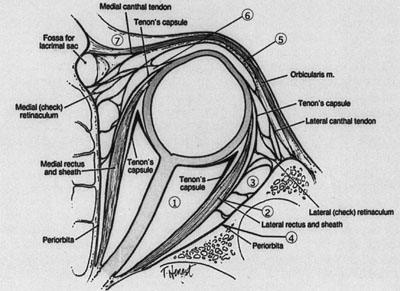
LATERAL ORBITOTOMY Lateral orbitotomy originally was popularized by Kronlein8 in 1888. The incision described by Kronlein was a reverse C-shaped incision
placed over the lateral rim and extending superiorly toward the
hairline and inferiorly toward the ear. This resulted in an unsightly
scar and a high likelihood of damage to the seventh cranial nerve. Subsequently, a
number of superior skin incisions have been devised to allow
exposure of the bony lateral orbital wall and access to the lateral
retrobulbar space.9–11 Currently, the lateral wall is most often approached through either a
canthotomy incision (modified Berke),12 or an upper eyelid crease incision extending into a lateral “laugh
line.”13 Rarely, a coronal incision in the hairline with subgaleal dissection of
a scalp flap carried down to the lateral rim is useful.14,15 Although percutaneous dissection and orbital bone removal can be performed
satisfactorily under local anesthesia, deeper orbital manipulation
requires retrobulbar injection, which would alter postoperative pupillary
evaluation and visual acuity checks. Because visual loss is a well-recognized
complication of orbital surgery,16 most lateral orbitotomies are performed under general anesthesia to allow
close postoperative monitoring of vision. EYELID CREASE LATERAL ORBITOTOMY This approach affords excellent exposure of the lateral orbital rim through
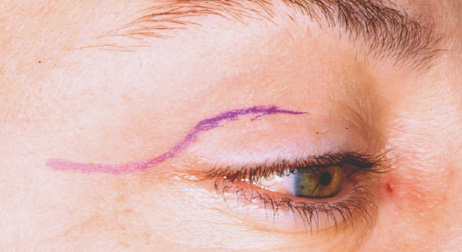
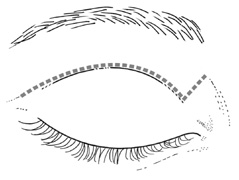
an incision placed within the upper eyelid crease, which may extended
laterally into a temporal “laugh line” (Fig. 6). Infiltration with local anesthetic with epinephrine (1:200,000) at
least 10 minutes before performing the skin incision minimizes
bleeding. |
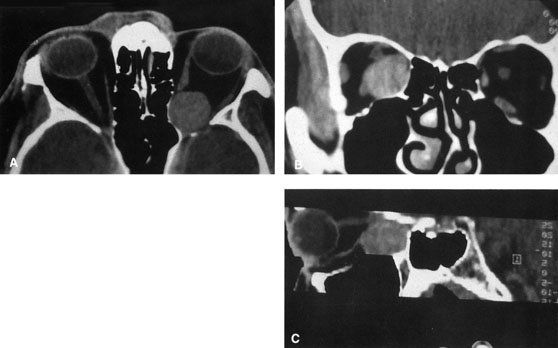
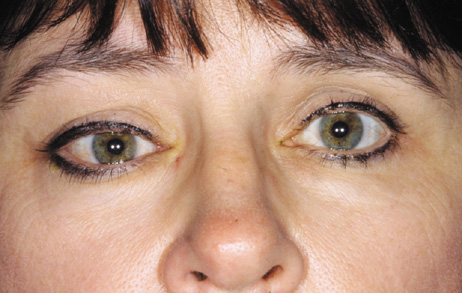
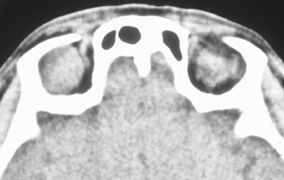
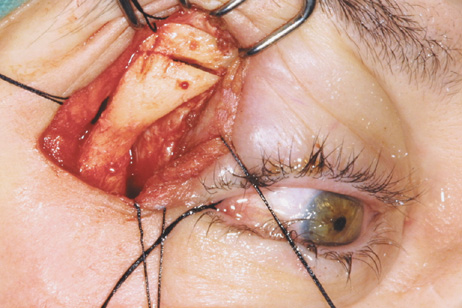
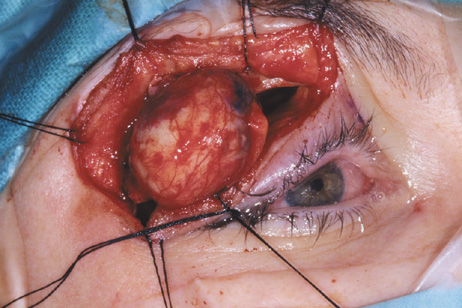
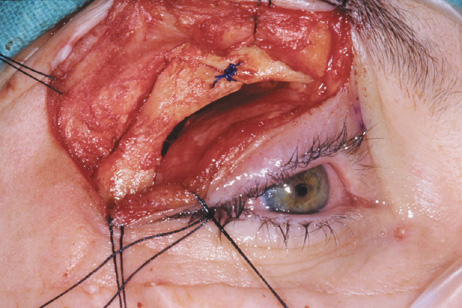
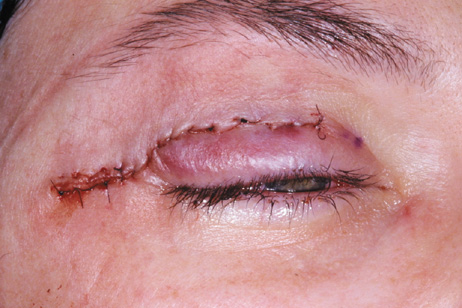
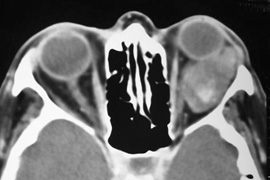
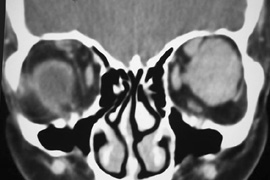
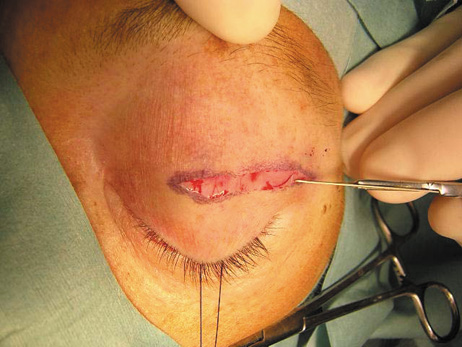
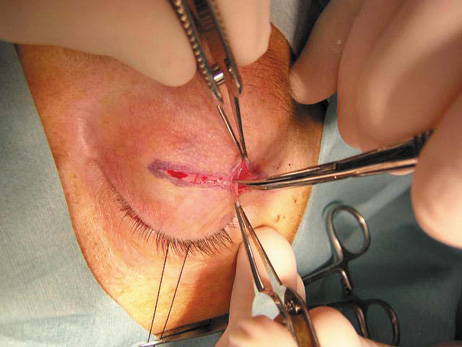
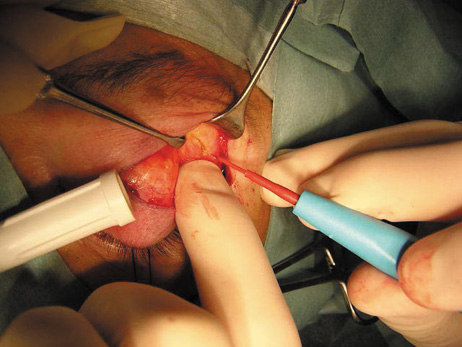
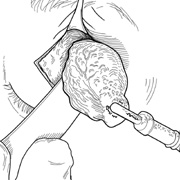
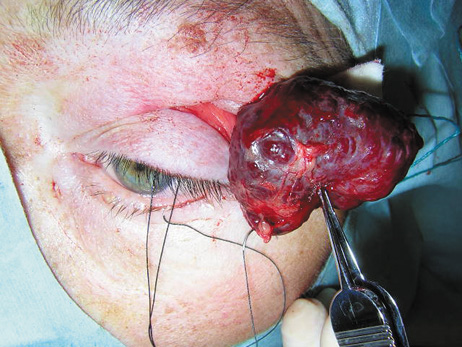
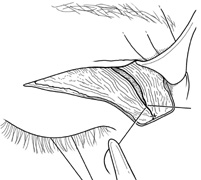
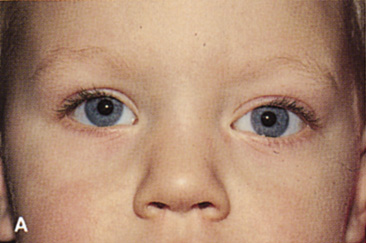
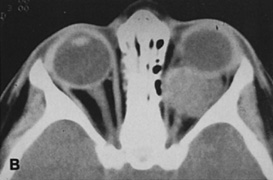
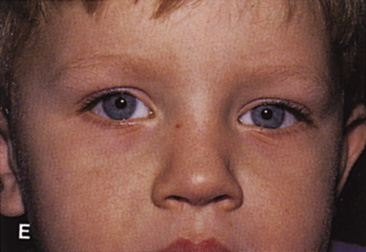
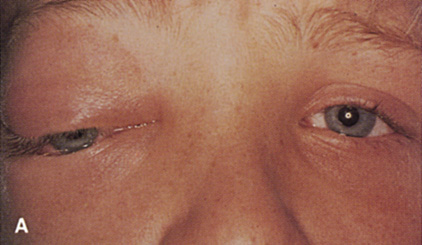
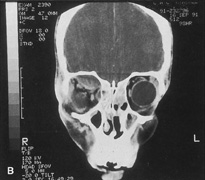
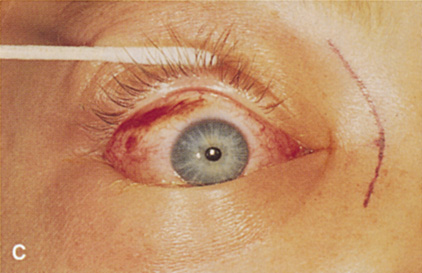
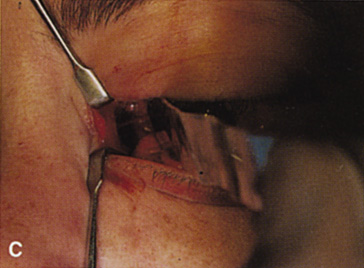
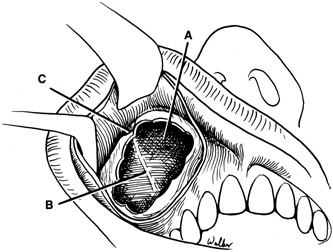
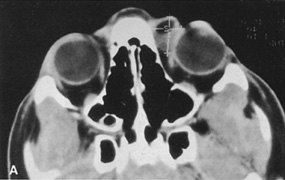
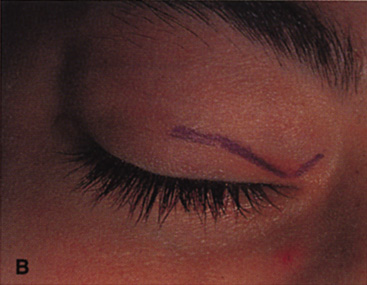
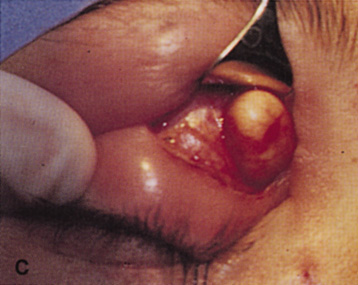
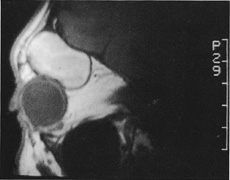
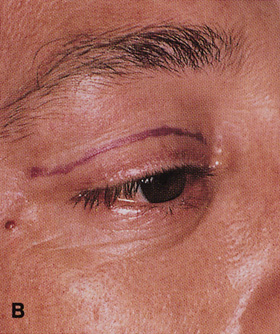
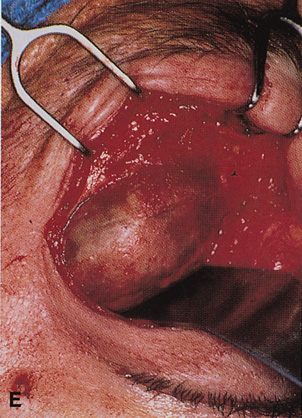
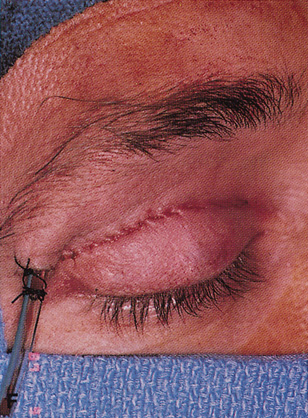
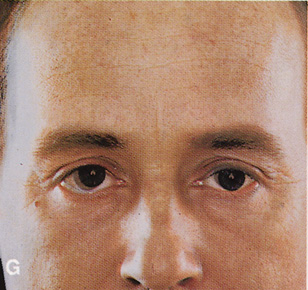
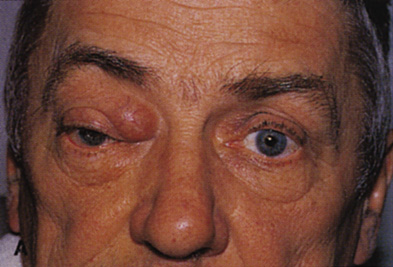
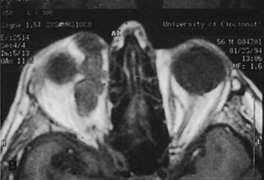
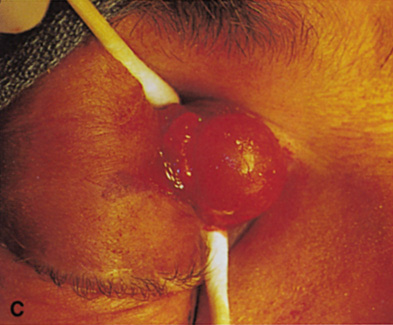
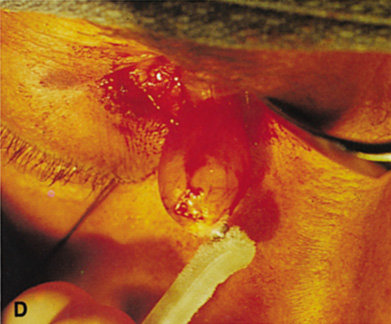
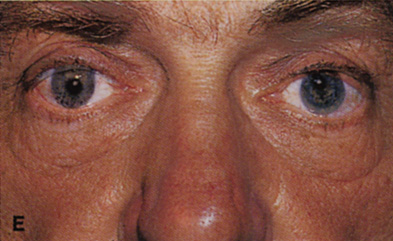
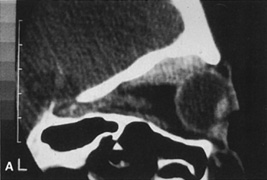
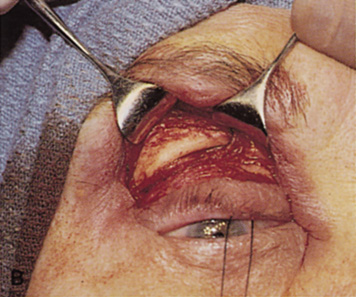
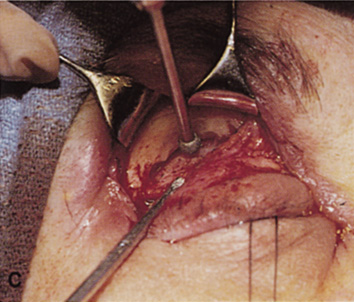
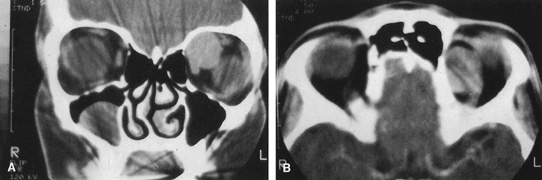
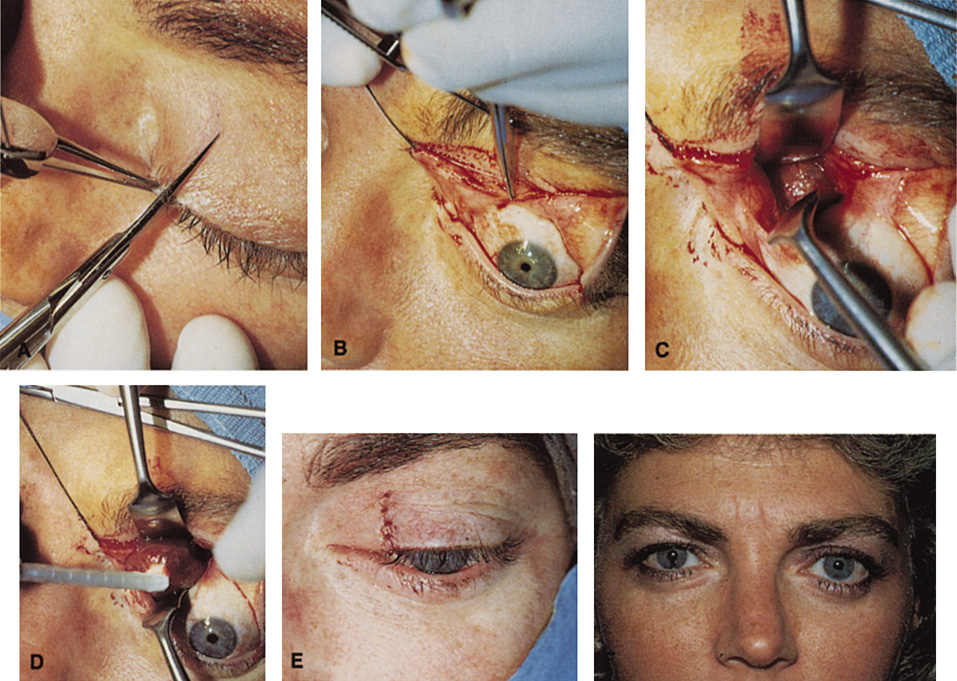
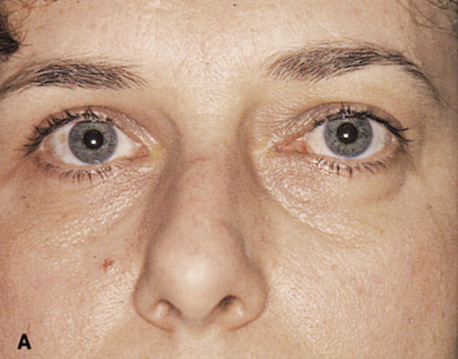
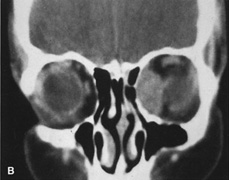
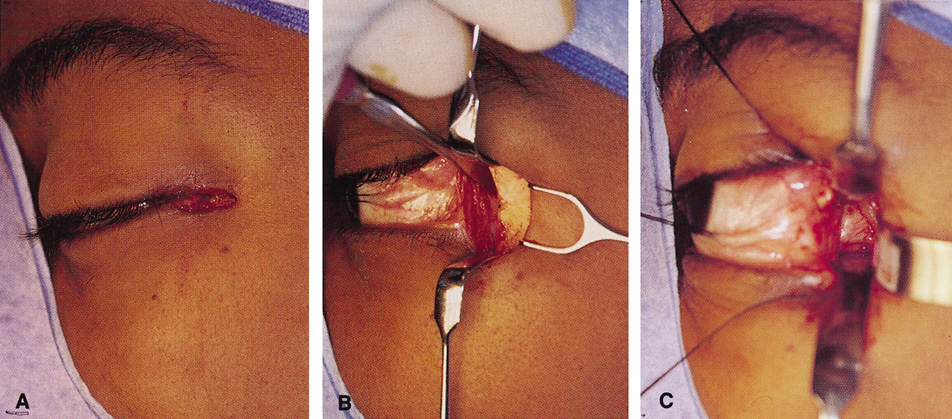
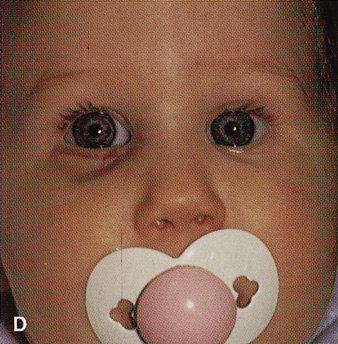
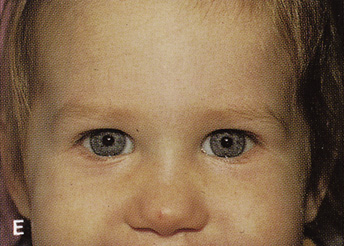
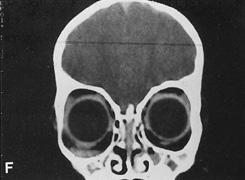
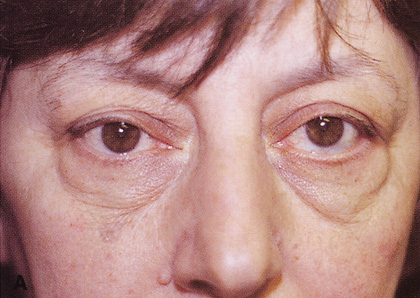
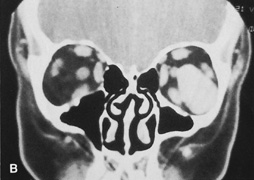
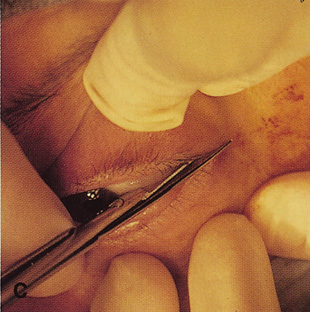
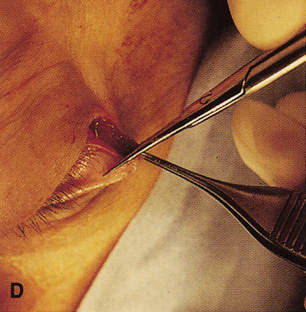
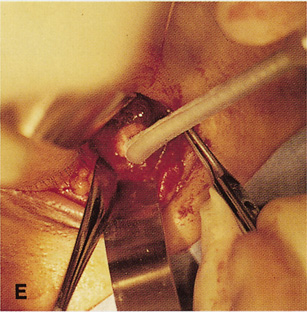
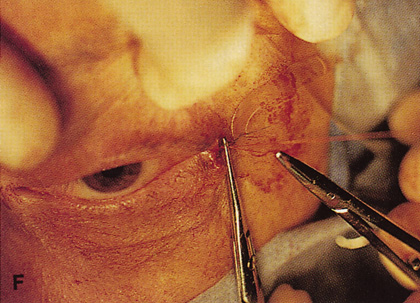
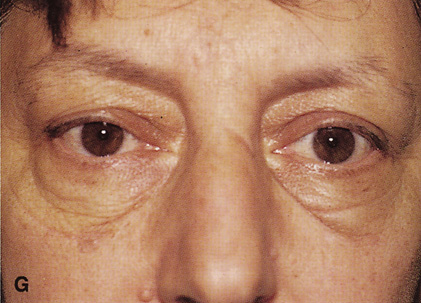
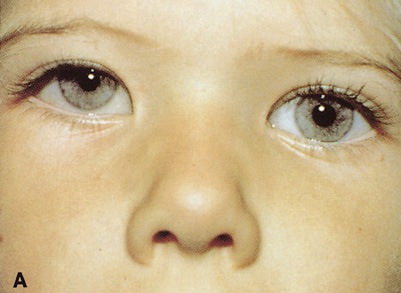
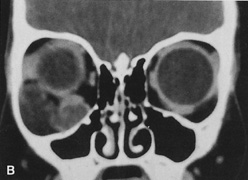
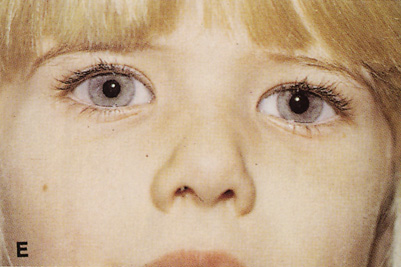
Fig. 6. Lateral orbitotomy through upper eyelid skin crease A. Photo demonstrating right globe ptosis present for more than 2 years. B. Axial CT scan showing a well outlined oval lesion in the lacrimal gland
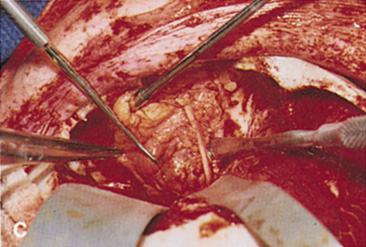
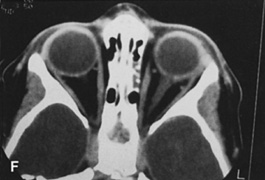
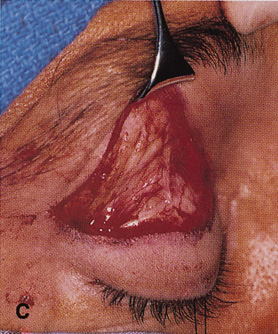
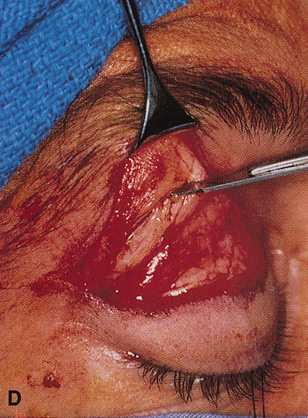
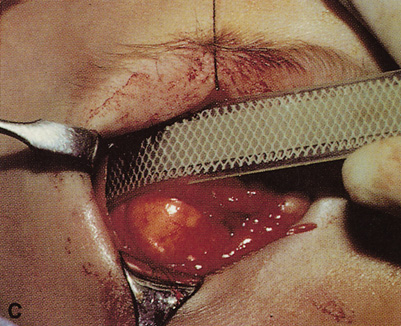
fossa. C. Coronal CT showing lesion pushing globe inferiorly. D. Skin crease excision marked for lateral orbitotomy. E. Lateral orbital rim exposed. Bone cuts made above frontozygomatic suture
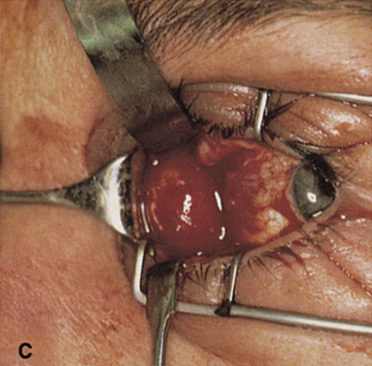
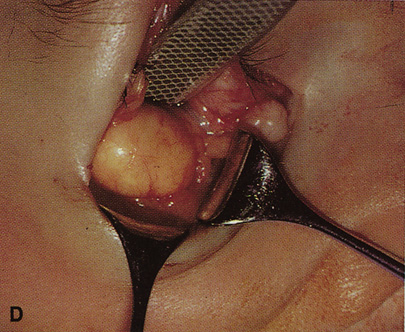
and at zygomatic arch. F. Lateral wall removed. Subperiosteal space exposed. Hard tumor could be
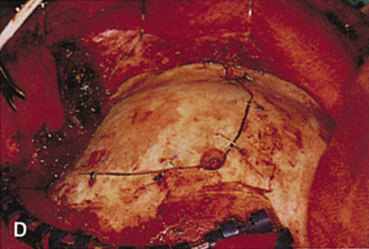
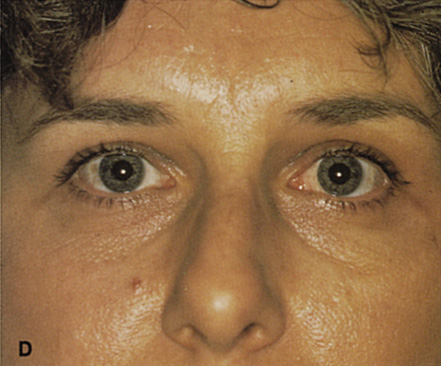
palpated in area of lacrimal gland. G. Benign mixed tumor of lacrimal gland removed. H. Bone sutured into place. I. Skin crease closed. |
The incision is made in the skin to the depth of the orbicularis muscle, and
skin and muscle dissected superficial to the orbital septum. Elevation
of a skin–muscle flap superiorly and laterally allows exposure
of the underlying superior and lateral orbital rims. Dissection
in this plane, deep to orbicularis muscle, can be carried inferiorly to
the level of the inferior orbital rim. In this fashion the superior
and lateral orbital rim can be exposed from a point just lateral to the
supraorbital nerve, superiorly, down to the junction of the lateral
wall and orbital floor, inferiorly. The periosteum then is incised with
the unipolar cautery just posterior to the arcus marginalis along the
orbital rim. A Cottle periosteal elevator can be used to elevate periosteum. If
the lateral bony rim is to be removed (see Fig. 6), then
periosteum usually is elevated first over the external surface
of the lateral rim to expose the underlying bone. The lateral rim periosteum
fuses with the superficial temporal fascia at the posterior border
of the lateral orbital rim. As periosteal elevation progresses posteriorly, the
superficial temporal fascia is encountered and cut with
unipolar cautery and the anterior temporalis muscle is elevated to expose
the temporal fossa. The temporalis muscle may be bluntly swept out
of the large temporal fossa by use of gauze wrapped around the surgeon's
index finger or a large periosteal elevator. Elevation of periosteum
and temporalis muscle is carried superiorly to a point 1 cm
above the zygomaticofrontal suture and inferiorly to the level of the
zygomatic arch, which is even with the orbital floor (see Fig. 6E). Once the outer surface of the lateral rim has been exposed, the periorbita
along the mesial surface of the lateral wall is similarly elevated
posteriorly within the orbit so that the bony lateral orbital rim can
be completely bared along its external and internal surfaces. The periorbita
is tightly adherent at the inner orbital rim (arcus marginalis), especially
over the lateral orbital tubercle. Periorbital
elevation in this area must be performed carefully to avoid buttonholing
and prolapse of orbital fat. Once the elevation has proceeded along
the inner surface posterior to the lateral orbital tubercle, the periorbita
elevates quite freely from the bone. The zygomaticotemporal and
zygomaticofacial neurovascular bundles are encountered about 1 cm posterior
to the rim (Fig. 7). Transecting the neurovascular bundles results in a small area of
postoperative hypesthesia over the lateral rim. These bundles usually
are cut with a unipolar cautery to allow periorbital elevation to continue
back to the inferior orbital fissure. The periorbita enters the
inferior orbital fissure at the junction of the lateral wall and floor
and is a landmark to establish the depth of dissection along the inner
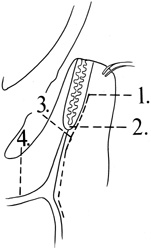
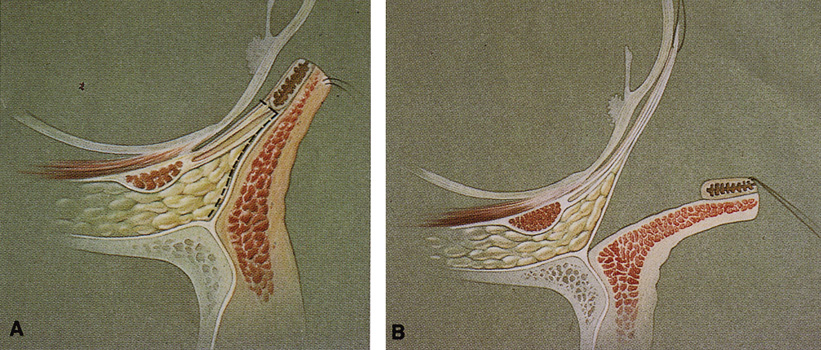
surface of the lateral rim. 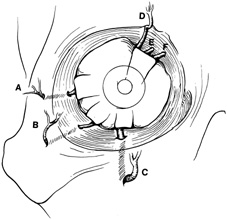 Fig. 7. Coronal schematic view demonstrating major vessels penetrating periorbita
and traversing the extraperiosteal space that may be encountered during
periorbital elevation. (A, zygomaticotemporal artery; B, zygomaticofacial artery; C, communicating branch of infraorbital artery: D, supraorbital artery: E, posterior ethmoidal artery: F, anterior ethmoidal artery.) Fig. 7. Coronal schematic view demonstrating major vessels penetrating periorbita
and traversing the extraperiosteal space that may be encountered during
periorbital elevation. (A, zygomaticotemporal artery; B, zygomaticofacial artery; C, communicating branch of infraorbital artery: D, supraorbital artery: E, posterior ethmoidal artery: F, anterior ethmoidal artery.)
|
After the periorbita has been elevated internally, and the periosteum reflected
laterally and superiorly to completely expose the lateral rim, a
large malleable retractor is placed between the orbital contents and
the mesial surface of the lateral orbital rim. This protects the orbital
contents while an air-driven saw is used to make two osteotomies. One
is placed superiorly about 0.5 cm above the zygomaticofrontal suture. Aligning
the saw blade toward the upper molars on the opposite side (an
angle of 45°) produces a wide opening while avoiding
inadvertent entry into the overlying frontal cranial fossa by the
saw blade. Finger palpation of the orbital roof beneath the brow reminds
the surgeon of the location of the frontal lobe and helps ensure that
the saw is not angled in a fashion to allow intracranial penetration. A
second osteotomy is made inferiorly through the lateral rim just
adjacent to the floor of the orbit above the zygomatic arch. The osteotomies
may be shifted superiorly or inferiorly depending on the exact
location of the orbital lesion, but an opening in the lateral wall of 2.5 to 3 cm
is desirable. After the saw cuts have been made, a wire-passing
drill bit is used to preplace drill holes above and below each
of the two osteotomies so that the bone can be refixated at the completion
of the procedure. Care is taken again to angle the superior drill
hole to avoid intracranial penetration and to protect orbital contents
with a wide malleable retractor. The lateral rim is out-fractured with
large rongeur by gently “rocking” the rim outward, and
remaining strands of temporalis muscle at the posterior edge of the bone
fragment may be cut with the unipolar cautery. The rim is removed and
placed off the field in moist gauze. Great care is taken to avoid inadvertent
contamination of the lateral rim as it will be replaced at
the end of the procedure. After out-fracture of the lateral rim, the thinner
bone behind the rim can be removed with a rongeur to provide better
access to the orbital apex. Bone removal usually is carried posteriorly
to the pterion, the thicker wedge of cancellous bone that constitutes
the body of the greater wing of the sphenoid. Hemorrhage from the
cancellous bone may be brisk and may require tamponade with bone wax
for control. The temporalis fascia is retracted posteriorly with broad
malleable retractors so that the apical periorbita can be exposed. Relaxing
incisions can be made with the unipolar cautery through the superior
temporalis muscle if it is difficult to adequately expose the
apex. The lateral rectus muscle and lacrimal gland lie just beneath the periorbita
along the lateral orbit (see Fig. 6F), and damage to these
structures is avoided by opening the periorbita with blunt-tipped
scissors. A traction suture placed transconjunctivally beneath the insertion
of the muscle can be manipulated to help identify the belly of
the lateral rectus posteriorly in the orbit. A longitudinal periorbital
incision beginning anteriorly and extending back to the orbital apex
will allow extensive retraction of the periorbita. Additional vertical
relaxing incisions through the periorbita may be made anteriorly. The
periorbita is retracted, and the lateral rectus muscle and lacrimal
gland are identified. If the lesion lies in the intraconal space, sharp
dissection to release the intermuscular septum, either above or below
the lateral rectus muscle, is usually required to gain access to the
retrobulbar space. Sewall orbital retractors provide retraction of the
lacrimal gland and lateral rectus muscle. Depending on the location
of the lesion superiorly or inferiorly within the orbit, dissection is
carried out above the superior border or below the inferior border of
the retracted lateral rectus muscle. Deep orbital dissection requires
fiberoptic illumination and loupe magnification at a minimum, and often
it is best performed with the operating microscope to aid in identification
of the vital vascular and neural structures within the orbit. After removal or biopsy of the proposed lesion, the orbital rim is reinserted
and fixated with sutures passed through the predrilled holes, or
with rigid screw and microplate fixation (see Fig. 6H). Usually
it is not necessary or desirable to close the periorbita because
this allows for decompression of any postoperative retro-orbital hemorrhage
into the temporal fossa. After the bone has been resutured in place, the
anterior cut edges of periorbita and periosteum along the lateral
rim may be loosely reapposed over it to prevent the overlying orbicularis-skin
flap from adhering to the bare bony rim. The eyelid crease
incision can be closed with a running suture (see Fig. 6I) as
one would after upper blepharoplasty. A drain may be placed in the
temporal fossa and brought through the lateral aspect of the incision
or through a separate stab wound and connected to external suction
for the first 24 hours postoperatively if there is any question about
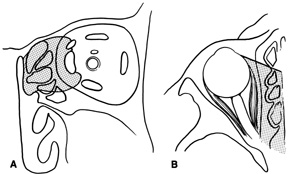
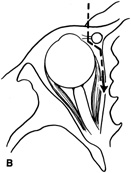
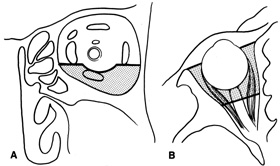
complete hemostasis. Indications Lateral orbitotomy provides excellent access to deep lesions in the subperiosteal, peripheral, or
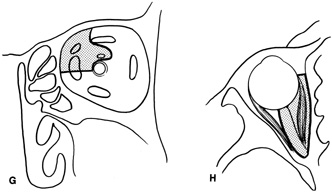
intraconal space lateral to the optic nerve (Fig. 8A, B). 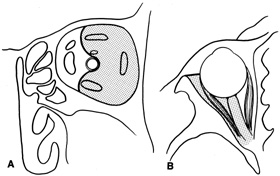 Fig. 8. Coronal (A) and axial (B) views in an illustration of areas (shaded) amenable to lateral
orbitotomy. Fig. 8. Coronal (A) and axial (B) views in an illustration of areas (shaded) amenable to lateral
orbitotomy.
|
Although intraconal lesions medial to the nerve sometimes can be approached
laterally, great care to identify and protect the optic nerve is
required during deep orbital dissection. Because the eyelid crease incision
allows such wide exposure of the superolateral orbit, it is often
possible to remove fairly large orbital lesions without removing the
lateral orbital wall (Fig. 9). Surgery in this case proceeds as described to exposure of the superior
and lateral bony orbital rims. It is not necessary to reflect
periosteum over the external surface of the rim. Instead, once periosteum
at the rim is exposed, it is cut with cautery and then only the mesial
periorbita need be elevated internally to expose orbital contents
with subsequent intra-orbital dissection carried out with the lateral
rim in place. Often it is preferable to initially attempt to remove intraconal
or lacrimal fossa lesions in this fashion. If exposure proves
inadequate, the periosteum over the external surface of the lateral
orbital rim can be elevated and osteotomies and removal of the lateral
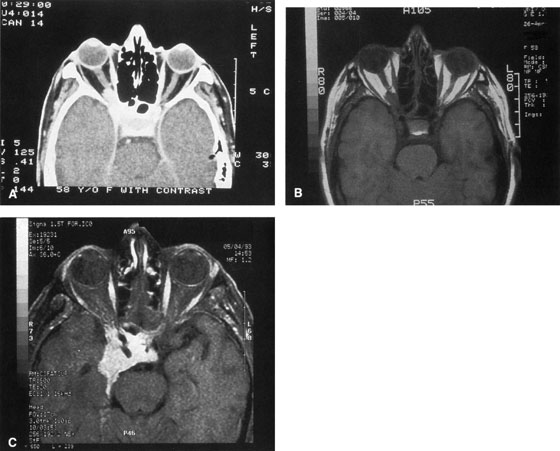
wall still can be carried out. | Fig. 9. A,B. Coronal and axial CT images of a large intraconal neoplasm. C. Because it was felt to represent a well-encapsulated cavernous hemangioma, this
lesion was a candidate for removal via an eyelid crease orbitotomy
without bone removal. The eyelid crease incision marked. D. Incision made with scalpel. E. Orbicularis muscle is tented up and incised to expose the underlying septum. F. Dissection of a skin-muscle flap deep to orbicularis exposes the orbital
septum and superior orbital bony rim. G. Cutting cautery is used to incise periosteum along the superior and lateral
rims; finger palpation of the bone helps to direct this incision. H. Periorbita is elevated along the mesial surface of the lateral orbital
rim in order to expose the deep orbital tissues. I. The cavernous hemangioma is visualized in the wound. Retraction is provided
by one or more malleable retractors. J. Cryoprobe is affixed to the hemangioma to facilitate manipulation of the
lesion. K. Large cavernous hemangioma after removal through the eyelid crease incision
which was accomplished without bone removal. L. Periorbita is reattached over the lateral rim. M. The eyelid crease incision is closed with a running suture. |
CANTHOTOMY APPROACH Many deep orbital lesions requiring removal of the lateral orbital rim
can be approached through a smaller lateral canthotomy incision. Although
Berke17 initially described a fairly extensive lateral canthal incision extending
back over the zygomatic arch for 5 to 7 cm, this longer incision often
leaves an unsightly scar and may risk damage to the seventh cranial
nerve. Because of the extensibility of the periocular tissues, exposure
of the lateral orbital rim usually can be accomplished through a
small lateral canthotomy incision measuring 1 to 1.5 cm in length (Figs. 10, 11, and 12). With wide undermining in the suborbicularis plane and retraction
of the skin and orbicularis, superior and inferior osteotomies can be
made quite readily despite the small external incision. The incision
always can be extended farther posteriorly if exposure is inadequate. 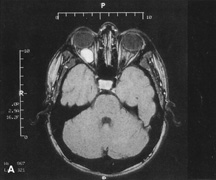 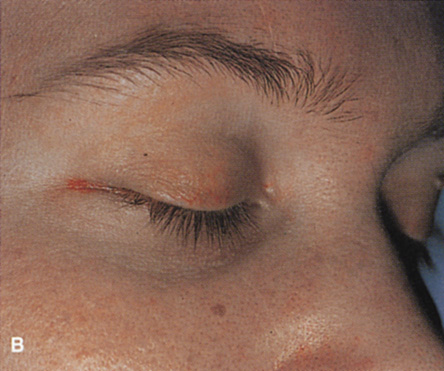 Figure 10. A. Large, well-encapsulated intraconal mass on MR scan. B. Small lateral canthotomy incision will be used to perform lateral orbitotomy
and remove the intraconal mass.
Figure 10. A. Large, well-encapsulated intraconal mass on MR scan. B. Small lateral canthotomy incision will be used to perform lateral orbitotomy
and remove the intraconal mass.
|
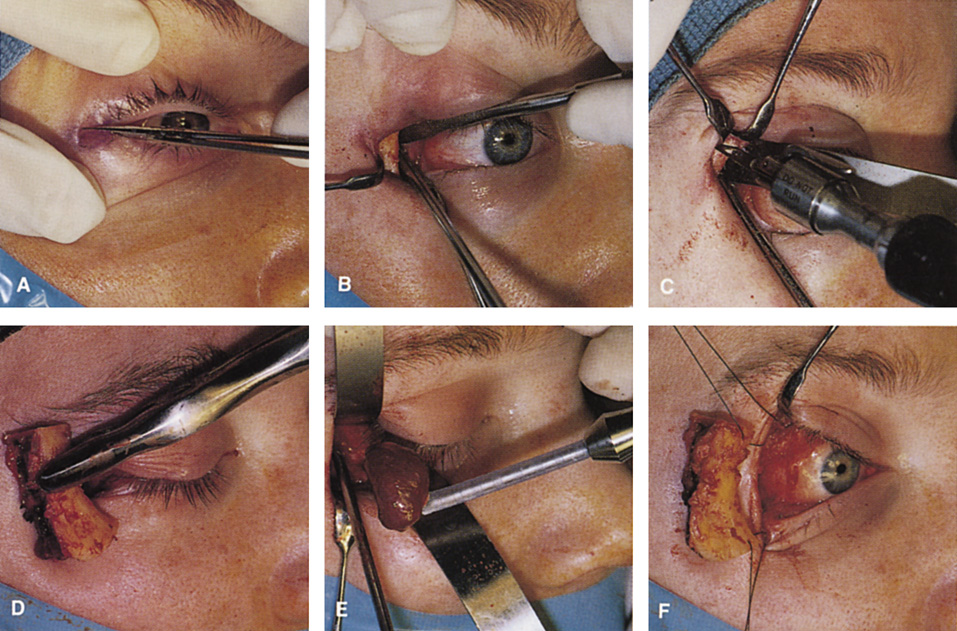 Fig. 11. A. Lateral canthotomy incision is made with straight iris scissors. B. Periosteum is elevated off of the lateral orbital rim. C. Wide undermining allows retraction of the skin incision to permit superior
and inferior osteotomies to be made with the air-driven saw. D. The bony rim has been outfractured. Because of the distensibility of the
skin, it is possible to remove a large bone flap through the small
canthotomy incision. E. The intraconal mass is extracted with the aid of the cryoprobe. F. The bone fragment is positioned for resuturing.
Fig. 11. A. Lateral canthotomy incision is made with straight iris scissors. B. Periosteum is elevated off of the lateral orbital rim. C. Wide undermining allows retraction of the skin incision to permit superior
and inferior osteotomies to be made with the air-driven saw. D. The bony rim has been outfractured. Because of the distensibility of the
skin, it is possible to remove a large bone flap through the small
canthotomy incision. E. The intraconal mass is extracted with the aid of the cryoprobe. F. The bone fragment is positioned for resuturing.
|
 Fig. 12. A. The lateral canthotomy incision is reapproximated with simple closure
of the superior and inferior crura of the lateral canthal tendon. A drain
from the temporal fossa has been brought out through a separate stab
incision posteriorly. 12B. Excellent postoperative scar camouflage is obtained by this approach.
Fig. 12. A. The lateral canthotomy incision is reapproximated with simple closure
of the superior and inferior crura of the lateral canthal tendon. A drain
from the temporal fossa has been brought out through a separate stab
incision posteriorly. 12B. Excellent postoperative scar camouflage is obtained by this approach.
|
Procedure The lateral canthotomy is made with straight iris scissors or a No. 15 blade, and
the unipolar cautery then can be used to extend the incision
deeply through orbicularis to expose periosteum along the length of
the lateral rim (see Fig. 11A). The periosteum is opened vertically
along the length of the rim, and large periosteal elevators are
used to elevate the periosteum superiorly and inferiorly to completely
expose the bony lateral rim. The inferior and superior crura of the
lateral canthal tendon generally are left attached to the periosteum
during dissection. If further exposure is needed, they can be sharply
disinserted. Once the bony lateral rim has been fully exposed, the subsequent
steps are otherwise identical to the eyelid crease approach as
described. At the completion of the procedure, the lateral canthus is reapproximated
with a single double-armed absorbable suture passed through the superior
and inferior crura of the lateral canthal tendon (see Fig. 12). It
is not necessary to refixate the canthus to the periosteum
except in older patients with pre-existing significant lower lid laxity. The
skin is then closed with one or two interrupted absorbable sutures. If
a drain is required in the temporal fossa, it can be brought
out through a separate stab incision posterior to the canthotomy or at
the posterior edge of the canthotomy incision. Indications The majority of lateral orbital lesions can be approached with this limited
skin incision. However, very large tumors, apical orbital lesions, and
lacrimal fossa lesions requiring periosteal removal and a wide field (e.g., benign mixed tumors of the lacrimal gland; see Fig. 8) are better
approached with a more extensive eyelid crease incision
Transcoronal Lateral Orbitotomy Access to the lateral orbital wall also can be achieved through an extended
incision placed at or behind the hairline in the scalp.14,15 The incision is carried full-thickness through skin and subcutaneous tissue
down to pericranium, and dissection then is carried forward deep
to the galea aponeurotica and frontalis muscle to expose the lateral
orbital rim and overlying temporalis muscle. Raney clips may be used to
tamponade bleeding vessels along the extended scalp incision. Inferior
extension of the coronal incision to the preauricular area usually
is required to sufficiently relax the coronal flap to allow exposure of
the lateral rim. The temporalis muscle must be disinserted along its
anterior and superior origin and retracted extensively so that the usual
osteotomies can be made through the lateral rim. Once the rim has
been exposed, osteotomies and bone removal are performed. Indications This approach provides access for removal of the lateral wall and access
to lateral orbital lesions. It has several disadvantages, however. It
requires a lengthy incision and extensive undermining with increased
potential for blood loss. Although it provides excellent scar camouflage
in patients with an intact hairline, the potential for male-pattern
hair recession makes it less satisfactory in male patients. There is
also the possibility of damage to the seventh cranial nerve if the dissection
plane strays too superficially over the temporalis muscle. Extensive
elevation and retraction of the temporalis muscle usually are
required, and this may be associated with cosmetically objectionable temporalis
atrophy in the postoperative period. Although the coronal approach
is ideal for lesions requiring removal of the orbital roof or extensive
bony reconstruction of the superior orbital rim, it is rarely
indicated for routine lateral orbitotomy, where upper eyelid crease or
lateral canthotomy incisions leave a virtually imperceptible scar while
avoiding these complications
TRANSCRANIAL ORBITOTOMY The superior orbit can be approached through a number of orbitotomy incisions; however, the
orbital apex, intracanalicular optic canal, and chiasmal
region may be adequately exposed only with use of a transcranial
orbitotomy. An anterior orbitotomy through the upper lid may be used
for the anterior one-half of the superior orbit. A lateral orbitotomy
with bone removal gives exposure of the lateral superior orbit, but
the nerves and vessels in the superior orbital fissure limit the extent
of the posterior exposure. A frontoethmoidal or transcaruncular incision
with removal of the ethmoid sinus gives a limited view of the orbital
apex. Only a transcranial orbitotomy provides extensive exposure
of the orbital apex, especially superior and medial to the optic nerve. Procedure The transcranial orbitotomy uses a frontal craniotomy with removal of a
portion of the orbital roof to expose the orbital apex or superior orbit. This
is best performed by a neurosurgeon familiar with skull-base
surgical approaches. In most cases, the supraorbital rim over the involved
side is removed en bloc with the frontal bone flap (Fig. 13). The anterior one-half or two-thirds of the orbital roof breaks
free with removal of the rim and frontal bone flap, and the remaining
posterior portion of the roof can be removed with rongeurs. Historically, it
was suggested that all orbital tumors be removed via craniotomy, because
before the imaging era it was difficult to anticipate the intraorbital
location of a mass.18 The transfrontal approach was first described by Jones10 in 1970. Jane and colleagues19 proposed the current technique in 1982. Refinements have been discussed
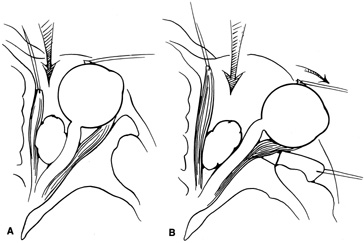
by Maroon and Kennerdell9 and Housepian.20 This operation has been termed the panoramic orbitotomy by Rootman21 because of the wide area of exposure offered by this procedure. 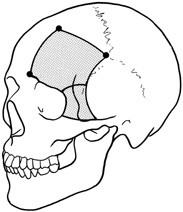 Fig. 13. Schematic diagram for transcranial orbitotomy in which the supraorbital
rim is removed en bloc with the frontal bone flap. This provides extensive
exposure to the superior and lateral orbit. Fig. 13. Schematic diagram for transcranial orbitotomy in which the supraorbital
rim is removed en bloc with the frontal bone flap. This provides extensive
exposure to the superior and lateral orbit.
|
Selected steps in the procedure are demonstrated in Figure 14. A coronal skin incision is made 2 to 3 cm behind the normal hairline. A
frontal scalp flap is raised, elevating pericranium off the frontal
bone. The supraorbital nerve is identified and, if exiting the skull
through a foramen rather than a notch, is chiseled out, mobilizing the
nerve. The subperiosteal plane is followed over the superior orbital
rim, elevating periorbita from the orbital roof and lateral orbital wall. The
temporalis muscle is dissected anteriorly out of the temporalis
fossa to expose the temporal bone. Once these soft tissues have been
separated from the bone, a high-speed drill is used to cut the bone flap.
|
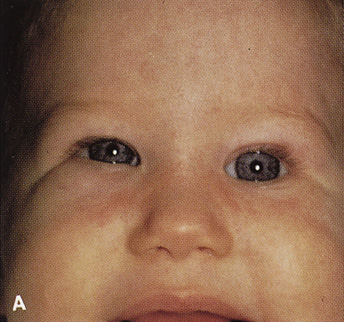
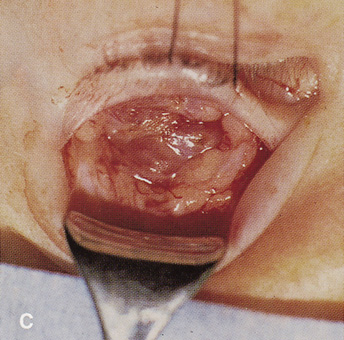
Fig. 14. A,B. Large intraorbital lymphangioma causing proptosis and optic nerve compression
in a 2-year-old child. C. View of the left orbit from above after removal of the frontal bone flap, including
the supraorbital rim and orbital roof. An extensive exposure
of the entire superior and lateral orbit is afforded. The levator
and superior rectus complex is being retracted laterally with a muscle
hook, whereas the Freer elevator retracts the superior oblique muscle
medially. The frontal nerve can be seen running from posterior to anterior
over the superior orbit. The orbital mass is exposed in this fashion. D. The fronto-orbital bone flap is wired back in place after completion of
the procedure. E. Postoperative appearance of the patient. F. The postoperative CT scan shows complete removal of the lymphangioma. This
large and diffuse lesion would have been difficult to remove with
any other approach. | A burr hole is placed in the midline just above the orbital rim. This burr
hole usually enters the frontal sinus. A second burr hole is placed
anteriorly in the temporalis fossa at the junction of the cranium and
orbit so that both compartments are exposed. Two or three additional
holes are made in the frontal bone connecting the first two holes. The
orbital rim is cut from the midline inferiorly, and the lateral orbital
rim is cut from the temporalis fossa anteriorly. The dura is freed
from the undersurface of the bone flap and is elevated superiorly, and
the orbital roof is cracked off. The frontal bone, orbital roof, and
supraorbital rim break off in one piece. The brain is retracted superiorly, and
the remaining orbital roof is removed with bone rongeurs (see
Fig. 14C). After removal of the bony roof, the periorbita is visible. Typically, the
periorbita is thin, and the levator rectus muscle and frontal nerve
are visible beneath it. If exposure of the posterior optic nerve is desired, the
dura can be elevated over the optic canal. The canal can be
unroofed to decompress or explore the optic nerve, and the dura may
be opened to view the intracranial optic nerve and the chiasm. At the
orbital apex, the annulus may be cut to allow more anterior dissection
and removal of the optic nerve in cases such as optic nerve glioma or
meningioma. Because the superior orbital fissure and its contents lie
lateral to the nerve, the intraconal space is entered on the medial side
of the optic nerve. The orbital dissection can be carried out with
a minimal amount of brain retraction after the en bloc removal of the
frontal bone, supraorbital rim, and orbital roof. After the dissection, the dura is closed and the frontal bone flap is plated
or wired back into position (see Fig. 14D). The orbital
roof is functionally restored with the replacement of the bone flap. The
sinuses must be sealed off with muscle, pericranium, or other tissue. The
coronal flap is closed in layers. The postoperative appearance
is unchanged because the bone flap is replaced in one piece. Problems
with globe ptosis, enophthalmos, pulsatile proptosis, or meningitis
are rare. However, extensive mobilization of the temporalis may result
in cosmetically significant temporal atrophy. Orbital apical dissection
often results in extraocular motility dysfunction as a result of traction
on the third, fourth, or sixth cranial nerves, but cranial nerve
function usually recovers unless the nerves have been transected. Indications Transcranial orbitotomy provides access to the superior two-thirds of all
the orbital compartments (Fig. 15). In some cases, the craniotomy is used only to provide access to
the orbit that is otherwise not possible, such as biopsy of an orbital
apex mass. Its primary use is for exploration of tumors involving the
orbital apex, or large tumors extending above and medial to the optic
nerve. The transcranial orbitotomy also is used as part of combined
procedures for approaching tumors involving the orbit as well as the anterior
and middle cranial fossae. Most commonly, this involves sphenoid
wing meningioma resection. 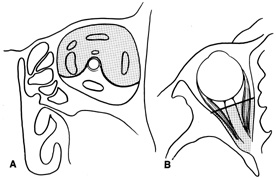 Fig. 15. Schematic of areas amenable to transcranial orbitotomy. Coronal (A) and axial (B) sections. Fig. 15. Schematic of areas amenable to transcranial orbitotomy. Coronal (A) and axial (B) sections.
|
The performance of a transcranial orbitotomy requires that the orbital
surgeon has a good working relationship with a neurosurgeon interested
in diseases of the orbitocranial junction. Often, the surgeons working
together on these procedures are part of a larger craniofacial team
that uses combined expertise to approach other complicated tumor extirpations
and reconstructions of the face and skull base. The transcranial orbitotomy offers unsurpassed exposure of the superior
orbit, orbital apex, and chiasm. Its relatively low morbidity makes this
orbitotomy the procedure of choice when safe, wide exposure of the
posterosuperior orbit is necessary. It is not indicated for orbital apex
lesions lying inferior to the optic nerve, in which an extended lateral
orbitotomy and temporal craniotomy may be required. |