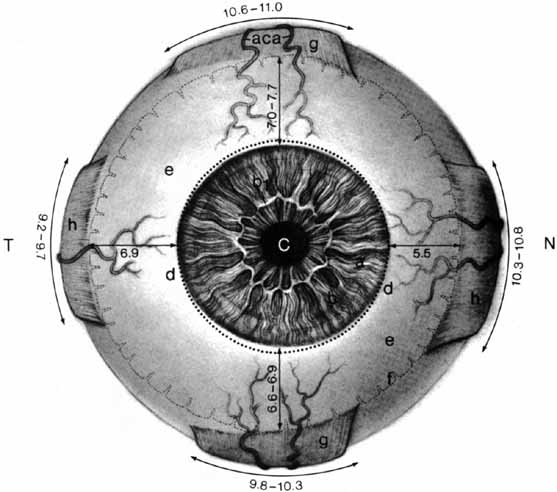
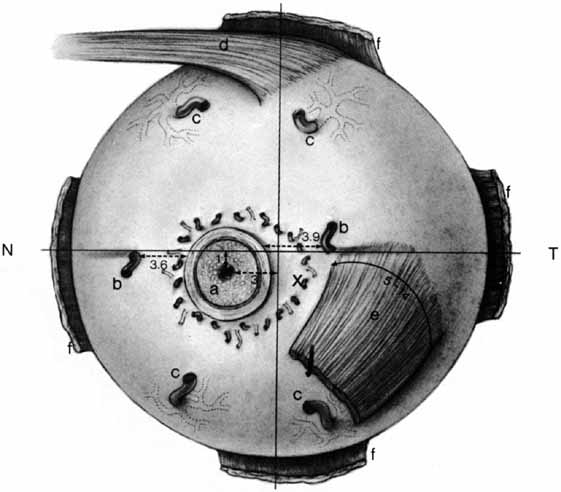
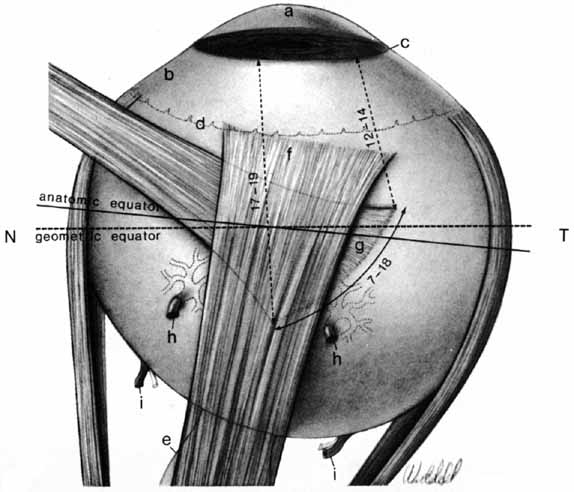
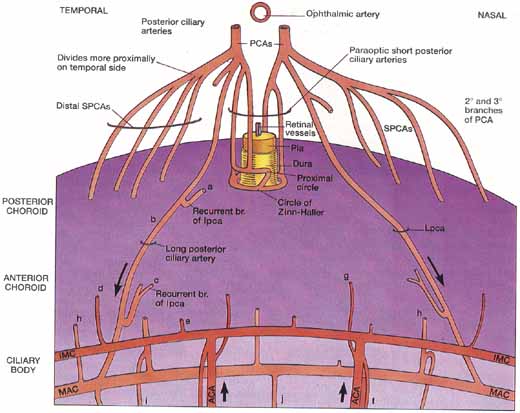
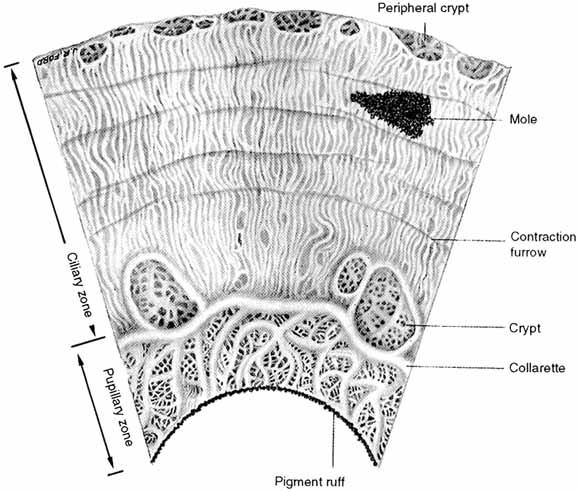
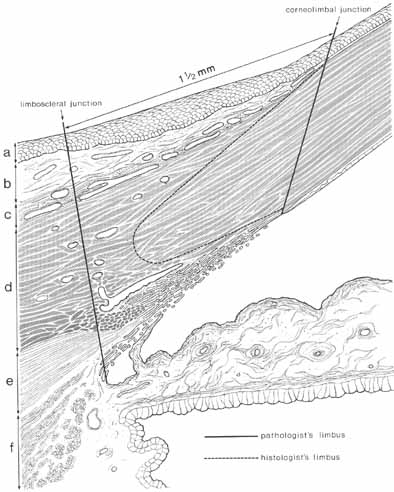
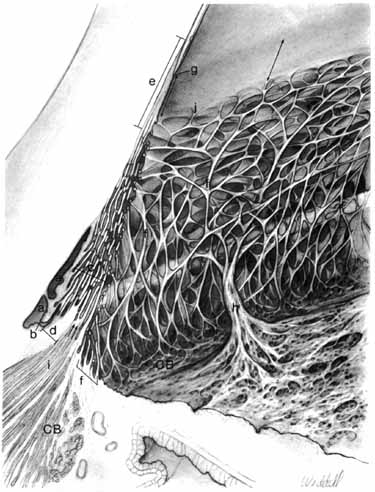
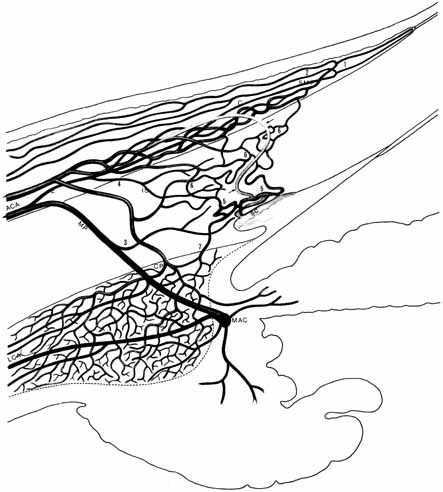
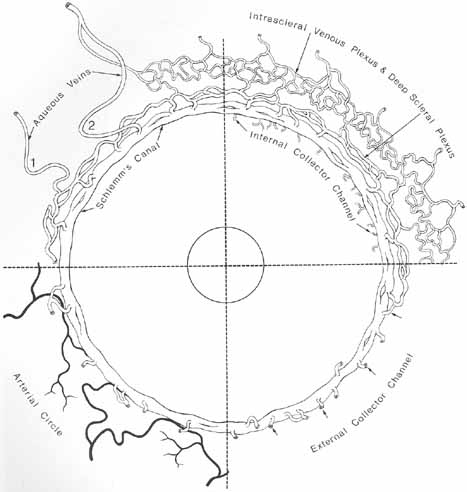
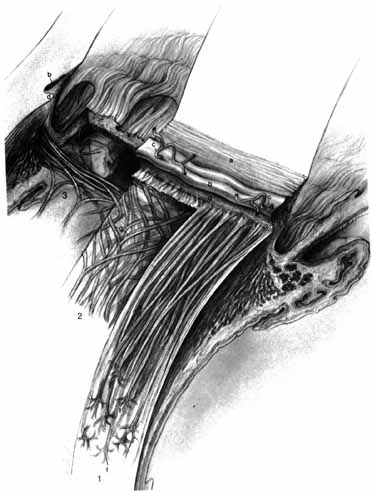
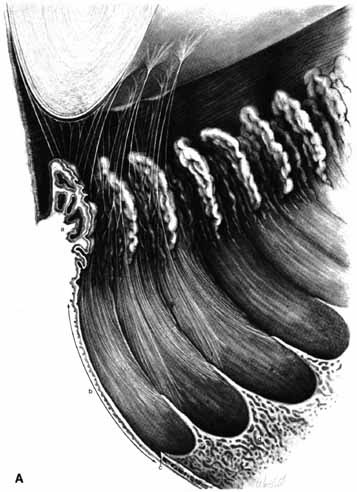
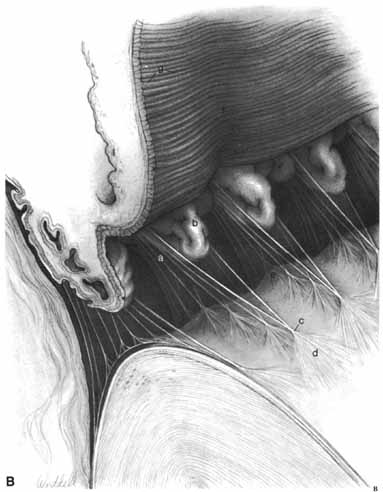
1. Duke-Elder S, Wybar K: The eye. In Duke-Elder S (ed): The Anatomy of the Visual System, Vol II. St. Louis: CV Mosby, 1961:75–386 2. Hogan M, Alvarado J, Weddell J: Histology of the Human Eye—An Atlas and Textbook. Philadelphia: WB Saunders, 1971 3. Whitnall S: The Anatomy of the Human Orbit and Accessory Organs of Vision. London: Oxford University Press, 1932 4. Stenström S: Untersuchungen über die Variation und Kovariation der optischen Elemente
des menshlichen Auges. Acta Ophthalmol (Copenh) 26:1, 1946 5. Sorsby A, Sheridan M: The eye at birth: measurement of the principal diameters in forty-eight
cadavers. J Anat 94:192, 1960 6. Testut L: Traité d'anatomie humaine, 5th ed. Paris, 1905 7. Weiss L: Uber das wachstum des menschlichen auges und uber die veranderung der muskelinsertionen
am wachsenden auge (anatomische hefte). Arb Anat Inst Weisbaden 8:191, 1897 8. Brubaker RF, Pederon JE: Ciliochoroidal detachment. Surv Ophthalmol 27:281, 1983 9. Migliori ME, Gladstone GJ: Determination of the normal range of exophthalmometric values for black
and white adults. Am J Ophthalmol Oct 15;98(4):438–442, 1984 10. Lemke BN: Anatomy of the ocular adnexa and orbit. In Smith BC (ed): Ophthalmic Plastic and Reconstructive Surgery, Vol. 1, Part 1. St. Louis: C.V. Mosby, 1987 11. Bron AJ, Tripathi RC, Tripathi BJ: Wolff's Anatomy of the Eye and Orbit, 8th ed. London: Chapman & Hall, 1997 12. Maurice D: Cornea and sclera. In Davson H (ed): Physiology of the Eye, 2nd ed. Boston: Little, Brown and Co, 1969:6,14,15 13. Vannas S, Teir H: Observations on struture and age changes in the human sclera. Acta Ophthalmol 38:268, 1960 14. Weale RA: A Biography of the Eye: Development, Growth Age. London: H.K. Lewis, 1982 15. Tripathi RC, Tripathi BJ: Functional anatomy of the anterior chamber angle. In Duane TD, Jaeger EA (eds): Biomedical Foundations of Ophthalmology. Philadelphia: Lippincott, 1982 16. Hayreh S, Vrabec F: The structure of the head of the optic nerve in the rhesus monkey. Am J Ophthalmol 61:136, 1966 17. Stevenson TC: Intrascleral nerve loops: a clinical study of frequency and treatment. Am J Ophthalmol 55:935, 1963 18. Fuchs E: Beitrage Beiträge zur normalen Anatomie des Augapfels. Albrecht Von Graefes Arch Klin Exp Ophthalmol 30:1, 1894 19. Rutin U: Fundus appearance in the normal eye. I. The choroid. Am J Ophthalmol 64:821, 1967 20. Von Noorden G: Burian-Von Noorden's Binocular Vision and Ocular Motility. St. Louis: CV Mosby, 1985 21. Apt L: An anatomical evaluation of rectus muscle insertions. Trans Am Ophthalmol Soc 78:365, 1980 22. Lang J, Horn T, von den Eichen U: Über die äusseren Augenmuskeln und ihre Ansatzzonen. Gegenbaurs Morphol Jahrb 126:817, 1980 23. Fink W: Surgery of the Vertical Muscles of the Eyes. Springfield, IL:. Charles C Thomas, 1962 24. Stallard H: Eye Surgery. Baltimore: Williams & Wilkins, 1950 25. Mishima S: Some physiologic aspects of the precorneal tear film. Arch Ophthalmol 73:233, 1965 26. Klyce SD, Beuerman RW: Structure and function of the cornea. In Kaufman HE, Barron BA, McDonald MB, Waltman SR (eds): The Cornea. New York: Churchill Livingston, 1988 27. Rapuano CJ, Fishbaugh JA, Strike DJ: Nine point corneal thickness measurements and keratometry readings in normal
corneas using ultrasound pachymetry. Insight 18:16, 1993 28. Eysteinsson T, Jonasson F, Sasaki H, Arnarsson A, Scerrisson T, Sasaki K, Stefansson E: Reykjavik Eye Study Group: Central corneal thickness, radius of the corneal
curvature and intraocular pressure in normal subjects using non-contact
techniques: Reykjavik Eye Study. Acta Ophthalmol Scand 80:11, 2002 29. Liu Z, Pflugfelder SC: The effects of long-term contact lens wear on corneal thickness, curvature, and
surface regularity. Ophthalmology 107:105, 2000 30. Myrowitz EH, Melia M, O'Brien TP: The relationship between long-term contact lens wear and corneal
thickness. CLAO J 28:217, 2002 31. Pflugfelder SC, Liu Z, Feuer W, Verm A: Corneal thickness indices discriminate between keratoconous and contact
lens-induced corneal thinning. Ophthalmology 109:2336, 2002 32. Sanchis-Gimeno JA, Lleo A, Alonso L, Rahhal MS, Martinez-Soriano F: Differences in corneal anatomy in a pair of monozygotic twins due to continuous
contact lens wear. Cornea 22:243, 2003 33. Perez JG, Meijome JM, Jalbert I, Sweeney DF, Erickson P: Corneal epithelial thinning profile induced by long-term wear of
hydrogel lenses. Cornea 22:304, 2003 34. Gonzalez-Meijome JM, Gonzales-Perez J, Cervino A, Yebra-Pimentel E, Parafita MA: Changes in corneal structure with continuous wear of high-Dk soft
contact lenses: a pilot study. Optom Vis Sci. 80:440, 2003 35. von Bahr G: Corneal thickness: Its measurement and changes. Am J Ophthalmol 42:251, 1956 36. Martola E, Baum J: Central and peripheral corneal thickness. Arch Ophthalmol 79:28, 1968 37. Koch DD, Haft EA: Introduction to corneal topography. In Sanders DR, Koch DD (eds): An Atlas of Corneal Topography. Thorofare, NJ:. SLACK Inc., 1993 38. Guirao A, Redondo M, Artal P: Optical aberrations of the human cornea as a function of age. J Opt Soc Am A Opt Image Sci Vis 17:1697, 2000 39. Snell RS, Lemp MA: Clinical Anatomy of the Eye. Cambridge, MA:. Blackwell Scientific Publications, Inc., 1989 40. Morrison J, van Buskirk EM: Anterior collateral circulation in the primate eye. Ophthalmology 90:707, 1983 41. Brancato R, Bandello F, Lattanzio :. Atlas of Iris Fluorescein Angiography. Milano: Ghedini Editore, 1995 42. Hayreh S: The ophthalmic artery. III. Branches. Br J Ophthalmol 46:212, 1962 43. Virchow H: Graefe-Saemisch handbuch der gesammten Augenheilkunde. Leipzig: Engelmann, 1910 44. Tousimis A, Fine B: Ultrastructure of the iris: The intercellular components. Am J Ophthalmol 48:397, 1959 45. Tousimis A, Fine B: Ultrastructure of the iris: The intracellular stromal components. AMA Arch Ophthalmol 62:974, 1959 46. Ehinger B: Ocular and orbital vegetative nerves. Acta Physiol Scand (suppl 268) 67:1, 1966 47. LaGrange F: Un moyen de relevement rélévement de la tension oculaire. Ann Ocul (Paris) 157:447, 1920 48. Cosar CB, Senar AB: Orbscan corneal topography system in evaluating the anterior structures
of the human eye. Cornea 22:118, 2003 49. Aizawa K: The depth of the anterior chamber. Nippon Ganka Gakkai Zasshi 62:2283, 1958 50. Tornquist R: Shallow anterior chamber in acute glaucoma. Acta Ophthalmol (Copenh) (suppl 39) 31:1, 1953 51. Weekers R, Grieten J, Lavergne G: Study of the dimensions of the human anterior chamber. Ophthalmologica 142:361, 1961 52. Weekers R, Grieten J: The measurement of the depth of the anterior chamber in clinical practice. Bull Soc Belge Ophtalmol 129:361, 1961 53. Weekers R, Grieten J, Lekeux M: Etude des dimension de la chambre anterieure antérieure de l'oeil
humain. IV. L'intumescence cristallinienne et ses conséquences
chirurgicales. Ophthalmologica 146:57, 1963 54. Touzeau O, Allouch C, Borderie V, Kopito R, Laroche L: Correlation between refraction and ocular biometry. J Fr Ophtalmol 26:355, 2003 55. Wong TY, Foster PJ, Ng TP, Tielsch JM, Johnson GJ, Seah SK: Variations in ocular biometry in an adult Chinese population in Singapore: the
Tanjong Pagar Survey. Invest Ophthalmol Vis Sci 42:73, 2001 56. Fontana ST, Brubaker RF: Volume and depth of the anterior chamber in the normal aging human eye. Arch Ophthalmol 98:1803, 1980 57. Lyhne N, Sjolie AK, Kyvik KO, Green A: The importance of genes and environment for ocular refraction and its determiners: a
population based study among 20–45 year old twins. Br J Ophthalmol 85:1470, 2001 58. Garner LF, Yap MK: Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol Opt 17:12, 1997 59. Stamper R: Aqueous humor: Secretion and dynamics. In Tasman W, Jaeger E (eds): Duane's Foundations of Clinical Ophthalmology, Vol 2., Philadelphia: JB Lippincott, 1992 60. Brubaker RF, Nagataki S, Townsend DJ, Burns RR, Higgins RG, Wentworth W: The effect of age on aqueous humor formation in man. Ophthalmology 88:283, 1981 61. Caprioli J: The ciliary epithelia and aqueous humor. In Hart WM (ed): Adler's Physiology of the Eye: Clinical Application, 9th ed. St. Louis: Mosby, 1992 62. DiMatteo J: Active transport of ascorbic acid into lens epithelium of the rat. Exp Eye Res 66:731, 1998 63. Hoskins HD, Kass MA: Aqueous humor outflow. In Hoskins HD, Kass MA (eds): Becker-Schaffer's Diagnosis and Therapy of the Glaucomas. St. Louis: Mosby, 1989 64. Johnson F, Maurice D: A simple method of measuring aqueous humor flow with intravitreal fluoresceinated
dextrans. Exp Eye Res 39:791, 1984 65. Van Buskirk EM: The anatomy of the limbus. Eye 3:101, 1989 66. Luo YR, Luo WB: A study of the surgical anatomy of the corneo-scleral limbus. Zhonghua Yan Ke Za Zhi 29:362, 1993 67. Burian H, Braley A, Allen L: External and gonioscopic visibility of the ring of Schwalbe and the trabecular
zone. Trans Am Ophthalmol Soc 52:389, 1955 68. Tamm E, Flugel C, Stefani FH, Rohen JW: Contractile cells in the human scleral spur. Exp Eye Res 54:531, 1992 69. Stegman Z, Sokol J, Liebmann JM, Cohen H, Tello C, Ritch R: Reduced trabecular meshwork height in juvenile primary open-angle
glaucoma. Arch Ophthalmol 114:660, 1996 70. Salzmann M: The Anatomy and Histology of the Human Eyeball. Brown EVL (trans): Chicago: University of Chicago Press, 1912 71. Allen L, Burian H, Braley A: The anterior border ring of Schwalbe and the pectinate ligament. Arch Ophthalmol 53:799, 1955 72. Ashton N, Brini A, Smith R: Anatomical studies of trabecular meshwork of normal human eye. Br J Ophthalmol 40:257, 1956 73. Holmberg A: Schlemm's canal and the trabecular meshwork. An electron microscopic
study of the normal structure in man and monkey (cerecopithecus
ethiops). Doc Ophthalmol (Den Haag) 19:339, 1965 74. Moses RA, Grodzki WJ Jr : The scleral spur and scleral roll. Invest Ophthalmol Vis Sci 16:925, 1977 75. Unger H, Jankovsky F: Sehnen und Stützelemente im Trabeculum corneosclerale. Albrecht Von Graefes Arch Klin Exp Ophthalmol 170:355, 1966 76. Flocks M: The anatomy of the trabecular meshwork as seen in tangential section. Arch Ophthalmol 56:708, 1956 77. Garron L, Feeney M, Hogan M, McEwen W: Electron microscopic studies of the human eye. I. Preliminary investigations
of the trabeculae. Am J Ophthalmol 46:27, 1958 78. Garron L, Feeney M: Electron microscopic studies of the human eye. II. Study of the trabeculae
by light and electron microscopy. Arch Ophthalmol 62:966, 1959 79. Fine B: Structure of the trabecular meshwork and the canal of Schlemm. Trans Am Acad Ophthalmol Otolaryngol 70:777, 1966 80. Gabelt BT, Gottanka J, Lutjen-Drecoll E, Kaufman PL: Aqueous humor dynamics and trabecular meshwork and anterior ciliary muscle
morphologic changes with age in rhesus monkeys. Invest Ophthalmol Vis Sci 44:2118, 2003 81. McEwen W: Application of Poiseuille's law to aqueous outflow. Arch Ophthalmol 60:290, 1958 82. Tripathi RC, Tripathi BJ: Human trabecular endothelium, corneal endothelium, keratocytes, and scleral
fibroblasts in primary cell culture. A comparative study of growth
characteristics, morphology, and phagocytic activity by light and scanning
electron microscopy. Exp Eye Res 35:611, 1982 83. Allingham RR, de Kater AW, Ethier CR: Schlemm's canal and primary open angle glaucoma: correlation between
Schlemm's canal dimensions and outflow facility. Exp Eye Res 62:101, 1996 84. Ashton N: Anatomical study of Schlemm's canal and aqueous veins by means of
neoprene casts, Part 1. Aqueous veins. Br J Ophthalmol 35:291, 1951 85. Rohen J: Das Auge und seine Hilfsorgane. In Bargman W (Kiel) (ed): Handbuch der mikroskopischen anatomie des Menschen, Vol III, Haut und Sinnesorgane, Part IV, Suppl to Vol III/2. Berlin: Springer-Verlag, 1964 86. Parc CE, Johnson DH, Brilakis HS: Giant vacuoles are found preferentially near collector channels. Invest Ophthalmol Vis Sci 41:2984, 2000 87. Theobald G: Schlemm's canal. Its anastomosis and anatomic relations. Trans Am Ophthalmol Soc 32:574, 1934 88. Ascher K:. The Aqueous Veins. Springfield, IL:. Charles C Thomas, 1961 90. Sondermann R: The formation, morphology and function of Schlemm's canal. Acta Ophthalmol (Copenh) 11:280, 1933 91. Francois François J, Neetens A, Collette J: Microradiographic study of the inner wall of Schlemm's canal. Am J Ophthalmol 40:491, 1955 92. Unger H: Anatomy of the angle of the anterior chamber. Klin Monatsbl Augenheilkd 135:161, 1959 93. Iwamoto T: Light and electron microscopy of Sondermann's channels of the human
trabecular meshwork. Albrecht Von Graefes Arch Klin Exp Ophthalmol 172:197, 1967 94. Iwamoto T: Further observation on Sondermann's channels of the human trabecular
meshwork. Albrecht Von Graefes Arch Klin Exp Ophthalmol 172:213, 1967 95. Prost M: Anatomy of the ciliary body and cyclocryotherapy. Ophthalmologica 188:9, 1984 96. Smith SG, Snowden F, Lamprecht EG: Topographical anatomy of the ciliary sulcus. J Cataract Refract Surg 13:543, 1987 97. Straatsma B, Foos R, Spencer L: The Retina—Topography and Clinical Correlations. Proceedings of The
New Orleans Academy of Ophthalmology Symposium on the Retina and Retinal
Surgery. St. Louis: CV Mosby, 1969 98. Silverman RH, Lizzi FL, Ursea BG, Rondeau MJ, Eldeen NB, Kaliscz A, Lloyd HO, Coleman DJ: High-resolution ultrasonic imaging and characterization of the ciliary
body. Invest Ophthalmol Vis Sci 42:885, 2001 99. McCulloch C: The zonules of Zinn: Its origin, course, and insertion and its relation
to neighboring structures. Trans Am Ophthalmol Soc 52:525, 1954 100. Beauviex M: La zonule (e é tude topographique et histologique). Arch Ophthalmol 39:484, 1922 101. Rohen J, Rentsch F: Der Konstructive Bau des Zonulaapparates beim Menschen und dessen funktionelle
Bedeutung. Graefes Arch Clin Exp Ophthalmol 178:1, 1969 102. Rohen J: Scanning electron microscopic studies of the zonular apparatus in human
and monkey eyes. Invest Ophthalmol Vis Sci 18:133, 1979 103. Holmberg A: Studies of the ultrastructure of the non-pigmented epithelium of
the ciliary body. Acta Ophthalmol Scand 33:377, 1955 104. Alm A, Bill A: Ocular and optic nerve blood flow at normal and increased intraocular pressure
in monkeys (Macaca iris): A study with radioactively
labelled microspheres including flow determination in brain and some
other tissues. Exp Eye Res 15:15, 1973 105. Alm A, Bill A: Ocular Circulation. St. Louis: CV Mosby, 1987:183–203 106. Caprioli J, Sears M, Mead A: Ocular blood flow in phakic and aphakic monkey eyes. Exp Eye Res 39:1, 1984 107. Woodlief N: Initial observations on the ocular microcirculation in man. I. The anterior
segment and extraocular muscles. Arch Ophthalmol 98:1268, 1980 108. Taniguchi Y: Fine structure of blood vessels in the ciliary body. Jpn J Ophthalmol 6:93, 1962 109. Calasans O: The architecture of the ciliary muscle of man. An Fac Med Univ Sao Paolo 27:3, 1953 110. Gullstrand A: Einführung in die Methoden der Dioptrik des Auges des Menschen. Leipzig, S Hirzel Verlag, 1911 111. Smith P: Diseases of the crystalline lens and capsule. Trans Ophthalmol Soc UK 3:79, 1883 112. Heine L: Physiologisch-anatomische Untersuchungen über die Accomodation
des Vogelauges. Albrecht Von Graefes Arch Ophthalmol 45:469, 1898 113. Scammon R, Hesdorffer M: Growth in mass and volume of human lens in postnatal life. Arch Ophthalmol 17:104, 1937 114. Fine B, Tousimis A: The structure of the vitreous body and the suspensory ligaments of the
lens. Arch Ophthalmol 65:95, 1961 115. Brown NP, Bron AJ: Lens Disorders: Clinical Manual of Cataract Diagnosis. Oxford: Butterworth-Heinemann Ltd, 1996 116. Hayreh S: Segmental nature of the choroidal vasculature. Br J Ophthalmol 59:631, 1975 117. Guyer DR, Schachat AP, Green WR: The choroids: structural considerations. In Ryan SJ (ed): Retina, 3rd ed., Vol. 1, Part 1. St. Louis: Mosby, Inc., 2001 118. Wolfrum M: Contributions to the anatomy and histology of the choroid in man and higher
animals. Albrecht Von Graefes Arch Ophthalmol 67:307, 1908 119. Sumita R: The fine structure of Bruch's membrane in the choroid. Nippon Ganka Gakkai Zasshi 65:1188, 1961 120. Hogan M: Ultrastructure of the choroid. Its role in the pathogenesis of choroidal
diseases. Trans Pacific Coast Oto Ophthalmol Soc 42:61, 1961 121. Straatsma B, Hall M, Allen R, Crescitelli F: The Retina—Morphology, Function and Clinical Characteristics. Berkeley: University of California Press, 1969 122. Cohen A: The Retina. St. Louis: CV Mosby, 1987:458–490 123. Thomson A: The Anatomy of the Human Eye. Oxford: Clarendon Press, 1912 124. Landau D, Schneidman EM, Jacobovitz T, Rozenman Y: Quantitative in vivo retinal thickness measurements in healthy subjects. Ophthalmology 104:639, 1997 125. Konno S, Akiba J, Yoshida A: Retinal thickness measurements with optical coherence tomography and the
scanning retinal thickness analyzer. Retina 21:57, 2001 126. Gorrand JM, Delori FC: Reflectance and curvature of the inner limiting membrane at the foveola. J Opt Soc Am A Opt Image Sci Vis 16:1229, 1999 127. Sigelman J, Ozanics V: Retina. In Jakobiec F (ed): Ocular Anatomy, Embryology, and Teratology. Philadelphia: Harper & Row, 1982 128. Hayreh S: The central retinal artery of the retina: Its role in the blood supply
of the optic nerve. Br J Ophthalmol 47:651, 1963 129. Michaelson I: Retinal circulation in man and animals. Springfield, IL:. Charles C Thomas, 1954 130. Toussaint D, Kuwabara T, Cogan D: Retinal vascular patterns. Arch Ophthalmol 65:575, 1961 131. Bek T, Jensen PK: Three-dimensional structure of human retinal vessels studied by
vascular casting. Acta Ophthalmol (Copenh) 71:506, 1993 132. Justice J, Lehmann R: Cilioretinal arteries: A study based on review of stereo fundus photographs
and fluorescein angiographic findings. Arch Ophthalmol 94:1355, 1976 133. Weinberg DV, Egan KM, Seddon JM: Asymmetric distribution of arteriovenous crossings in the normal retina. Ophthalmology 100:31, 1993 134. Bairati A Jr, Orzalesi N: The ultrastructure of the pigment epithelium and of the photoreceptor-pigment
epithelium junction in the human retina. J Ultrastruct Res 9:484, 1963 135. Tso M, Friedman E: The retinal pigment epithelium. I. Comparative histology. Arch Ophthalmol 78:641, 1967 136. Friedman E, Tso M: The retinal pigment epithelium. II. Histologic changes associated with
age. Arch Ophthalmol 79:315, 1968 137. Tso M, Friedman E: The retinal pigment epithelium. III. Growth and development. Arch Ophthalmol 80:214, 1968 138. Zinn KM, Benjamin-Henkind J: Retinal pigment epithelium. In Jakobiec F (ed): Ocular Anatomy, Embryology, and Teratology. Philadelphia: Harper & Row, 1982 139. Hudspeth AJ, Yee AG: The intercellular junctional complexes of the retinal pigment epithelia. Invest Ophthalmol 12:354, 1973 140. Cohen A: Rods and cones. In Fuortes M (ed): Handbook of Sensory Physiology, Vol 7/2. Berlin: Springer-Verlag, 1972 141. Osterberg G: Topography of the layer of rods and cones in the human retina. Acta Ophthalmol (suppl) 6:8, 1936 142. Ahnelt PK: The photoreceptor mosaic. Eye 12:531, 1998 143. Rostgaard J, Qvortrup K: A note about retinal structure and visual acuity. A light microscopic study
of the cones in fovea centralis. Acta Ophthalmol Scand 77:45, 1999 144. Yamada E: Some structural features of the fovea centralis in the human retina. Arch Ophthalmol 82:151, 1969 145. Arey L: Retina, Choroid and Sclera. New York: Paul B. Hoeber, 1932:743 146. Jones AL, Sheen NJ, North RV, Morgan JE: The Humphrey optical coherence tomography scanner: quantitative analysis
and reproducibility study of the normal human retinal nerve fibre layer. Br J Ophthalmol 85:673, 2001 147. Varma R, Bazzaz S, Lai M: Optical tomography-measured retinal nerve fiber layer thickness
in normal Latinos. Invest Ophthalmol Vis Sci 44:3369, 2003 148. Mok KH, Lee VW, So KF: Retinal nerve fiber layer measurement of the Hong Kong Chinese population
by optical coherence tomography. J Glaucoma 11:481, 2002 149. Kanamori A, Escano MF, Eno A, Nakamura M, Maeda H, Seya R, Ishibashi K, Negi A: Evaluation of the effect of aging on retinal nerve fiber layer thickness
measured by optical coherence tomography. Ophthalmologica 217:273, 2003 150. Polyak S: The Retina. Chicago: University of Chicago Press, 1941 151. Hogan M: The vitreous, its structure and relation to the ciliary body and retina. Invest Ophthalmol 2:418, 1963 152. Foos R: Vitreoretinal juncture: Topographical variations. Invest Ophthalmol 11:801, 1972 153. Oppel O: Microscopic examinations of the number and thickness of the myelinated
nerve fibers in the human optic nerve. Albrecht Von Graefes Arch Klin Exp Ophthalmol 166:19, 1963 154. Kupfer C, Chumbley L, Downer J: Quantitative histology of optic nerve, optic tract, and lateral geniculate
of man. J Anat 101:393, 1967 155. Quigley HA, Brown AE, Morrison JD, Drance SM: The size and shape of the optic disc in normal human eyes. Arch Ophthalmol 108:51, 1990 156. Agarwal HC, Gulati V, Sihota R: The normal optic nerve head on Heidelberg Retina Tomograph II. Indian J Ophthalmol 51:25, 2003 157. Jonas JB, Thomas R, George R, Berenshtein E, Muliyil J: Optic disc morphology in south India: the Vellore Eye Study. Br J Ophthalmol 87:189, 2003 158. Brown GC, Tasman WS: Congenital Anomalies of the Optic Disc. New York: Grune & Stratton, 1983 159. Digre KB, Corbett JJ: Practical Viewing of the Optic Disc. Burlington: Elsevier Science, Inc., 2003 160. Anderson D, Braverman S: Reevaluation of the optic disc vasculature. Am J Ophthalmol 82:165, 1976 161. Hayreh S: Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and
oedema of the optic disc. Br J Ophthalmol 53:721, 1969 162. Lieberman M, Maumenee A, Green W: Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol 82:405, 1976 163. Wolter J: The human optic papilla. A demonstration of new anatomic and pathologic
findings. Am J Ophthalmol 44:48, 1957 164. Dutton JJ: Atlas of Clinical and Surgical Orbital Anatomy. Philadelphia: W.B. Saunders Company, 1994 165. Anderson D: Ultrastructure of the human and monkey lamina cribrosa and optic nerve
head. Arch Ophthalmol 82:800, 1969 166. Anderson D: Ultrastructure of the optic nerve head. Arch Ophthalmol 83:63, 1970 167. Anderson D, Hoyt W: Ultrastructure of the intraorbital portion of human and monkey optic nerve. Arch Ophthalmol 82:506, 1969 168. Anderson D, Hoyt W, Hogan M: The fine structure of the astroglia in the human optic nerve and optic
nerve head. Trans Am Ophthalmol Soc 65:275, 1967 169. Ducournau D: A new technique for the anatomical study of the choroidal blood vessels. Ophthalmologica 184:190, 1982 170. Visser-Heerema J: Über das spezifische Gewicht der bei der Operation von Netzhautablösungen
gewonnenen Flüssigheit. Arch Augenheilkd 109:543, 1936 171. Redslob E: Le corps vitré. Son dévelopment, sa structure, ses propiétes
physicochimiques. Paris: Masson et Cie, 1932 172. Richards O, Hague E: Vitreous as removed with large and small needles. Am J Ophthalmol 55:151, 1963 173. Guggenheim I, Franceschetti A: Refraktometrische Untersuchungen des Glaskörpers von Kaninchen un
Mensch unter physiologischen und pathologischen Bedingungen. Arch Augenheilkd 98:448, 1928 174. Paufique L, Moreau P: Les greffes de vitre vitré lyophilise lyophilisé. Ann Ocul (Paris) 186:873, 1953 175. Balazs E: Physiology of the vitreous body. In Schepens C (ed): Importance of the Vitreous Body in Retina Surgery with Special Emphasis
on Reoperations. St. Louis: CV Mosby, 1960 176. Berman E, Michaelson I: The chemical composition of the vitreous body as related to age and myopia. Exp Eye Res 3:9, 1964 177. Sebag J: The Vitreous: structure, function, and pathobiology. New York: Springer-Verlag, 1989 178. Goldman H: Biomicroscopy of the vitreous body. Arch Ophthalmol 127:334, 1954 178a. Michels RG, Wilkinson CP, Rice TA: Retinal Detachment. St. Louis: Mosby, Inc., 1990 179. Ergglet H: Klinische Befunde bei focaler Beleuchtung mit der Gullstrandischen Nernst-Spaltlampe. Klin Monatsbl Augenheilk 53:449, 1914 180. Busacca A: Observations biomicroscopiques sur le corps ciliaire normal et pathologique. Bull Soc Ophthalmol Fr 68:295, 1955 181. Green WR: Vitreo retinal juncture. In Ryan SJ (ed): Retinal Disease. St. Louis: CV Mosby, 1989 182. Friedenwald J, Stiehler R: Structure of the vitreous. Arch Ophthalmol 14:789, 1935 183. Vogt A: Ueber Berührungspunkte der senilen und der myopichen Bulbusdegeneration. Klin Monatsbl Augenheilkd 72:212, 1924 184. Jack RL: Regression of the hyaloid artery system. An ultrastructural analysis. Am J Ophthalmol 74:261, 1972 185. Bloom GD, Balazs EA, Ozanics V: The fine structure of the hyaloid arteriole in bovine vitreous. Exp Eye Res 31:129, 1980 186. Reeser FH, Aaberg T: Vitreous humor. In Records PE (ed): Physiology of the Human Eye and Visual System. Hagerstown, MD:. Harper & Row, 1979 187. Gartner J: Electronmicroscopic study on the fibrillar network and fibrocyte-collagen
interactions in the vitreous cortex at the ora serrata of human
eyes with special regard to the role of disintegrating cells. Exp Eye Res 42:21, 1986 188. Brini A, Bronner A, Gerhard J-P, Nordmann J: Biologie et Chirurgie du Corps Vitre. Paris: Masson et Cie, 1968 189. Fine B: Retinal structure: Light and electronmicroscopic observations. In McPherson A (ed): New and Controversial Aspects of Retinal Detachment. New York: Harper & Row, 1968 190. Pau M: Zur Entwicklung der Glaskörperstrukturen und der Zonula. Ophthalmologica 134:320, 1957 191. Gärtner J: Klinische Beobachtungen über Glaskörperadhärenzen am hinteren
Augenpol. Klin Monatsbl Augenheilkd 140:161, 1962 192. Schepens C: Clinical aspects of pathologic changes in the vitreous body. Am J Ophthalmol 38:8, 1954 193. Gärtner J: Klinische Beobachtungen über den Zusammenhang der Glaskörpergrenzmembran
mit Glaskörpergerüst und Netzhautgefässen in
der Ora- Aequatorgegend. Klin Monatsbl Augenheilkd 140:524, 1962 194. Rieger H: Zur Histologie der Glaskörperabhebung, Teir II. Über die Beziehungen
des aabgehobenen Glaskörpers zur Netzhaut. Albrecht Von Graefes Arch Klin Exp Ophthalmol 146:447, 1944 |