PRIMARY FIBER FORMATION
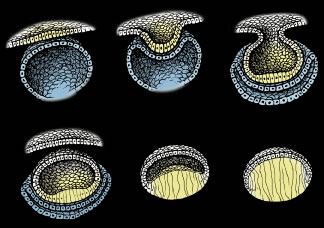
Lens formation begins in the latter part of the first trimester as surface ectodermal cells, immediately overlying the developing optic vesicle, are induced to thicken and form the lens placode (Fig. 1). As the optic vesicle begins to fold inward and form the optic cup, the lens placode invaginates toward the developing optic cup until it eventually pinches off as an inverted (inside-out) lens vesicle. The apical surfaces of the lens vesicle cells are directed toward the lumen, whereas their basal surfaces are directed toward the outer surface. Throughout life, the basal surfaces of lens cells produce a progressively thicker basement membrane, the lens capsule, that envelops the lens. Lens development proceeds as cells of the lens vesicle approximating its retinal half are induced to terminally differentiate. A striking consequence of lens terminal differentiation is that the originally cuboidal lens vesicle cells are transformed into long fiber-like cells, or fibers. Fiber elongation proceeds until they fill in the lumen of the lens vesicle. Because these fibers are the first lens cells to be transformed into fibers, they are referred to as the primary fibers.
SECONDARY FIBER FORMATION
The cells of the lens vesicle that were not induced to form primary fibers remain as a monolayer, the lens epithelium, that covers the anterior surface of the primary fiber mass. Lens development and growth continues throughout life, in a manner similar to other stratified epithelia with the lens epithelium constituting the basal layer. However, whereas typically stratified epithelia have their progenitor cells distributed throughout the basal layer, the lens is unique in that its progenitor cells are sequestered as a distinct subpopulation within the lens epithelium known as the germinative zone (GZ). The remainder of the lens epithelial cells are sequestered in three additional regions known as the central zone (CZ), pregerminative zone (PGZ), and transitional zone (TZ). CZ epithelial cells comprise a broad polar cap of the lens epithelium covering most (approximately 80%) of the anterior surface of the lens. These cells are arrested in the G0 stage of the cell cycle and are not recruited to terminally differentiate into fibers.1 PGZ cells comprise a narrow, latitudinal band (approximately 5% of the lens epithelium) peripheral to the CZ. Although a small number of these cells undergo mitotic division, only rarely are any of their daughter cells induced to terminally differentiate and become additional fibers. Rather, these daughter cells add to the lens epithelial population as the anterior surface of the lens increases in size because of continued growth and aging.2,3 GZ cells comprise a narrow, latitudinal band (approximately 10% of the lens epithelium) peripheral to the PGZ. These cells undergo mitotic division, and some daughter cells are selected to terminally differentiate into additional fibers. Because these are the second fibers to develop, they are referred to as secondary fibers. Finally, TZ cells comprise a narrow, latitudinal band (approximately 5% of the lens epithelium) peripheral to the GZ. These cells are the nascent fibers having already begun the process of elongation.
FIBER ELONGATION
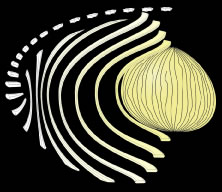
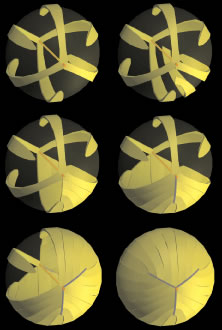
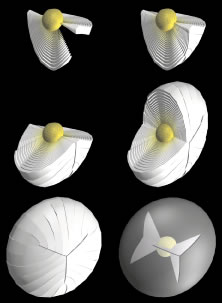
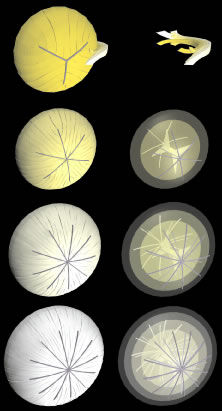
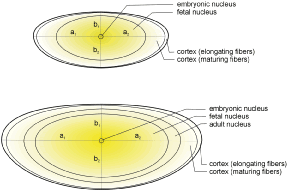
The secondary fibers comprise the layers, or strata, of the lens. An understanding of how fibers are added onto the existing lens throughout life is necessary to comprehend the complex structure of the lens that is paramount in the establishment and maintenance of its transparency (Fig. 2).
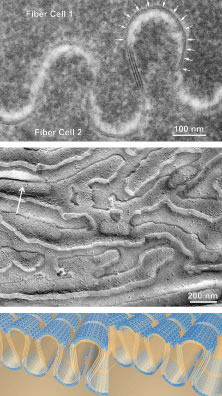
As the cells within the TZ migrate posteriorly, they rotate 90 degrees about their polar axis while elongating bidirectionally. As fiber elongation continues, the anterior ends are insinuated between the lens epithelium and the primary fiber mass while the posterior ends are insinuated between the primary fiber mass and the posterior lens capsule. Elongation is complete when the anterior and posterior ends of newly formed secondary fibers break contact with the apical surfaces of CZ epithelial cells anteriorly and the capsule posteriorly, respectively.The fact that all fibers detach from the basement membrane, the lens capsule, is direct evidence that the lens is a stratified, rather than a simple epithelium because in simple epithelia, all cells permanently retain contact with the basement membrane.
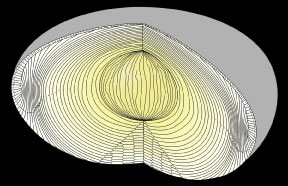
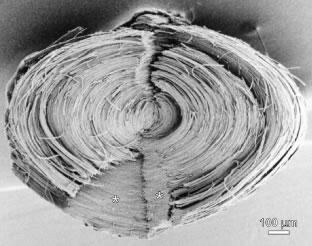
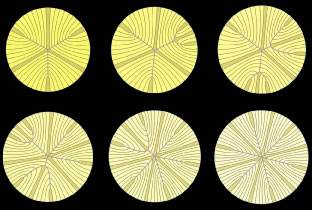
The anterior ends of fully elongated fibers abut and overlap with one another, as do the posterior ends, to form a growth shell. Because secondary fiber formation, or stratification, occurs throughout life, the net result is the establishment of successive growth shells, surrounding the original primary fiber mass. Thus, a view through a lens split along its anteroposterior or visual axis reveals concentric growth shells and/or radial cell columns (Fig. 3). The inside-out development scheme of the lens dictates that growth shells become progressively more internalized and, therefore, fibers cannot be sloughed off. In fact, all primary and secondary fibers formed are retained and must be supported for a lifetime. Failure to preserve the viability of any fiber is presumed to lead to pathology.
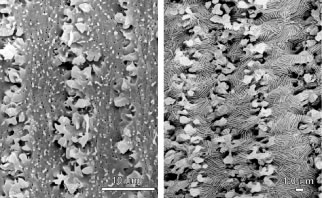
After fiber elongation is complete, terminal differentiation continues as the newly formed fibers routinely eliminate their nuclei, Golgi bodies, rough endoplasmic reticulum, and most smooth endoplasmic reticulum and mitochondria.4–11 The removal of these organelles is often described as necessary because their retention would cause a significant diffraction of light and thereby compromise lens function. However, it should be noted that other stratified epithelia (e.g., skin) also routinely eliminate these same organelles from cells in upper or older layers as a function of terminal differentiation. Fiber maturation continues with the production of specialized cytoplasmic proteins, the crystallins, as well as specialized cytoskeletal and plasma membrane components. As fibers age, the structural and biochemical nature of the previously described fiber characteristics are altered.12