of patient's cornea is not only dependent on having normal barrier functions, but also on having a normal IOP. Therefore, a loss of corneal barrier function, an IOP ≥55 mm Hg, or a combination of the two results in corneal edema (Fig. 19).77
|
Corneal edema is a term often used loosely and nonspecifically by clinicians, but literally refers to a cornea that is more hydrated than normal (i.e., >78% water). The topic of corneal edema is important for clinicians to understand because it affects the architecture and function of the epithelium and the stroma. Epithelial edema clinically causes a hazy microcystic appearance to occur in the epithelium in mild to moderate cases, significantly decreasing vision and increasing glare. It also can cause the development of large, painful, subepithelial bullae in severe cases. These changes correlate histopathologically with hydropic basal epithelial cell degenerative changes and the development of extraepithelial cellular fluid filled spaces (e.g., cysts and bullae). Interestingly, if bullae are chronically present, a fibrocollagenous degenerative pannus often times will grow into the subepithelial space, decreasing vision further while reducing the pain.
Stromal edema clinically appears as a painless, hazy thickening of the corneal stroma resulting in a mild to moderate reduction in visual acuity and an increase in glare. At the same time, Descemet's membrane folds commonly appear on the posterior surface of the cornea. Histopathologically, these changes correlate with the light microscopic findings of thickening of the corneal stroma in the posterior cross-sectional direction with loss of artifacteous stromal clefting.78 Ultrastructural and biochemical studies have further shown that stromal edema causes an increase in the distance and disruption of spatial order between collagen fibrils,78 a decrease in the refractive index of the extracellular matrix,79 hydropic degenerative changes or cell lysis in the resident keratocyte population,80 and a loss of proteoglycans.81
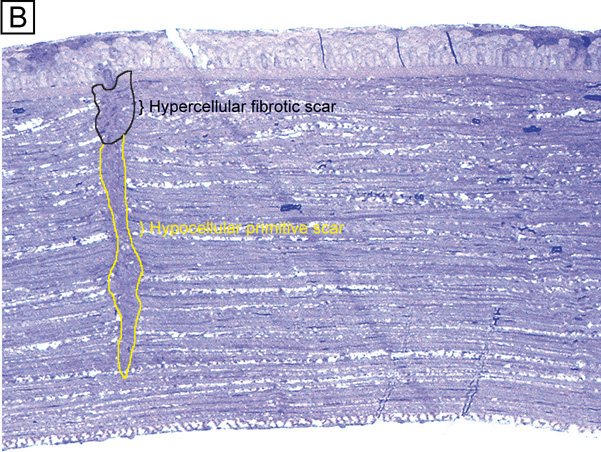
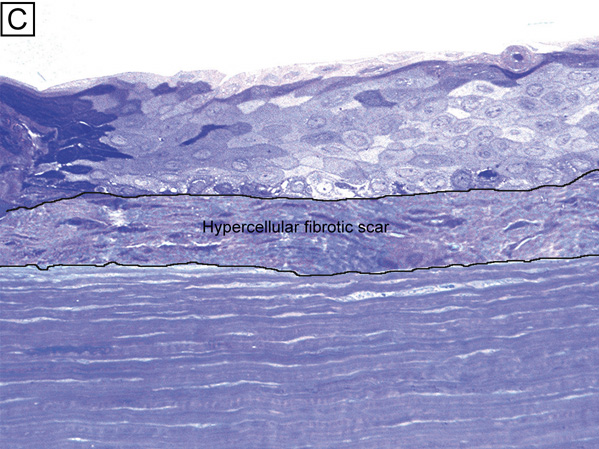
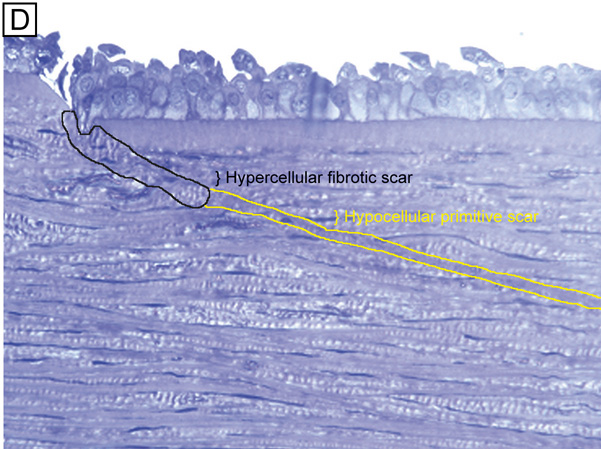
Although different proportions of the two types of negatively-charged proteoglycan may account for the higher hydration levels in the posterior stroma compared to the anterior stroma,46,82 it appears that the directional orientation of the collagen fibrils probably has the greatest influence on how much each region thickens, or swells, as a result of increased hydration levels. Because the collagenous architecture of the stroma (i.e., limbus-to-limbus directional orientation of collagen fibrils) highly resists circumferential expansion, only anterior-posterior expansion occurs in the human cornea, mostly in the posterior direction. This latter fact occurs because extensive lamellar interweaving occurs in the anterior portions of the corneal stroma, whereas weak bridging filaments (i.e., type VI and FACIT collagens) occur diffusely throughout the entire corneal stroma. Furthermore, this lamellar interweaving also explains why the anterior third of the cornea mildly swells and actually maintains its anterior corneal curvature even when the remaining stroma swells to up three times its normal thickness.80 Because fibrotic corneal scars have random directionally-oriented interweaving collagen fibrils, they also have been found to resist swelling under edematous conditions.83
Therefore, although it is commonly stated that corneal thickness and interfibrillar spacing increase in a linear fashion to the hydration level of the corneal stroma,75,78 one needs to be aware that this relationship mainly applies to the mid and posterior stromal regions.
Finally, although both epithelial and stromal edema commonly co-exist together, there are two notable exceptions that are important to understand. Because the epithelium lacks fixed negatively-charged proteoglycans and has a different set of cohesive mechanical strengths than the stroma (e.g., intercellular desmosomal junctions), its state of hydration is mainly dictated by IOP levels.84 Conversely, because collagen fibrils in corneal stroma are anchored at the limbus for 360 degrees, they exert increasing cohesive tensile mechanical forces on the corneal stroma (i.e., compression of stromal tissue) as the IOP elevates above normal. This results in the transmission of edema to the epithelial surface in cases of high IOP. Therefore, if IOP is ≥55 mm Hg with normal endothelial barrier and pump function, epithelial edema usually occurs by itself. In contrast, if endothelial cell dysfunction and hypotony (IOP ∼0 mm Hg) occur together, then stromal edema occurs alone (see Fig. 19).
MICROSCOPIC ANATOMY, ULTRASTRUCTURE, AND PHYSIOLOGY OF THE ENDOTHELIUM
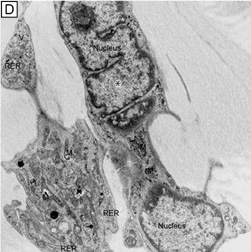
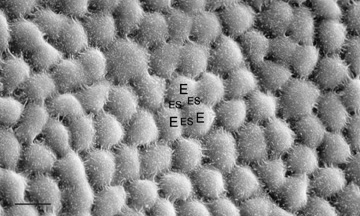
The endothelium of the infant cornea is composed of a single layer of approximately 500,000 neural crest-derived cells, each measuring around 5 μm in thickness by 20 μm in diameter and cover a surface area of 250 μm2.58,85 The cells lie on the posterior surface of the cornea and form an irregular polygonal mosaic. The tangential appearance of each corneal endothelial cell is uniquely irregular, usually uniform in size to one another, and typically six-sided (i.e., hexagons). They abut one another in an interdigitating fashion with 20 nm wide intercellular space between each other. The intercellular space is known to contain discontinuous apical tight junctions (macula occludens) and lateral gap junctions, thereby forming an incomplete barrier to diffusion of small molecules. As corneal endothelial cells have numerous cytoplasmic organelles, particularly mitochondrial organelles, they have been inferred to have the second highest aerobic metabolic rate of cells in the eye next to retinal photoreceptors.58
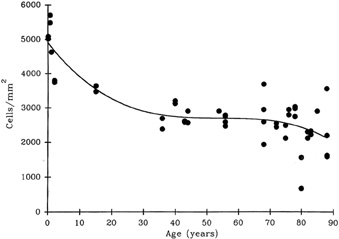
At birth, the central endothelial cell density of the cornea is around 5,000 cells/mm2.85 Because the corneal endothelium has very limited regenerative capabilities, there is a well-documented decline in central endothelial cell density with age that typical involves two phases: a rapid and a slow component.85,86 Due to corneal growth and age-related, or developmental, selective cell death, during the fast component, the central endothelial cell density decreases exponentially to about 3,500 cells/mm2 by age 5 years and 3,000 cells/mm2 by age 20 years.86 Thereafter, a slow component occurs where central endothelial cell density decreases to a linear steady rate of 0.6% per year, resulting in cell counts around 2,500 cell/mm2 in senescence (Fig. 20).85.86 Because endothelium maintains its continuity by migration and expansion of surviving cells, it is not surprising that the percentage of hexagonal cells decreases (pleomorphism) and the coefficient of variation of cell area increases (polymegathism) with age.86
|
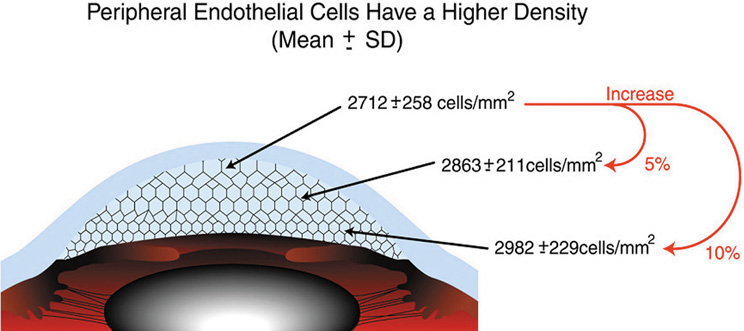
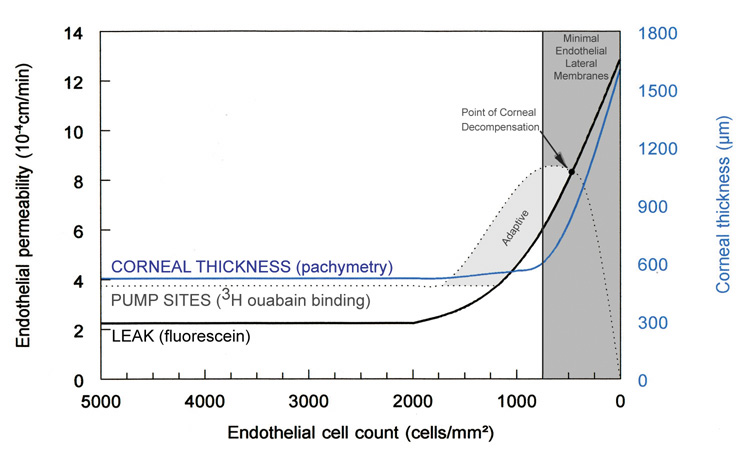
It is important when reviewing this information to realize that these are average central corneal endothelial cell measurements from predominantly Caucasian U.S. populations, as racial and geographic differences can exist. Also, understand that this data applies only to central corneal endothelial measurements because recent work has shown that higher cell densities can typically be found in more peripheral aspects of the cornea (Fig. 21).87 Therefore, based on these studies, it appears that central corneal endothelial cell numbers decrease on average about 50% from birth to death in normal subjects. Because corneal decompensation typically does not occur until central values reach around 500 cells/mm2, there appears to be plenty of cellular reserve potential remaining after an average human life span.86 Estimates suggest that healthy, normal human corneal endothelium could maintain corneal clarity up to a minimum of 215 years of life, if humans lived that long.85
|
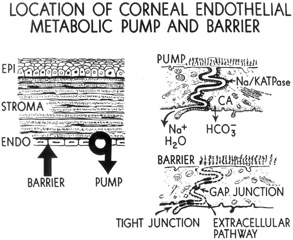
The primary function of the corneal endothelium is to maintain the health, deturgescence, and clarity of the cornea through a pump-leak mechanism first described by David Maurice (Fig. 22).88 Secondarily, it is also known to secrete an anteriorly-located basement membrane called Descemet's membrane and a posteriorly-located glycocalyx.58
|
Having a sufficient number of endothelial cells to cover the posterior surface of the cornea along with having integrity of their cell junctions (tight and gap junctions), which are present in the intercellular spaces between endothelial cells, establishes the barrier function of endothelium (Figs. 12 and 22, 23, and 24). Clinically, the barrier function of the cornea can be assessed in vivo by the use of specular microscopy or confocal microscopy (endothelial cell density) or fluorophotometry (permability). In healthy human eyes, this barrier prevents the bulk flow of fluid from the aqueous humor to the corneal stroma, but does allow moderate diffusion of nutrients, water, and other metabolites to cross into the stroma through the 20 nm wide intercellular space. This leaky endothelial barrier may initially seem inefficient, but when one considers that most nutrients for all layers of the cornea come from the aqueous humor, the situation is reasonable.
|
|
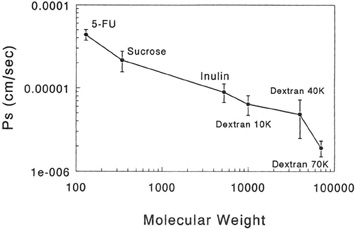
Additionally, despite the normal loss of endothelial cells that occurs with age, there appears to be no increase in the permeability of normal, healthy-aged corneas. Only when the endothelium is severely reduced in density, acutely damaged, and/or has disrupted cell junctions can the permeability, as measured with carboxyfluoresceing increase up to sixfold (12.85 × 10−4 cm/min) from normal (2.26 × 10−4 cm/min).89 Recent studies have inferred that during the fifth month of gestation the tight junctions completely form and decrease endothelial permeability.90
A number of factors have been known to acutely affect the barrier function of the endothelium, including the following: reversible disruption of cell junctions (calcium-free solutions or glutathione-restricted solutions [Fig. 25]), mechanical damage (e.g., trauma, intraocular lens [IOL] insertion, surgical instruments), or chemical injury (e.g., noncomplete toxic intraocular solutions, preservatives). Fortunately, if enough of the remaining viable cells are able to migrate, recover the posterior corneal surface by spreading out over a larger surface area, and reestablish the intercellular cell junctions, the barrier function of the corneal endothelium is restored.
|
The first evidence to suggest that corneal endothelial cells have metabolic pumps was the observation that stromal edema (i.e., corneal clarity) is temperature-dependent.91 Lowering the temperature arrested the endothelial metabolic pump causing corneal stromal edema, whereas restoring it back to physiologic levels resolved the edema. Subsequently, it was determined that the lateral plasma membrane of endothelial cells contains ATP-dependent transport proteins that catalyze the movements of ions from the stroma to the aqueous humor, creating an osmotic gradient that draws water from the stroma.92 It is important to note that this osmotic gradient occurs only if the endothelial cell barrier is not breached. The primary transport proteins creating this effect were later determined as Na+/K+-ATPases (Fig. 22).93,94 Subsequently, the number and density of Na+/K+-ATPases sites has also been quantified using [3H]-ouabain, which calculated that approximately 3 million Na+/K+-ATPases sites are present around the lateral membrane of a single endothelial cell (average density of pump sites along lateral plasma membrane wall = 4.4 trillion sites/mm2).95 Recent studies have inferred that only by 5 to 7 months of gestation does the density of Na+/K+-ATPases increase to adult levels so that the cornea becomes dehydrated and transparent.96 Clinically, the metabolic pump of the corneal endothelium can be assessed in vivo by measuring the how quickly the corneal thickness (pachymetry) recovers after being purposefully swollen by wearing oxygen-impermeable contact lens.
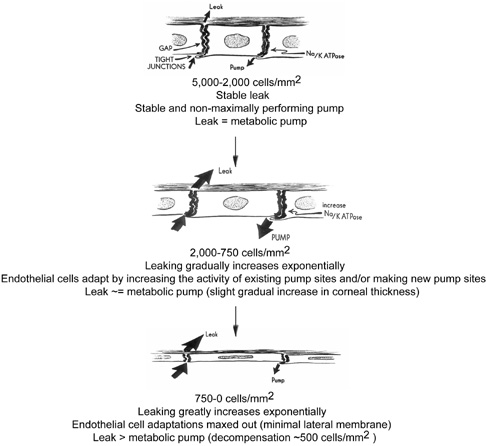
A number of factors are known to alter endothelial pump function: pharmacologic inhibition of Na/K-ATPase (e.g., ouabain), decreased temperature, lack of bicarbonate or carbonic anhydrase inhibitors, and chronic reduction in endothelial cell numbers from mechanical injury, chemical injury, or disease states. Fortunately, physiologic compensatory mechanisms prevent corneal edema from occurring to a certain degree (central endothelial cell densities between 2,000 to 750 cells/mm3) by increasing the activity of pump sites already present (requiring more ATP production by the cell) and/or by increasing the total number and density of pump sites on the lateral membranes of endothelial cells (Figs. 26 and 27).95 A similar phenomenon occurs in the cells of the kidney's proximal tubule to adjust for an increased salt load. For example, with Fuchs' endothelial dystrophy, the cornea has been known to remain clear and of normal thickness, despite having very low endothelial cell counts and increased endothelial flourescein permeability (2.89 × 10−3 to 5.30 × 10−3mm/min).95 Apparently, this occurs because the density of the Na+/K+ pump sites increases (mean of 10.2 × 109 sites/mm2) to compensate for increased permeability.97 The point at which compensatory mechanisms appear to ultimately fail is when central endothelial cell density reaches levels around 500 cells/mm3 (range of 250 to 750 cells/mm3) (Figs. 26 and 27).85,98 At this low cell number, the permeability has greatly increased to such a point that the endothelial cells, which are spread so thin, do not have enough room on their lateral cell membranes for more metabolic pump sites and all the current pumps are maximally active. Therefore, the metabolic pump fails to balance the leak and corneal edema results.
|
|
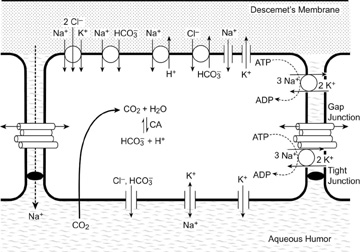
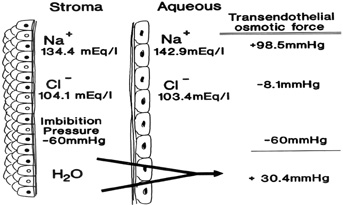
A diagram summarizing the current understanding of the endothelial cell transport system is shown in Figure 28. This subject was most recently reviewed in detail by Bonanno.99 When the corneal endothelial barrier and metabolic pump are functioning, the corneal stroma has a total Na+ concentration of 179 mEq/L (134.4 mEq/L free and 44.6 mEq/L bound to stromal PGs), whereas the aqueous humor has a total Na+ concentration of 142.9 mEq/L (all free).100 Therefore, after accounting for the chloride activity and stromal imbibition pressure, an osmotic gradient of 30.4 mm Hg exists, causing water to diffuse from the stroma to the aqueous humor (Fig. 29). Additionally, the corneal endothelial cells contain high concentrations of carbonic anhydrase, which forms HCO3− (bicarbonate) from metabolic CO2−. The HCO3− diffuses down its concentration gradient into the extracellular space or across the membrane via the Cl−/HCO3− exchanger101 or through Cl− (anion) channels.102,103 Bicarbonate can also enter the cells via a Na+/HCO3− co-transporter,105 and the intracellular pH can be regulated, at least in part, by a basal Na+/H+ exchanger.104 Endothelial Cl− transport occurs through both transporters and channels, with Cl− entering the cell from the stroma via a basal Na+/K+/2Cl− transporter and the HCO3−/Cl− exchanger, and exiting into the aqueous humor via apical anion channels.105,106
|
|
In addition to the transporting proteins, several types of ion channels have been characterized in the endothelial cells. These include two K+ channels, a nonselective cation channel, a large conductance anion channel the CFTR channel, a Ca++-activated Cl− channel (also permeable to HCO3−, and a tetrodotoxin-blockable Na+ channel).107–109 It is believed that these channels act as pathways to help maintain ionic balance within the cells. It has been shown that at least one of the K+ channels is important in maintaining the resting voltage of the endothelium, whereas the Cl− channels may also assist in HCO3− transport.107–109
As with the other cell types of the cornea, endothelial cells have intercellular connections via gap junctions. These junctions have been visualized by transmission electron microscopy110 and freeze fracture microscopy,7 and their permeability and gating characteristics have been measured by dye spread and patch clamp techniques.111–113 Connexin-43 is one of the major gap junction proteins present in human gap junction complexes.114 As in the corneal epithelium, endothelial gap junctions have increased connectivity in the presence of bicarbonate.15
Although this review is primarily concerned with healthy, normal corneal endothelium, there are many exogenous stresses that could potentially damage the corneal endothelium. Perhaps the most common interventions that affect the cornea/endothelium include contact lens wear, excimer laser refractive surgery (LASIK, PRK), and intraocular surgery (cataract surgery, refractive IOL surgery, corneal transplantation). Contact lens wear does not appear to cause loss of endothelial cell density, but can acutely induce reversible corneal edema and/or can cause chronic signs of endothelial cell stress (increased polymegathism and decreased pleomorphism).58,114 Contact lens-induced endothelial cell changes are thought to occur because of hypoxia, as they are not observed with more oxygen permeable lenses.114 Excimer-based refractive surgery has only been found to induce acute, transient endothelial cell stress (increased polymegathism and decreased pleomorphism) and loss of barrier function if performed on a cornea with a residual corneal thickness ≤200 μm, presumably because of the shockwave produced by the laser ablation.85 Otherwise, no long-term effects have been linked to laser refractive surgery.85
By comparison, all intraocular surgeries have been found to cause varying degrees of both acute and, more importantly, long-term chronic damage to endothelium. Modern small incision cataract surgery (Kelman phacoemulsification [KPE] + foldable IOLs) has been shown to cause an exponential reduction in the central endothelial cell density; at 1 year after surgery, endothelial cell density loss averages 10.5%.115 Additionally, no significant change in polymegathism or pleomorphism has been found.115 This fast component period of cell loss is similar to extracapsular cataract extraction (ECCE), another common way of performing cataract surgery, which results in a 9.1% reduction in endothelial cell density at 1 year after surgery.115 Capsule rupture with vitreous loss, hard cataracts, and age at the time of surgery are factors that have been shown to significantly increase endothelial cell loss rates in KPE cases compared to ECCE cases.115 Longer-term slow component cell loss data is currently unknown on KPE, whereas ECCE data shows that a 2.5% linear rate of cell loss occurs from 1 to 10 years after surgery.114 Early data from refractive IOL procedures seem to show even less damage to the cornea/endothelium than cataract surgery.
Finally, corneal transplants have been found to have the greatest long-term decreases in central endothelial cell densities of all the intraocular anterior segment procedures, perhaps because of the peripheral loss of adult stem-like cells that are located on or near Schwabe's line.116 Long-term longitudinal studies out to 20 years postoperatively show that endothelial cell loss after corneal transplantation occurs in two phases: a fast and a slow component. During the fast component, the central endothelial cell density decreases exponentially with 36.7% cell loss at 1 year and 8.4% cell loss at 5 years after surgery.117 Thereafter, a slow component occurs where central endothelial cell density decreases at a linear steady rate of 4.2% per year.117 Concurrently, polymegathism gradually increased and pleomorphism gradually decreased throughout the longitudinal follow-up period.
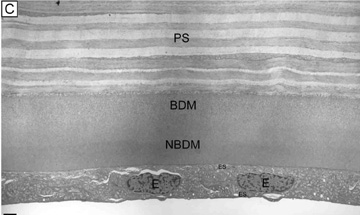
A secondary function of endothelium is its ability to secrete an extracellular matrix. The secreted and deposited extracellular matrix along the basal surface of the endothelium is known as Descemet's membrane (Fig. 12) and is essentially a lifelong accumulated basement membrane of the endothelial cells, similar to that of a tree trunk with accumulating tree rings. Although some collagen fibrils from the posterior stroma are embedded in the Descemet's membrane, it has no junctional or adhesional complexes. Descemet's membrane is highly elastic and strong as it is primarily composed of collagen (type IV and VIII) and glycoproteins (fibronectin, laminin, thrombospondin). At birth, it is 4-μm thick. On electron microscopy, this fetal Descemet's membrane is composed of many wide-spaced, 110-nm banded collagens.118 After birth, collagen is gradually added to this initial fetal layer throughout life, being notably different from the fetal layer as it is nonbanded and contains small diameter collagen fibrils that arrange into a lattice matrix.118 Typically, the Descemet's membrane at senescence in normal adults free of eye disease measures 10- to 15-μm thick (4-μm thick banded layer and a 6- to 11-μm thick non-banded layer). With disease (e.g., Fuchs' endothelial dystrophy, bullous keratopathy), the Descemet's membrane may become focally or diffusely thicker than normal from abnormal collagen deposition. This newly deposited abnormal collagen is called the posterior collagneous layer of the Descement's membrane and is classified as one of three types: banded, fibrillar, and fibrocellular.119 Presumably, this posterior collagenous layer is deposited because endothelial cells become stressed from damage or disease.
Finally, the endothelium is also known to secrete a 0.5-μm thick glycocalyx layer on its apical surface (Fig. 24).85 Functionally, it appears to protect endothelium, particularly in regard to anterior segment surgery.
CORNEAL METABOLISM
Epithelium
Because of the cornea's exposure to the external environment, metabolism is carried out under a broad range of temperatures. The average temperature of the human cornea has been estimated to be 34.8°C but will vary with the extremes of the environmental temperature.120
The corneal epithelium primarily uses glucose and glycogen for energy production. Glucose reaches the cells by diffusion from the aqueous humor, and the corneal epithelial cells store high levels of glycogen. Epithelial glycogen is rapidly depleted under stress, such as hard contact lens wear or trauma.121–124 Glucose is metabolized in the corneal epithelium primarily by anaerobic glycolysis (Embden-Meyerhof pathway); however, up to 35% of glucose enters the hexose monophosphate (HMP) shunt. The HMP shunt converts hexoses to pentoses required for nucleic acid synthesis and produces the reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH), a high-energy reducing agent required for fatty-acid synthesis and membrane repair. NADPH and pentoses are both required for a tissue with a high mitotic index, like the corneal epithelium. Glucose in the cornea may also enter the sorbitol pathway, which produces sorbitol and fructose. In the presence of excess glucose, sorbitol may accumulate in the cornea as it does in the lens and peripheral nerves, causing osmotic cell damage. However, the role of sorbitol in corneal epithelial damage in diabetes is not clear.125,126
The corneal epithelium receives its oxygen from the atmosphere under open-eye conditions where it is exposed to a partial oxygen pressure of 155 mm Hg in the tears. When the eyelids are closed, the oxygen pressure drops to 55 mm Hg, which is apparently adequate to maintain the epithelium, although a degree of epithelial edema does occur during sleep.127 Corneal thickness is increased in the morning following overnight eyelid closure.
The cornea consumes approximately 3.5 μL of oxygen/cm2/hour.128–129 Under aerobic conditions, pyruvate from glycolysis can enter the tricarboxylic acid cycle (Krebs cycle), although the activity of the pathway is low in the epithelium because of the paucity of mitochondria.123,128,130 Under hypoxic conditions (e.g., during contact lens wear), pyruvate is converted to lactate which, as described earlier, is transported from the cells to maintain intracellular pH at between 7.3 to 7.4. Lactate cannot diffuse across the apical barrier and builds up in the stroma.131 Associated with this lactic acid is anoxia of the epithelial cells, leading to epithelial edema that clinically can cause halo formation, glare, and reduced contrast sensitivity. The acidification of the extracellular fluid may interfere with cellular metabolism and mitosis, leading to epithelial thinning and erosion.132–133
The cytochrome P450 system is also functional in the corneal epithelium. Under conditions of hypoxia or inflammation, arachidonic acid is metabolized through this pathway with the production of two eicosanoids, 12(R)-hydroxyeicosa-tetraenoic acid (12(R)HETE) and 12(R)-hydroxyei-cosatrienoic acid (12(R)HETrE).134–135 Both of these eicosanoids have pathophysiologic importance. 12(R)HETE has the potential to diffuse from the epithelium to the endothelium and inhibit Na+/K+ ATPase and the endothelial metabolic pump, ultimately causing corneal swelling.136 12(R)HETrE can serve as a chemoattractant and induce stromal neovascularization.137 Contact lenses that do not fit well or have low oxygen permeability can lead to inflammation and hypoxia from 12(R)HETE or lactate, which can have an adverse effect on the structure and function of the corneal endothelium.138
Stroma
Very little is currently known about the metabolism of corneal stroma besides traditional cross-sectional ultrastructural analysis of stromal keratocytes that show these cells as metabolically quiescent cell types.69 Only recently has tangential ultrastructural analysis been performed, which suggests that the resting metabolic state of the stromal keratocyte is probably much higher than initially thought as these cell must produce and maintain the extracellular matrix of the corneal stroma.69
Endothelium
The endothelium utilizes the same carbohydrate metabolism pathways as the epithelium. The transport function of the cells in the endothelial monolayer requires oxidative activity that is five to six times that of the cells in the epithelium.139 Atmospheric oxygen is the primary source of oxygen to the endothelium. Interruption of this oxygen supply by low-oxygen transmissibility contact lenses or a low-oxygen environment will result in a shift to anaerobic metabolism, a concurrent increase in stromal lactic acid and CO2, and a drop in stromal pH.131 In addition, this hypoxia can stimulate epithelial production of 12(R)HETE, a potent inhibitor of the endothelial Na+/K+ ATPase.136,140 Acute reversible clinical changes seen with hypoxia include stromal swelling, endothelial dysfunction, and endothelial blebbing. Chronic hypoxia can lead to irreversible endothelial polymegathism and pleomorphism. Pulse et al have shown chronic hypoxia in humans alters the endothelium's ability to reverse induced swelling. 141
It has been estimated that glycolysis accounts for 93% of the conversion of glucose-6-phosphate to pyruvate in the endothelium. The aerobic tricarboxylic acid cycle converts 30% of the pyruvate to ATP, whereas the remaining 70% is converted to lactic acid by the anaerobic pathway of lactate dehydrogenase.139 Geroski and colleagues142 found that 37% of the glucose in the rabbit corneal endothelium is metabolized by the pentose phosphate shunt pathway. Adenosine has been found to stimulate this pathway in bovine endothelial cells.143
The endothelium also has the ability to extract and metabolize dehydro-L-ascorbic acid from the aqueous humor. Bode et al found that bovine endothelial cells can take up dehydro-L-ascorbic acid, the oxidized form of ascorbic acid, and reduce it to ascorbic acid. 144 Ascorbic acid is a free-radical scavenger that may act to protect the cells from radiation-induced free-radical damage.
WOUND HEALING
Epithelium
Following injury, the epithelium heals through three distinct steps: cell migration, cell proliferation, and cell adhesion. Before entering these steps, a 4- to 6-hour latent phase occurs where the epithelium responds by desquamating damaged cells, polymerizing actin filaments, synthesizing structural proteins, and releasing all hemidesmosomal attachments to the basal lamina extending 50 to 70 μm from the wound edge and significantly reducing those up to 200 μm from the wound edge.145 This is followed by a linear cell migration phase where a flattened monolayer of epithelial cells slide over the abraded areas and re-establish a barrier.146 Cell sliding, or migration, occurs at a constant rate of 60 to 80 μm/hour until the wound closure.145–148 The normal number of cell layers is subsequently re-established by limbal stem cell proliferation, basal epithelial cell centripetal migration, and vertical basal epithelial cell proliferation.115 As discussed earlier, the epithelium usually heals to normal thickness around 50 μm approximately 1 to 4 weeks after injury, but alternatively, it may thin over elevations or thicken over defects in the underlying stroma. Finally, adhesion complexes are usually regenerated by 1 to 2 months after injury, thereby permanently anchoring the healed epithelium firmly to the underlying stroma.149
The migratory step is a process that is independent of cell proliferation and is an energy-consuming step as glycogen is depleted from epithelial cells at the wound edge during healing.146,149 Both basal and suprabasal cells participate in the migratory process as epithelial cells appear to prefer to migrate centripetally as one continuous coherent sheet.149–150 The formation of lamellipodia and filopodia mark the beginning of cell migration. These areas of the cells as well as at the leading edge of the migrating epithelial cells seem to contain dense networks of contractile actin filaments that are important in the mechanics of epithelial cell motility.151 Experimentally, blocking polymerization of actin has resulted in loss of cell migration.152 Lamellipodial and filopodial activity continues until wound closure is complete with at least a continuous monolayer of epithelial cells recovering the defect. Commonly, individual or small groups of epithelial cells migrate independently from the coherent sheet and form predominantly clockwise whorls on the surface of the healing cornea.149 This unique pattern of healing has been termed hurricane keratopathy. It is postulated to occur because the migrating epithelial cells respond in this fashion to electromagnetic field generated by the electrical potential of the eye.153 Despite acting independently, cell proliferation appears to complement cell migration as epithelial cells away from the wound increase their rate of cell proliferation.150 Therefore, a wave of cells moves from the periphery to the wound, while the epithelial cells in the wound cease proliferating until a continuous monolayer of cells is re-established.
Although epithelial cell migration occurs without hemidesmosomal adhesion complexes, adhesion is still maintained via transient macromolecular focal contacts that are constantly formed and released during cell migration.149 After epithelial wounding, fibronectin, fibrin, laminin, tenascin, and dimeric glycoproteins accumulate on the denuded corneal stroma surface if the basal lamina is not left intact after injury.154 Migratory epithelial cells form focal temporary cell-to-substrate macromolecular contacts known as adhesion plaques. Intracellular actin mediates such attachments by aggregating into stress fibers oriented in the direction of migration and with the help of several cell surface adhesion molecules (i.e., vinculin, talin, integrin, fimbrin, and α-actin) that are synthesized by the epithelum during wound healing and bind to the extracellular substrates. Vinculin, a 110-kD cytoplasmic protein that links actin to talin, is concentrated in focal adhesion plaques on the plasma membrane at the wound edge where talin in turn is linked to integrin.155–157 It appears that the increase in vinculin synthesis during migration promotes the interaction of contractile protein (actin) with adhesion proteins (talin and integrin) so that the migrating cells can adhere to extracellular substrates in the absence of hemidesmosomes. The temporary focal contacts enable the cells to slide forward, then the epithelial cells release plasminogen activator at these focal contact areas. This in turn converts plasminogen to plasmin, which lyses previously used as adhesion plaques, allowing the cell to advance and form new adhesion plaques. The actual strength of these adhesion plaques is apparently relatively weak because regenerating epithelium can be easily peeled off as a sheet.158 Temporary adhesion plaques cease forming after wound closure from epithelial cell contact inhibition, at which time permanent hemidesmosomal adhesion complexes start being produced. The rapidity with which these permanent adhesion complexes form depends on whether the epithelial basal lamina remains intact. In rabbits, permanent adhesion complexes have been found to be regenerated within 1 week of wound closure if the basal lamina was left intact after injury, whereas it takes 6 weeks to occur if the basal lamina had to be resynthesized.158 Besides requiring energy, epithelial healing also appears to require calcium since cell migration is inhibited by calmodulin inhibitors.159 Additionally, corneal nerves also have a effect on epithelial wound healing, as is evident from denervated corneal epithelial tissue which shows a markedly reduced proliferation rate.153
Usually, an epithelial injury heals rapidly and uneventfully. Occasionally, however, persistent epithelial defects or recurrent corneal erosions develop, which is either due to deficiencies in the migratory or proliferation steps, or to the cell adhesion step, respectively. This has led to a search for therapeutic agents that promote or enhance wound healing. Various agents known to be involved in epithelial growth, adhesion, and differentiation have been studied experimentally and in clinical trials. These include topically applied epidermal growth factor, fibronectin, vitamin A, and many other agents.149,160–164 Although promising experimental results have been obtained, no agent has been identified to date that can consistently stimulate epithelial repair. In particular, there is no uniform confirmation of the utility of these agents in human disease.
As described earlier, limbal corneal epithelial stem cells produce the progenitor cells that are responsible for corneal epithelial cell regeneration. The stem cells are normally slow-cycling, long-lived cells that can be stimulated to replicate to a higher degree after wounding to produce many rapidly-dividing transient amplifying cells (TA) that help in healing corneal surface injuries. Because it has been known for some time that corneal injuries can be replaced by vascular conjunctival epithelium, it also understood that under normal circumstances the limbal epithelium acts as inhibitory barrier to the migration of conjunctival epithelium onto the cornea.165 However, when corneal epithelial injury also includes the limbus, this barrier can be permanently destroyed. This often is followed by permanent vascular conjunctival epithelial migration onto the corneal surface.166 Although conjunctival epithelium can theoretically undergo a slow transformation back to normal corneal epithelium called conjunctival transdifferentiation,167 animal models suggest that the transdifferentiated conjunctival epithelium is still far from normal and at best represents squamous metaplasia with loss of goblet cells.168–170 The main problem with this type of conjunctival epithelium is that it is thinner than normal, stains with fluorescein, attracts new blood vessels, and is prone to recurrent corneal erosions. Therefore, it significantly affects the optical qualities of the cornea and can be quite irritating to the patient.
The knowledge gained from the basic science discoveries of a limbal corneal epithelial stem cell population and the problems encountered by limbal stem cell deficiency has led to the development of limbal stem cell transplantation. As described earlier, the concept of using autologous conjunctival epithelial transplants to treat unilateral chemical injury was first described by Thoft in 1977.115 This concept was first put into clinical practice in 1989 by Kenyon and Tseng.171 Currently, limbal stem cell transplantation uses both autograft or allografts with amniotic membrane as a carrier for the cells.172–173 Because autografts are limited mainly to unilateral disease and allografts require significant and sometimes unsuccessful immunosuppression, cultured autologous limbal, corneal, and even oral mucosa epithelial sheets are being experimented with for corneal epithelial transplantation.174–175 The amniotic membrane, through its host of growth factors and matrix proteins, appears to facilitate epithelialization and to reduce inflammation, vascularization, and scarring, and can be used alone (with no corneal or limbal epithelial cells) to treat corneal epithelial disorders such as those associated with symptomatic bullous keratopathy or corneal burns.176–177
Stroma
The first published report specifically addressing the cellular reactions in the cornea stroma after injury occurred in 1958.178 It described the morphologic changes of stromal cells after different types of trauma and found that stromal cells lose their interconnecting, dendritic processes immediately after injury with many cells subsequently developing signs of degeneration. That report also described the appearance of morphologically-unique spindle-shaped corneal fibroblastic cells invading into the wound region during later stages of stromal healing. Since that time, many excellent animal corneal wound healing studies have further addressed the changes in the extracellular matrix179–182 and the stromal cells183–185 after stromal injury. They suggest that corneal stromal injury is immediately followed by a zone of keratocyte apoptosis around the site of stromal injury (Fig. 30A) with a subsequent influx of transient mixed acute and chronic inflammatory cell infiltration, proliferation and migration of surviving keratocytes (Fig. 30B), and finally differentiation of the keratocytes into a transiently metabolically activated cell type called an activated keratocyte. This latter cell type is functionally important because it synthesizes and deposits the extracellular matrix of the stromal scar, while also degrading and remodeling the cellular and extracellular tissues around the wound. Interestingly, epithelial injury alone can also cause transient cellular injury to the underlying stroma (i.e., apoptosis, proliferation, migration, and possibly activation of keratocytes) along with causing stromal edema, presumably from factors in the tears.184,186
|
Electrophysiologic studies evaluating corneal keratocytes have examined the migration of keratocytes in an electric field.187 These studies found a positive galvanic response in keratocytes. Studies in other wounded tissues have found similar electrical fields generated in the area of the wound. Such electrical fields may act to attract keratocytes to the wound site. It is possible that ion channels have a role in the activation of and/or responses of keratocytes in the wounded corneal stroma. Interestingly, keratocytes isolated from wounded corneas (myofibroblasts) lose their major ionic conductances (Na+ and K+) and gain a Cl− channel that is not found in quiescent keratocytes. This Cl− channel can be activated via a receptor-mediated response by lysophosphatidic acid (LPA) and other members of the lipid growth factor (PLGF) family, including sphingosine-1-phosphate (S1P).188–192 Rabbit studies have also shown that many of these PLGFs become elevated in the aqueous humor and in tear following corneal injury.193
Epithelial-stromal interactions appear to be important to the process of stromal wound healing as it causes an increase in the number of proliferating and migrating keratocytes within the wound, some of which differentiate beyond the activated keratocyte stage into myofibroblastic cell types (Figure 30C).194 Many studies have focused on initiation of keratocyte activation and the substances secreted by these activated cells. Several substances associated with the corneal wound healing process have been shown to activate keratocytes. These activating factors include epithelial growth factor (previously called mesodermal growth factor),195–196 fibroblast growth factor (FGF),197 interleukin-1, transforming growth factor-β,198 insulin,199 retinoic acid,200–201 and LPA.190 Transforming growth factor-β is currently the major epithelial growth factor of this group that is also known to stimulate myofibroblast transformation and enhance the normal repair process.184,194,198 Once activated, keratocytes exhibit a wide range of cellular responses, including increased tritiated thymidine uptake (indicating increased cell proliferation),199,201 initiation of protease and collagenase activity,202–203 phagocytosis,204–205 interferon, prostaglandin and complement factor I production,206–208 as well as fibronectin, collagen, and proteoglycan secretion.209–215 Epithelial-stromal interactions appear augment the wound healing process by causing the production of a thicker and stronger extracellular scar matrix than that found in deeper stromal wound regions that receive no epithelial cell factors.216
When reviewing the literature, it is important to realize that human studies217–220 concur with animal studies on most issues with the following notable differences: adult human corneas heal less aggressively, more slowly, and not as completely as animal corneas. However, both animal and human studies show that corneal stromal wounds heal in two distinct phases: (a) an active phase that results in the production of a stromal scar (over the first 6 months after injury in humans), and (b) a remodeling phase that improves corneal transparency and increases wound strength (occurs up to 3.5 years after injury in humans). Overall, the long-term result in human corneas is the production of a hypercellular fibrotic stromal scar type in wound regions where epithelial-stromal interactions occur and a hypocellular primitive stromal scar type in wound regions where keratocyte injury pathways work alone. These two histologic wound types have functional significance as the hypercellular fibrotic stromal scar is strong, but can look clinically hazy because of myofibroblastic cells populating this scar type.221 In contrast, the hypocellular primitive stromal scar is transparent, but is very weak in tensile strength and serves as a potential space for fluid, inflammatory cells, and microbes. An additional variable to consider in this simplified scheme is the fact that more precisely realigned wounds (i.e., sutured wounds or unsutured wounds with minimal gaping and no epithelial cell plugging) heal better than poorly-aligned wounds (i.e., wounds with wide wound gaping, epithelial plugging, or incarceration of adjacent corneal tissue).
The location and the variable amounts of scar types along with the variable degrees of wound realignment in the human cornea is best exemplified when one reviews published human histopathologic studies that describe findings in corneas that have had common ophthalmic procedures such as cataract extraction (CE), penetrating keratoplasty (PKP), radial keratotomy (RK), AK, PRK, or LASIK. Sutured and unsutured clear corneal cataract wounds are corneal stromal incisions constructed at oblique angles to corneal surface so that they self-seal. They usually heal with well-aligned external wound margins and wound edges, which results in a small, 50 to 75 μm in depth, subepithelial zone of hypercellular fibrotic stromal scarring and a remaining deeper zone of hypocellular primitive scarring (Fig. 31A). Occasional small epithelial plugs are found in the external wound of unsutured clear corneal cataract wounds and commonly Descemet's membrane is found partially detached or poorly realigned along the internal wound margin so that some stromal ingrowth occurs. In marked contrast, limbal and scleral tunnel cataract incisions heal because fibrovascular granulation tissue from the episclera completely grows into the wound by 15 days after surgery and finishes remodeling by 2.5 years after surgery.222–224 PKP wounds heal similar to sutured clear corneal cataract wounds with the notable exceptions of having significant wound compression because of the 0.25- to 0.5-mm oversized nature of the donor button and wound edge mismatch caused by the irregular, asymmetric nature found between donor and host wound edges. Additionally, a high percentage of PKP cases have overriding external or internal wound edges with Bowman's layer or Descemet's membrane incarceration.225 Although partial thickness (70% to 95% depth) and constructed perpendicular to the corneal surface, RK and AK wounds heal similarly to that of unsutured clear corneal cataract wounds.226–230 The most notable difference from unsutured cataract wounds is the more commonly present and more widely variable degree of external wound gaping found in these corneas (Fig. 31B). Additionally, when wound gaping occurred, epithelial cells almost universally migrated into these areas and formed epithelial plugs that rarely resolved (∼90% of cases).
|
PRK consists of submicron accurate anterior keratectomy using the excimer laser resulting in a surface wound that heals entirely under the influence epithelial-stroma interactions. Therefore, a disc-shaped hypercellular fibrotic stroma scar is produced, which usually is 15% to 25% in thickness of the amount initially ablated (Fig. 31C).231–232 As LASIK involves a partial-thickness lamellar incision (mechanical or laser created) followed by excimer laser guided intrastromal excision of tissue, it heals most similar to that of unsutured clear corneal cataract incisions. A subepithelial zone of hypercellular fibrotic scarring occurs at the flap wound margin and the remainder heals by producing a hypocellular primitive stromal scar usually with a thickness around 3% to 10% of that amount ablated by the excimer laser (Fig. 31D).233–235 Around 50% of the LASIK cases were found have at least some microscopic epithelial ingrowth present.
Endothelium
The corneal endothelium is the primary regulator of corneal hydration and, in turn, its transparency. Therefore, any injury to the endothelium must be compensated for in order to maintain a clear cornea.
Following injury to the central endothelium, both the central and peripheral cell densities (cells/mm2) and percent hexagonal cells are reduced, whereas the coefficient of variation of cell size (an indicator of polymegathism) is increased.236–238 In the rabbit, where wound healing is accompanied by both endothelial migration and extensive endothelial cell proliferation, these parameters shift toward preoperative values during the time course of wound healing. In cat corneas, where minimal cell division occurs following endothelial wounding, cell density remains depressed; a similar situation occurs in the human cornea.
Several studies have examined endothelial function following wound healing via corneal thickness measurements. Yee examined the permeability characteristics of the endothelium over the time course of wound healing while incorporating an ouabain-binding analysis to quantitate endothelial Na+/K+ ATPase pump-site densities.238 From this study, it was concluded that the endothelium of the rabbit following a transcorneal freeze heals in three stages. Stage 1 (0 to 3 days) is characterized by an initial coverage of the wound by pleomorphic spindle-shaped cells that form a functional but incomplete barrier and have minimal pump site density. In stage 2 (4 to 7 days), the cells assume a flattened configuration, have an irregular polygonal shape, and establish normal pump-site density and barrier function. Stage 3 (8 to 30 days) is characterized by a continuation of the remodeling of the monolayer. During the final stages of wound healing, cell rearrangement occurs (exchange of neighbors among cells by sliding past one another).
Endothelial wound healing in the cat cornea is slower and less complete in terms of returning to preoperative values. Ling and co-workers found that removal of the central 6 mm of endothelium in the cat cornea results in stromal swelling that returns to preoperative values 35 days after wounding.239 Central endothelial cell density is decreased by 25% at 4 weeks after wounding, and the coefficient of variation increases by 60%. In a separate study, it was found that stromal swelling occurred in the cat when cell density was reduced to below 40% to 45% of control values.240 Humans appear to tolerate more cell loss before stromal swelling occurs. Human corneal decompensation occurs when the cell density drops below 500 cells/mm2.
IN-VITRO HUMAN CORNEAL MODEL
It is now possible to study human corneal wound healing and other physiological and pathophysiological corneal functions using a bioengineered human cornea developed by Griffith, Doillon, and Watsky.241–242 The stroma of this bioengineered cornea is comprised of type I collagen and chondroitin sulfate, whereas the cells are all cell lines derived from human corneal epithelium, keratocytes, and endothelium. It has a vascularized sclera surrounding it, and most recently has been functionally innervated.243 Functional studies demonstrate that it can volume-regulate and its wounds heal in a manner similar to that of human corneas in vivo. As such, it is also being used as a substitute for animal cornea studies in industrial toxicology applications. Future modifications of this bioengineered tissue could lead to a substitute for human donor corneas for penetrating keratoplasty procedures.
CORNEAL INNERVATION AND SENSITIVITY
The epithelium of the cornea is the most densely innervated surface epithelium of the body, with about 2,500 nerve terminals/mm2 (300 to 600 times more dense than skin).244 As the surface area of the adult cornea is approximately 138 mm2, it is estimated that each corneal contains around 315,000 to 630,000 nerve endings. Most of the nerve fibers in the cornea are sensory in origin and are derived from the ophthalmic branch of the trigeminal nerve (cranial nerve [CN] III1), which supplies the eye mainly through two long ciliary nerves.245 Although all mammalian species have been found to receive variable proportions of nerve fibers in the cornea from the sympathetic and parasympathetic autonomic nervous system, humans corneas appear to be on extreme end of this spectrum, as these corneas have a very small proportion of their nerve fibers derived from the autonomic nervous system.245
Innervation of the cornea (corneal epithelium and the anterior third of the corneal stroma) arises from the long ciliary nerves that penetrate the sclera around the scleral canal and begin to branch in the outermost layers of the choroid, or epichoroidal space, as they pass beneath the ora serrata. The main nerve trunks are myelinated and provide branching networks that supply the sclera, episclera, and conjunctiva. The remaining branches form a circumcorneal network around the limbus called the annular plexus where 60 to 80 radial nerve trunks enter the peripheral cornea at or above the midpoint of the corneal stroma (Fig. 32A). Within 1 mm of the limbus, these stromal nerve trunks lose their myelin sheaths and perineurium but retain their Schwann cell sheaths. These radial nerve trunks then branch extensively in the anterior third of stroma interconnecting among themselves in the subepithelial plexus. Few if any of the nerves pass posteriorly to innervate the area of the deeper stroma and none pass through Descemet's membrane to supply the endothelial cells.
|
Although only a few nerve fibers in subepithelial plexus terminate in anterior third of the corneal stroma, parts of the stromal nerves in the subepithelial plexus that have gaps in the Schwann cell sheaths also act as receptor elements. When the nerves bundles abruptly turn 90 degrees to penetrate the Bowman's layer throughout the peripheral and central cornea, they lose their Schwann cell sheaths. They then turn 90 degrees again to continue parallel to the corneal surface forming the subbasal nerve plexus that is composed of nerve bundles that contain straight and beaded nerve fibers (Fig. 32B, C). Only the beaded fibers branch from the subbasal nerve plexus and form epithelial nerves, which course between superficial epithelial cells before finally terminating into 10 to 20 unspecialized free nerve endings (Fig. 32C). The terminals generally extend up to the most superficial epithelial cell layers. Since different types of epithelial nerve fibers can be distinguished on the basis of their ultrastructure, it appears that epithelial nerves are primarily A-delta and C fibers (touch and pain sensations). The free nerve endings in the cornea are able to generate action potentials from nonspecific stimuli. For example, generator potentials may be created from the inward diffusion of Na+, the outward diffusion of K+, or an osmotic change. The nerve ending may also be altered by pH, which can affect the ionic diffusion necessary to elicit generator potentials. It should be noted that nerve fibers from the conjunctiva and episclera also may enter the peripheral cornea and ramify, usually 2 to 3 mm past the limbus. This explains why areas of cold sensitivity can be found in the cornea.
Because the corneal nerve fibers ultimately terminate in the brainstem, it appears that interneurons, or intermediate pathways must relay the information to the sensation areas of cerebrum. Additionally, there must also be intermediate relays to efferent systems that trigger the reflex pathways of involuntary blinking (orbicularis motor innervation of CN VII) and reflex tearing (parasympathetic innervation of lacrimal gland). The intricate details of these pathways are currently unknown.
Corneal nerves appear to be very important in maintaining the health of the corneal epithelium and the ocular surface via trophic influences and/or other factors.245 Since the earliest experimental studies by Magendie,246 it has been shown that dysfunction of corneal innervation produces a degenerative condition to the corneal epithelium called neurotrophic keratitis. Although many disorders can cause neurotrophic keratitis such as trigeminal nerve damage or herpes keratitis, keratorefractive surgery has gained the most attention lately for transiently causing this condition, particularly LASIK.247 This usually results in symptoms of dry eye after the surgery which is more common, more severe, and longer in duration following LASIK than PRK. This is because the total surface area and depth of corneal nerve injury are less after PRK than LASIK, so reinnervation takes less time with PRK than LASIK.248 In general, PRK is immediately followed by a loss of corneal sensation and corneal nerves in the ablated region of the cornea. Subsequently, regrowth of the subbasal nerve plexus and epithelial nerves starts around the first month after the PRK with corneal sensation recovering to normal levels by 3 months postoperatively.245,248 In contrast, LASIK is immediately followed by loss of corneal sensation over the flap and gradual disappearance of most of the corneal nerves in the flap over the first 2 days after surgery. Regrowth of the subbasal nerve plexus starts between 3 to 6 months after the procedure with corneal sensation recovering to normal levels by 6 to 12 months postoperatively.245,248 Interestingly, the total length and morphologies of the corneal nerve fibers and corneal sensation only reaches maximum levels 1 to 2 years after both of these surgeries and never completely returns to normal preoperative levels in both cases.
Corneal sensitivity is usually tested clinically in a semiquantitative fashion with a Cochet and Bonnet esthesiometer, which is a thin, flexible, nylon filament of variable length. When the filament is long, it applies very little pressure to the cornea surface because it bends easily, whereas when short it applies a proportionally higher pressure. If one maps corneal sensitivity, it is found that the cornea is most sensitive in the central 5 mm of the cornea as compared to periphery. Additionally, it is more sensitive along the horizontal meridian and least sensitive in the vertical meridian, and more sensitive in the morning than in the evening. Corneal sensitivity may be evaluated subjectively by asking the patients when they feel touch upon the cornea or objectively when a blink response is triggered. The subjective and objective thresholds are quite similar in normal corneas with the subjective threshold being slightly lower. In the center of the normal cornea, the average subjective threshold is usually 15 mg. Near the limbus this increases to approximately 31 mg for the subjective threshold and 34 mg for the objective touch threshold.249
Corneal sensitivity has been found to decrease with age as the threshold of corneal stimulation normally from childhood to age 50 is around 10 to 15 mg, and gradually increases from age 50 to reach a value of 40 to 50 mg over the age of 60.250 It also is variably decreased following full recovery of surgical procedures on the anterior segment of the eye. As nerve regeneration occurs at the rate of approximately 1 mm per month, it may take 6 to 12 months or longer for corneal reinnervation to completely occur and corneal sensation to recover. After complete recovery, the sensitivity in the portion of the cornea involved by the procedure often is variably less than that which was present prior to surgery. For example, after complete recovery of corneal sensation from LASIK or PRK surgeries, the subjective threshold increases back to about 95% of that measured preoperatively.251 The remarkable neural recovery abilities of cornea is perhaps most uniquely demonstrated following penetrating keratoplasty (donor tissue) as corneal sensation increases in the graft over the first couple postsurgical years. It appears that host corneal nerves can innervate the donor tissue by penetrating the full thickness scar and reinnervating the graft stroma and epithelium.
The mechanisms by which corneal nerves maintain the ocular surface and promote healing after eye injuries is currently under active research in several laboratories. The most recent, detailed review on this subject was published in 2003.245