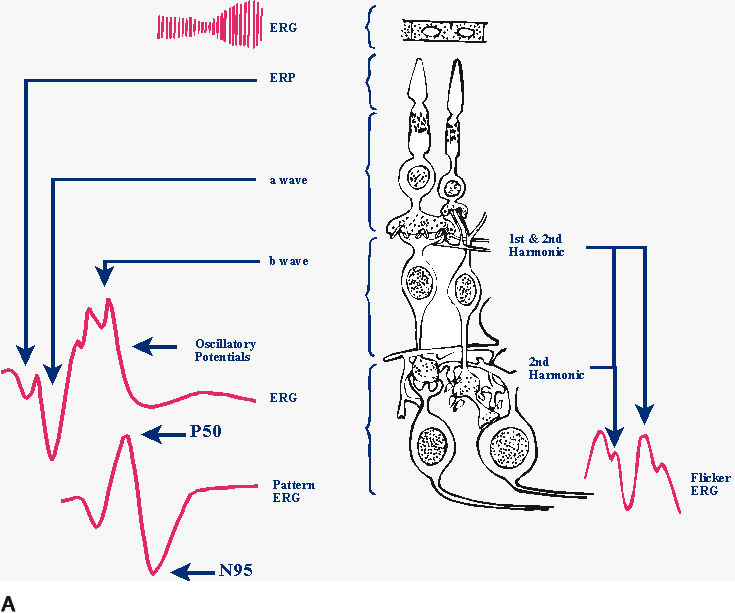
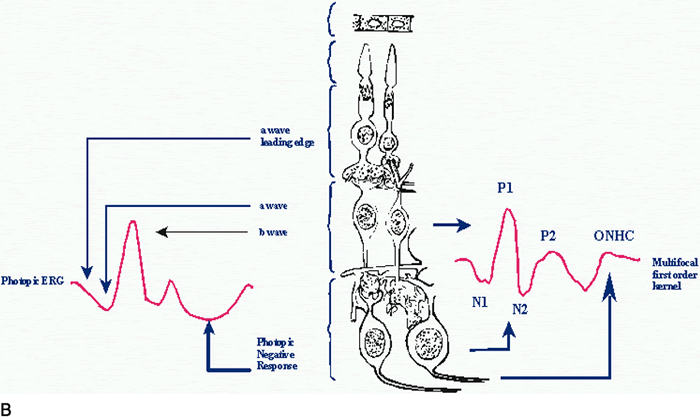
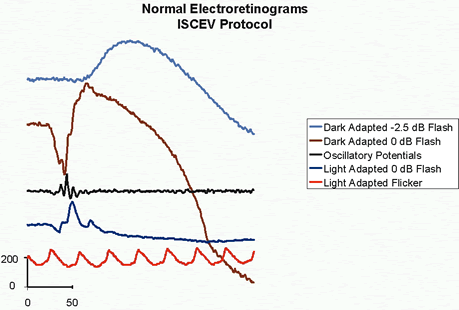
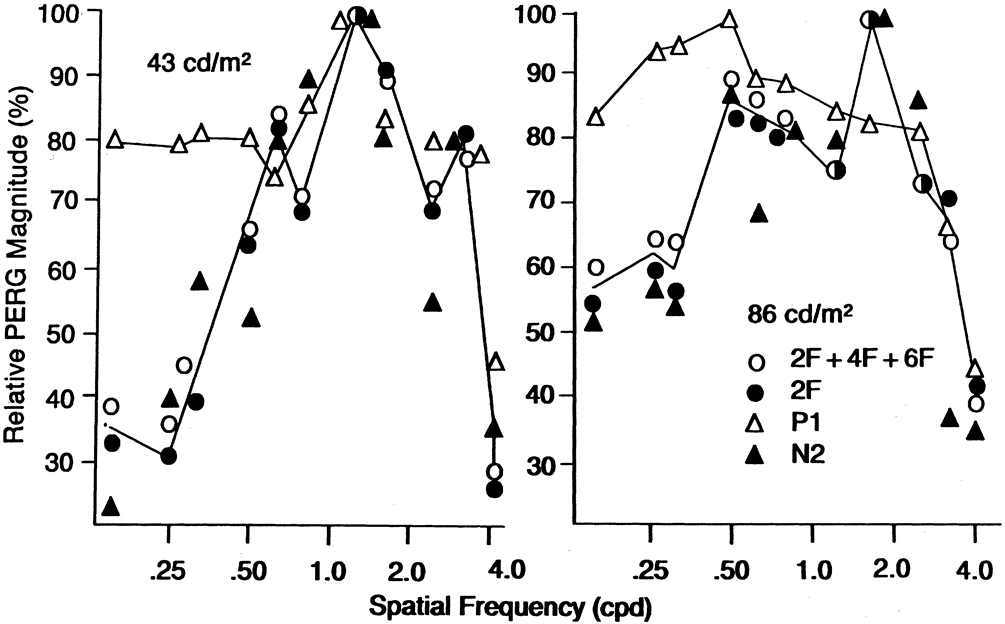
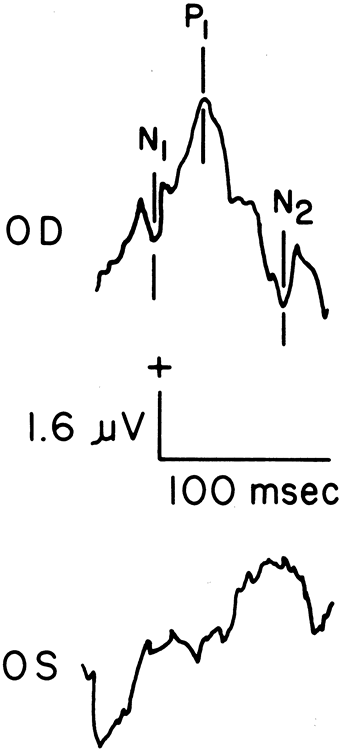
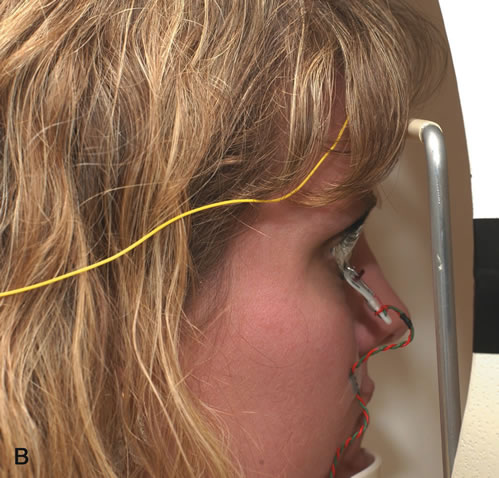
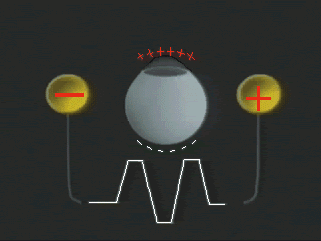
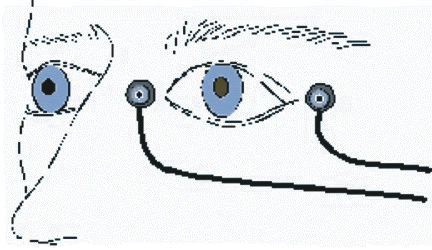
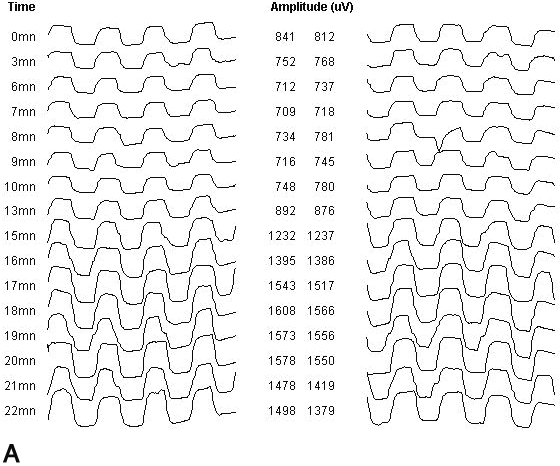
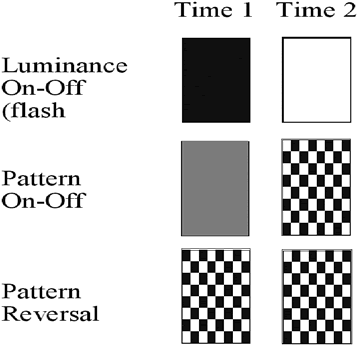
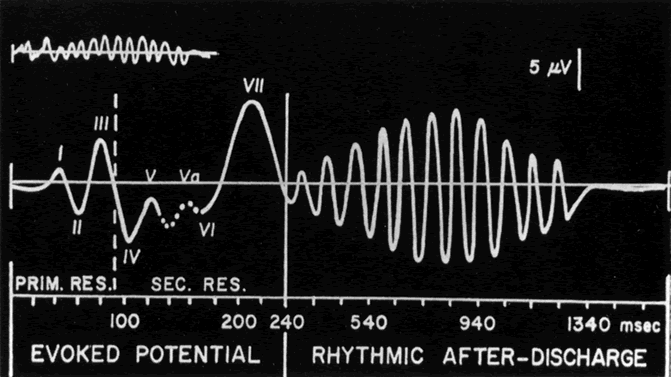
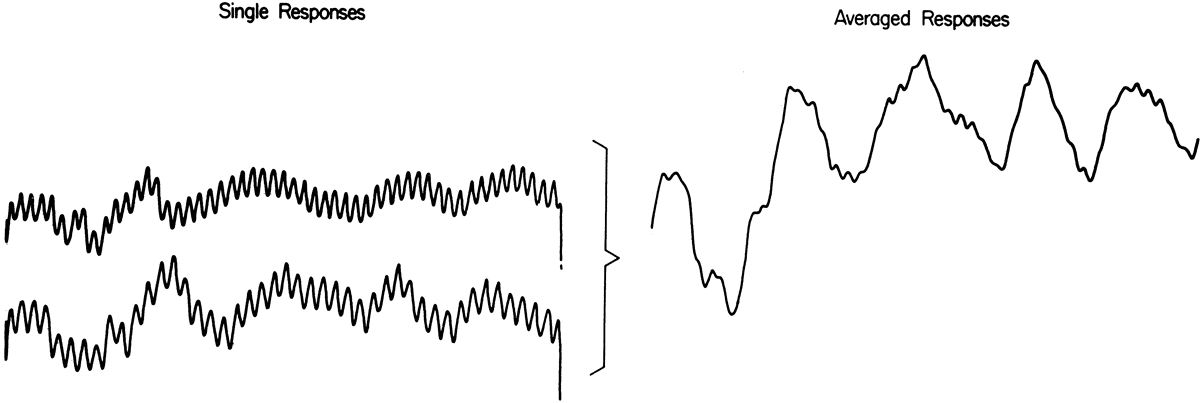
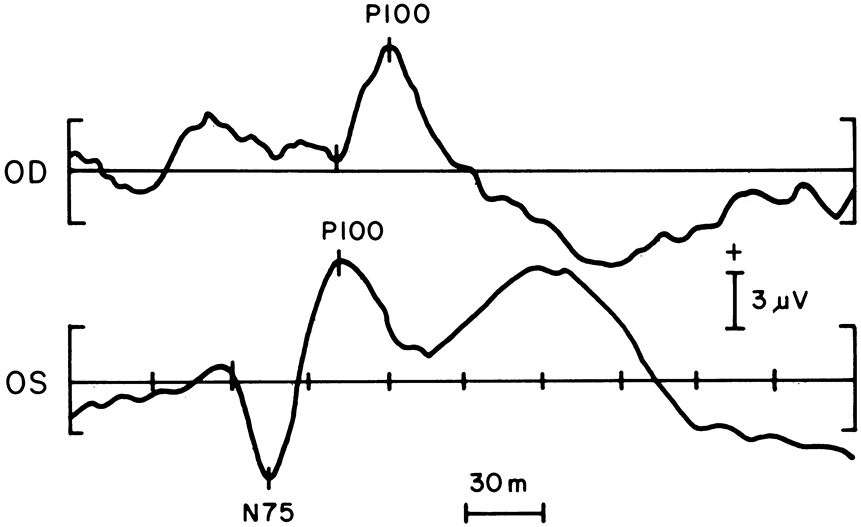
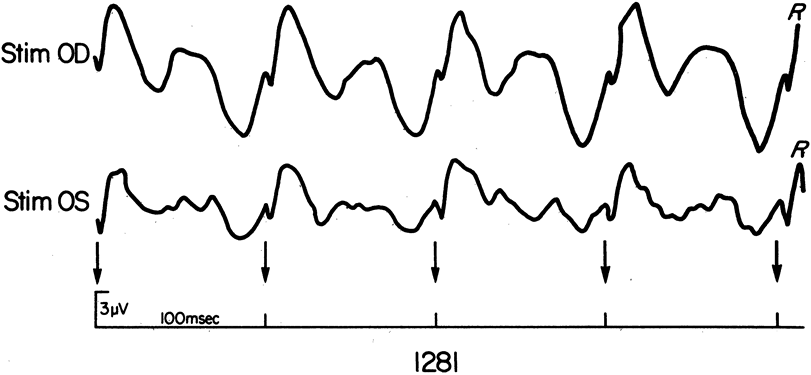
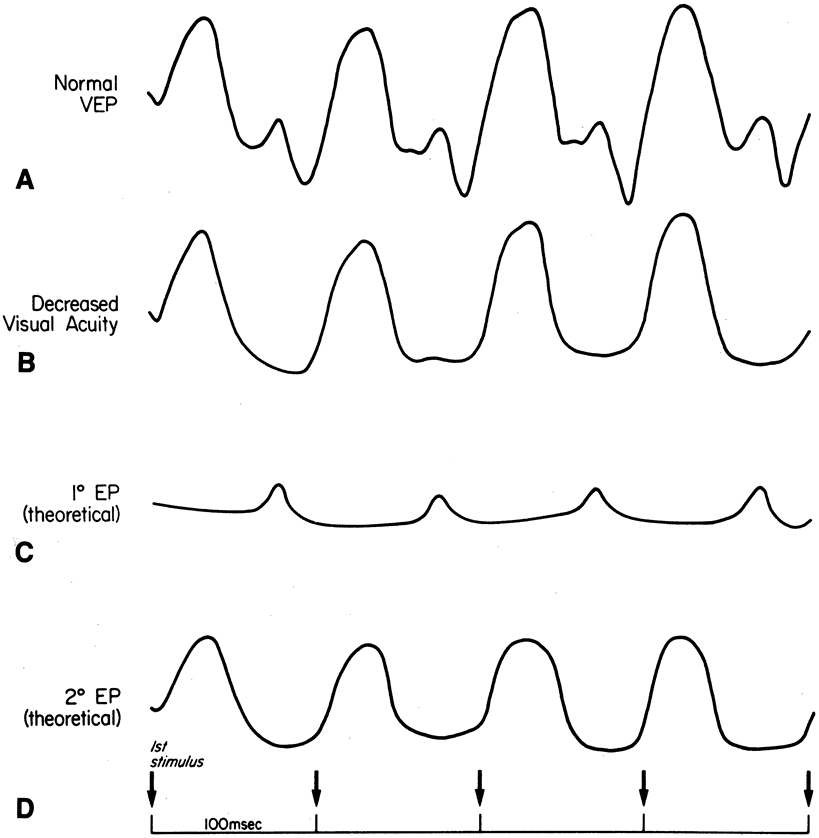
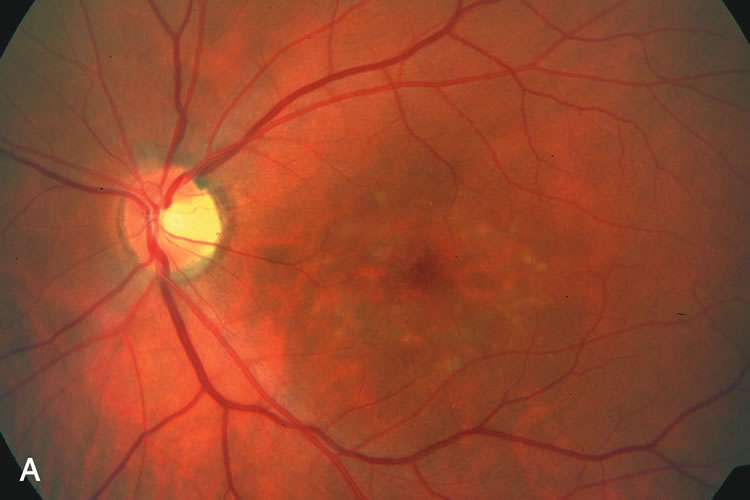
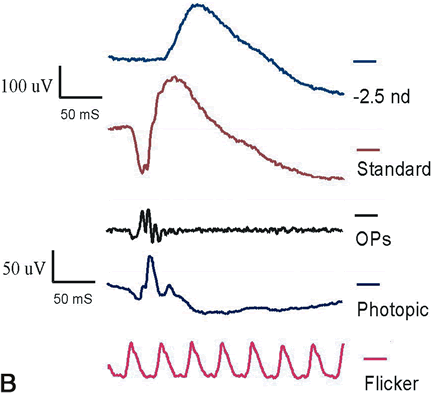
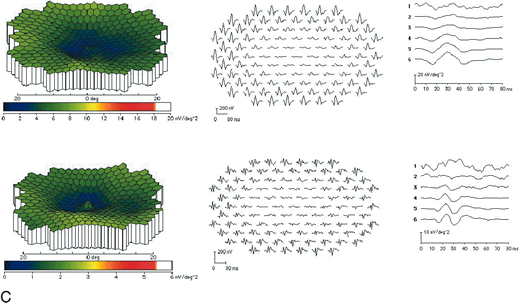
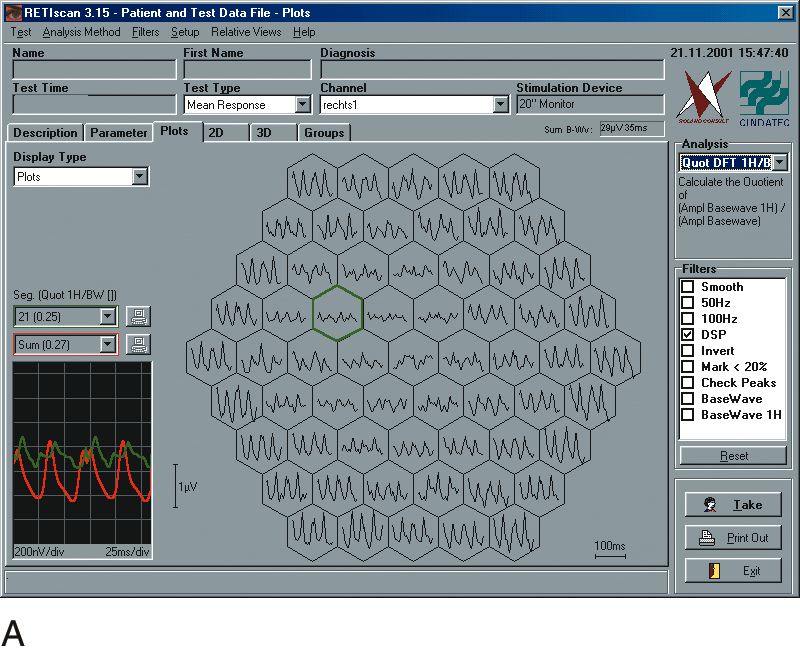
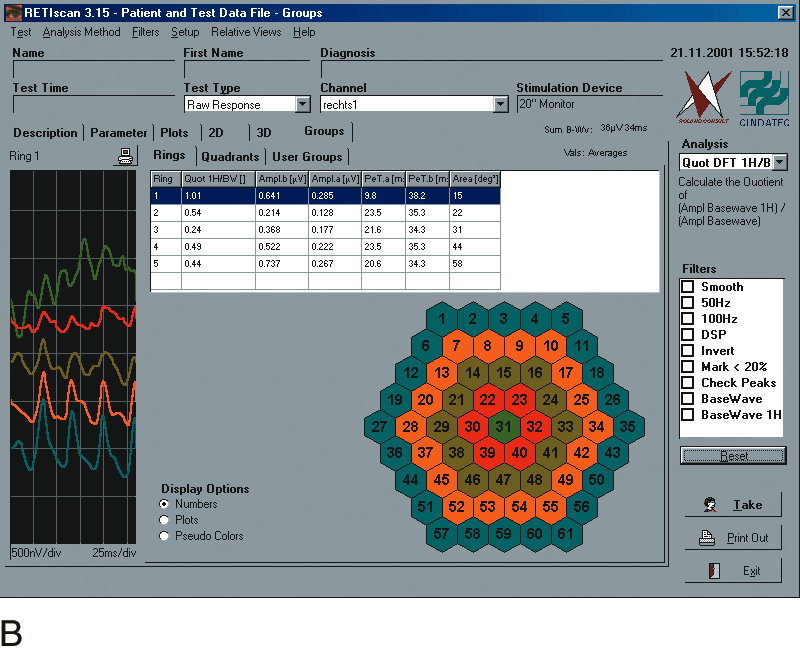
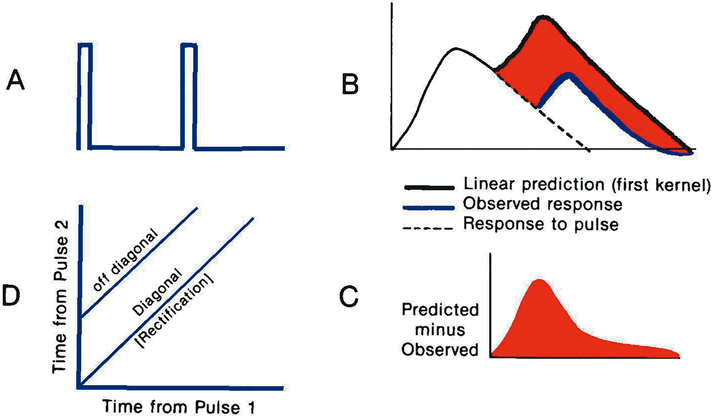
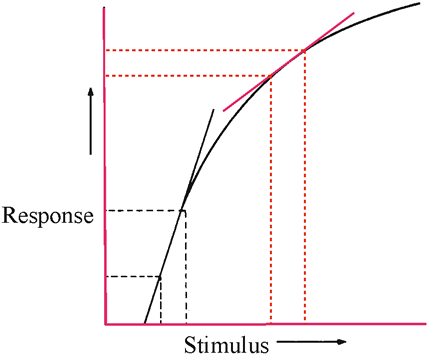
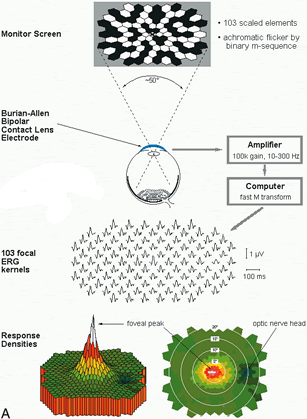
1. Carr RE, Heckenlively JR: Hereditary pigmentary degenerations of the retina. In Tasman W, Jaeger EA (eds): Clinical Ophthalmology. Philadelphia, Lippincott and Co, 1991 2. Cavender JC, Ai E: Hereditary macular dystrophies. In Tasman W, Jaeger EA (eds): Clinical Ophthalmology. Philadelphia, Lippincott and Co, 1991 3. Glaser JS, Goodwin JA: Neuro-ophthalmologic examination: The visual sensory system. In Tasman W, Jaeger EA (eds): Clinical Ophthalmology. Philadelphia, Lippincott and Co, 1991 4. Sanborn GE, Magargal LE: Arterial destructive disease of the eye. In Tasman W, Jaeger EA (eds): Clinical Ophthalmology. Philadelphia, Lippincott and Co, 1991 5. Gouras P, Charles S: Physiology of the retina. In Tasman W, Jaeger EA (eds): Clinical Ophthalmology. Philadelphia,Lippincott and Co, 1991 6. Carr RE, Siegel IM: Electrodiagnostic Testing of the Visual System: A Clinical Guide. Philadelphia, FA Davis Co, 1990 7. Fishman GA, Birch DG, Holder GE, Brigell MG: Electrodiagnostic Testing in Disorders of the Retina, Optic Nerve, and
Visual Pathway. 2nd ed. San Francisco, American Academy of Ophthalmology, 2001 8. Brigell M, Celesia GG: Electrophysiological evaluation of the neuro-ophthalmology patient: An
algorithm for clinical use. Semin Ophthalmol 7:65, 1991 9. Birch DG: Clinical electroretinography. Ophthalmol Clin North Am 2:469, 1989 10. Weinstein GW, Odom JV, Cavender S: Visually evoked potentials and electroretinography in neurologic evaluation. Neurol Clin 9:225, 1991 11. Heckenlively JR, Arden GB (eds.): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 12. Fulton AB: Intensity relations and their significance. In Heckenlively JR, Arden GB (eds): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 13. Weinstein GW, Weinberg RS, Hobson RR: Constant amplitude electroretinography for the determination of retinal
sensitivity in normal and abnormal subjects. Am J Ophthalmol 69:836, 1970 14. Bocquet X, Charlier J, Zanlonghi X, Odom JV: A new method for recording cone electroretinogram: The fast sweep ERG. Invest Ophthalmol Vis Sci 30(Suppl):512, 1989 15. Marmor MF, Arden GB, Nilsson SEG, Zrenner E: Standards for clinical electroretinography. Arch Ophthalmol l07:8l6, 1989 16. Marmor MF, Zrenner E: Standard for clinical electroretinography (1999 update). Doc Ophthalmol 97:143, 1999 17. Marmor MF, Zrenner E: Standard for clinical electro-oculography. Doc Ophthalmol 85:115, 1993 18. Marmor MF: Standardization notice: EOG standard reapproved. Doc Ophthalmol 95:91, 1998 19. Harding GFA, Odom JV, Spileers W, Spekreijse H: Standard for visual evoked potentials. Vision Res 36:3567, 1996 20. Marmor MF, Holder GE, Porciatti V, et al: Guidelines for basic pattern electroretinography. Recommendations by the
International Society for Clinical Electrophysiology of Vision. Doc Ophthalmol 91:291, 1996 21. Bach M, Hawlina M, Holder GE, et al: Standard for pattern electroretinography. Doc Ophthalmol 101:11, 2000 22. Marmor MF, Hood D, Keating D, et al: Guidelines for basic multifocal electroretinography (mfERG). Doc Ophthalmol, in press 23. Brigell M, Bach M, Barber C, et al: Guidelines for calibration of stimulus and recording parameters used in
clinical electrophysiology of vision. Doc Ophthalmol 95:1, 1998 24. Galloway N, Arden G, Odom JV (committee members): Visual Electrodiagnostics: A Guide To Procedures Commissioned by the International Society for Clinical
Electrophysiology of Vision (ISCEV), to assist practitioners and
administrators. Web document http://www.iscev.org/standards 25. De Rouck AF: History of the electroretinogram. In Heckenlively JR, Arden GB (eds): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 26. Frishman L: The scotopic threshold response. In Heckenlively JR, Arden GB (eds): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 27. Granit R: The components of the retinal action potential and their relation to the
discharge in the optic nerve. J Physiol 77:207, 1933 28. Bush RA, Sieving PA: Do photoreceptors alone contribute to the primate photopic ERG a-wave?Invest Ophthalmol Vis Sci 33:836, 1992 29. Sieving PA, Murayama K, Naarendorp F: Push-pull model of the primate photopic electroretinogram: A role
for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519, 1994 30. Sieving PA: Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91:701, 1993 31. Frishman LJ, Steinberg RH: Origin of negative potentials in the light-adapted ERG of cat retina. J Neurophysiol 63:1333, 1990 32. Viswanathan S, Frishman LJ, Robson JG, et al: The photopic negative response of the macaque electroretinogram: Reduction
by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124, 1999 33. Viswanathan S, Frishman LJ, Robson JG, Walters JW: The photopic negative response of the flash electroretinogram in primary
open angle glaucoma. Invest Ophthalmol Vis Sci 42:514, 2001 34. Colotto A, Falsini B, Salgarello T, et al: Photopic negative response of the human ERG: Losses associated with glaucomatous
damage. Invest Ophthalmol Vis Sci 41:2205, 2000 35. Drasdo N, Aldebasi YH, Chiti Z, et al: The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:1266, 2001 36. Miller RF, Dowling JE: Intracellular responses of the Müller (glial) cells of mudpuppy retina: Their
relation to b-wave of the electroretinogram. J Neurophysiol 33:323, 1970 37. Baron WS, Boynton RM, Hammon RW: Component analysis of the foveal local electroretinogram elicited with
sinusoidal flicker. Vision Res 19:479, 1979 38. Odom JV, Reits D, Burgers N, Riemslag FCC: Flicker electroretinograms: A systems analytic approach. Optom Vis Sci 69:106, 1992 39. Bush RA, Sieving PA: Inner retinal contributions to the primate photopic fast flicker electroretinogram. J Opt Soc Am A 13:557, 1996 40. Kim SH, Bush RA, Sieving PA: Increased phase lag of the fundamental harmonic component of the 30 Hz
flicker ERG in Schubert-Bornschein complete type CSNB. Vision Res 37:2471, 1997 41. Kondo M, Sieving PA: Primate photopic sine-wave flicker ERG: Vector modeling analysis
of component origins using glutamate analogs. Invest Ophthalmol Vis Sci 42:305, 2001 42. Marmor MF, Jacobson SG, Foerster MH, et al: Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and
enhanced S cone sensitivity. Am J Ophthalmol 110:124, 1990 43. Roman AJ, Jacobson SG: S cone-driven but not S cone-type electroretinograms in the
enhanced S cone syndrome. Exp Eye Res 53:685, 1991 44. Hood DC, Cideciyan AV, Roman AJ, Jacobson SG: Enhanced S cone syndrome: Evidence for an abnormally large number of S
cones. Vision Res 35:1473, 1995 45. Greenstein VC, Zaidi Q, Hood DC, et al: The enhanced S cone syndrome: An analysis of receptoral and post-receptoral
changes. Vision Res 36:3711, 1996 46. Yamamoto S, Hayashi M, Takeuchi S: Electroretinograms and visual evoked potentials elicited by spectral stimuli
in a patient with enhanced S-cone syndrome. Jpn J Ophthalmol 43:433, 1999 47. Marmor MF, Tan F, Sutter EE, Bearse MA Jr: Topography of cone electrophysiology in the enhanced S cone syndrome. Invest Ophthalmol Vis Sci 40:1866, 1999 48. Jacobson SG, Roman AJ, Roman MI, et al: Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am J Ophthalmol 111:446, 1991 49. Kellner U, Foerster MH: Pattern of dysfunction in progressive cone dystrophies—an extended
classification. Ger J Ophthalmol 2:170, 1993 50. Colotto A, Falsini B, Salgarello T, et al: Photopic negative response of the human ERG: Losses associated with glaucomatous
damage. Invest Ophthalmol Vis Sci 41:2205, 2000 51. Drasdo N, Aldebasi YH, Chiti Z, et al: The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:1266, 2001 52. Severns ML, Johnson MA: The care and fitting of Naka-Rushton functions to electroretinographic
intensity-response data. Doc Ophthalmol 85:135, 1993 53. Gangadhar DV, Wolf BM, Tanenbaum HL: Naka-Rushton equation parameters in electroretinogram analysis of daunomycin
effects on retinal function. Doc Ophthalmol 72:61, 1989 54. Evans LS, Peachey NS, Marchese AL: Comparison of three methods of estimating the parameters of the Naka-Rushton
equation. Doc Ophthalmol 84:19, 1993 55. Anastasi M, Brai M, Lauricella M, Geracitano R: Methodological aspects of the application of the Naka-Rushton equation
to clinical electroretinogram. Ophthalmic Res 25:145, 1993 56. Massof RW, Wu L, Finkelstein D, et al: Properties of electroretinographic intensity-response functions
in retinitis pigmentosa. Doc Ophthalmol 57:279, 1984 57. Wu LZ, Massof RW, Starr SJ: Electroretinographic intensity-response function in retinal disease. Chin Med J (Engl) 98:250, 1985 58. Sverak J, Peregrin J, Kralove H: Electroretinographic intensity-response curves in central retinal. Arch Ophthalmol 79:526, 1968 59. Hood DC, Birch DG: A quantitative measure of the electrical activity of human rod photoreceptors
using electroretinography. Vis Neurosci 5:379, 1990 60. Hood C, Birch DG: The A-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci 31:2070, 1990 61. Breton ME, Montzka DP: Empiric limits of rod photocurrent component underlying a-wave response
in the electroretinogram. Doc Ophthalmol 79:337, 1992 62. Breton ME, Schueller AW, Lamb TD, Pugh EN Jr: Analysis of ERG a-wave amplification and kinetics in terms of the
G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 35:295, 1994 63. Hood DC, Birch DG: Human cone receptor activity: The leading edge of the a-wave and
models of receptor activity. Vis Neurosci 10:857, 1993 64. Hood DC, Birch DG: Phototransduction in human cones measured using the a-wave of the
ERG. Vision Res 35:2801, 1995 65. Hood DC, Cideciyan AV, Halevy DA, Jacobson SG: Sites of disease action in a retinal dystrophy with supernormal and delayed
rod electroretinogram b-waves. Vision Res 36:889, 1996 66. Hood DC, Birch DG: Abnormalities of the retinal cone system in retinitis pigmentosa. Vision Res 36:1699, 1996 67. Smith NP, Lamb TD: The a-wave of the human electroretinogram recorded with a minimally
invasive technique. Vision Res 37:2943, 1997 68. Thomas MM, Lamb TD: Light adaptation and dark adaptation of human rod photoreceptors measured
from the a-wave of the electroretinogram. J Physiol 518:479, 1999 69. Paupoo AA, Mahroo OA, Friedburg C, Lamb TD: Human cone photoreceptor responses measured by the electroretinogram a-wave
during and after exposure to intense illumination. J Physiol 529:469, 2000 70. Friedburg C, Thomas MM, Lamb TD: Time course of the flash response of dark- and light-adapted
human rod photoreceptors derived from the electroretinogram. J Physiol 534:217, 2001 71. Yoshimura Y, Onoe S, Takahashi Y, et al: Electroretinogram c-wave and slow PIII of the rabbit: Changes in
peak time and amplitude under various stimulus durations. Doc Ophthalmol 69:187, 1988 72. Nagata M, Honda Y: Studies on focal electric response of the human retina. V. The effects
of varying stimulus durations upon the macular response [in Japanese]. Nippon Ganka Gakkai Zasshi 74:582, 1970 73. Miyake Y, Yagasaki K, Horiguchi M, Kawase Y: On- and off-responses in photopic electroretinogram in complete
and incomplete types of congenital stationary night blindness. Jpn J Ophthalmol. 31:81, 1987 74. Sieving PA: Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91:701, 1993 75. Sieving PA, Murayama K, Naarendorp F: Push-pull model of the primate photopic electroretinogram: A role
for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519, 1994 76. Quigley M, Roy MS, Barsoum-Homsy M, et al: On- and off-responses in the photopic electroretinogram in
complete-type congenital stationary night blindness. Doc Ophthalmol 92:159, 1996 77. Alexander KR, Fishman GA, Peachey NS, et al: ‘On’ response defect in paraneoplastic night blindness with
cutaneous malignant melanoma. Invest Ophthalmol Vis Sci 33:477, 1992 78. Alexander KR, Fishman GA, Barnes CS, Grover S: On-response deficit in the electroretinogram of the cone system
in X-linked retinoschisis. Invest Ophthalmol Vis Sci 42:453, 2001 79. Shinoda K, Ohde H, Mashima Y, et al: On- and off-responses of the photopic electroretinograms in X-linked
juvenile retinoschisis. Am J Ophthalmol 131:489, 2001 80. Odom JV, Maida TM, Dawson WW: Pattern evoked retinal responses (PERR) in human: Effects of spatial frequency, luminance
and defocus. Curr Eye Res 2:99, 1982 81. Odom JV, Norcia AM: Retinal and cortical potentials: Spatial and temporal characteristics. Doc Ophthalmol Proc Ser 40:29, 1984 82. Sutter EE, Vaegan : Lateral interaction component and local luminance nonlinearities in the
human pattern reversal ERG. Vision Res 30:659, 1990 83. Baker CL, Hess RR, Olsen BT, Zrenner E: Current source density analysis of linear and non-linear components
of the primate electroretinogram. J Physiol 407:l55, 1988 84. Sieving PA, Steinberg RH: Contribution from proximal retina to intraretinal pattern ERG: The M-wave. Invest Ophthalmol Vis Sci 26:1642, 1985 85. Firshman LJ, Sieving PA, Steinberg RH: Contributions to the electroretinogram of currents originating in proximal
retina. Vis Neurosci 1:307, 1988 86. Schuurmans RP, Berninger T: Luminance and contrast responses recorded in man and cat. Doc Ophthalmol 59:187, 1985 87. Viswanathan S, Frishman LJ, Robson JG: The uniform field and pattern ERG in macaques with experimental glaucoma; removal
of spiking activity. Invest Ophthalmol Vis Sci 41:2797, 2000 88. Holder GE: The significance of abnormal pattern electroretinography in anterior visual
pathway dysfunction. Br J Ophthalmol 71:166, 1987 89. Weinstein GW, Arden GB, Hitchings RA, et al: The pattern electroretinogram (PERG) in ocular hypertension and glaucoma. Arch Ophthalmol 106:923, 1988 90. Odom JV, Holder GE, Feghali JG, Cavender S: Pattern electroretinogram intrasession reliability: A two center comparison. Clin Vision Sci 7:263, 1992 91. Holder GE: Pattern electroretinography (PERG) and an integrated approach to visual
pathway diagnosis. Prog Retin Eye Res 20:531, 2001 92. Reeser F, Weinstein GW, Feiock KB, Oser R: Electro-oculography as a test of retinal function: The normal and
supernormal EOG. Am J Ophthalmol 70:505, 1970 93. Arden GB, Barrada A, Kelsey JH: New clinical test of retinal function based upon the standing potential
of the eye. Br J Ophthalmol 46:449, 1962 94. De Rouck A, Kayembe D: A clinical procedure for the simultaneous recording of fast and slow EOG
oscillations. Int Ophthalmol 3:179, 1981 95. Riemslag FCC, Verduyn Lunel HFE, Spekreijse H: The electrooculogram: A refinement of the method. Doc Ophthalmol 73:369, 1989 96. Steinberg RH, Linsenmeier RA, Griff ER: Three light-evoked responses of the retinal pigment epithelium. Vision Res 23:1315, 1983 97. Weleber RG: Fast and slow oscillations of the electro-oculogram in Best's
macular dystrophy and retinitis pigmentosa. Arch Ophthalmol 107:530, 1987 98. Weinstein GW, Ward B, Hobson RR: The super-normal EOG: Evidence of light induced retinal damage?Doc Ophthalmol Proc Ser 22:77, 1973 99. Regan D: Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York, Elsevier, 1989 100. Previc FH: Visual evoked potentials to luminance and chromatic contrast in rhesus
monkeys. Vision Res 26:1897, 1986 101. Schroeder CE, Tenke CE, Givre SJ, et al: Striate cortical contribution to the surface-recorded pattern-reversal
VEP in the alert monkey. Vision Res 31:1143, 1991 102. Schroeder CE, Tenke CE, Givre SJ: Subcortical contributions to the surface-recorded flash-VEP in the
awake macaque. Electroencephalogr Clin Neurophysiol 84:219, 1992 103. Schroeder CE, Mehta AD, Givre SJ: A spatiotemporal profile of visual system activation revealed by current
source density analysis in the awake macaque. Cereb Cortex 8:575, 1998 104. Ossenblok P, Reits D, Spekreijse H: Analysis of striate activity underlying the pattern onset EP of children. Vision Res 32:1829, 1992 105. Moskowitz A, Sokol S: Developmental changes in the human visual system as reflected by the latency
of the pattern reversal VEP. Electroencephalogr Clin Neurophysiol 56:1, 1983 106. Weinstein GW: Clinical aspects of the visually evoked potential. Trans Am Ophthalmol Soc 75:627, 1977 107. Weinstein GW: Clinical aspects of the visually evoked potential. Ophthalmic Surg 9:56, 1979 108. Spekreijse H, Estevez O, Reits D: Visual evoked potentials and the physiological analysis of visual processes
in man. In Desmedt JE (ed): Visual Evoked Potentials in Man: New Developments. Oxford, Clarendon Press, 1977 109. Regan D: Speedy assessment of visual acuity in amblyopia by the evoked potential
method. Ophthalmologica 175:159, 1977 110. Norcia AM, Tyler CW: Spatial frequency sweep VEP: Visual acuity during the first year of life. Vision Res 25:1399, 1985 111. Zemon V, Hartmann EE, Gordon J, Prunte-Glowazki A: An electrophysiological technique for assessment of the development of
spatial vision. Optom Vis Sci 74:708, 1997 112. Regan MP, Regan D: Objective investigation of visual function using a nondestructive zoom–FFT
technique for evoked potential analysis. Can J Neurol Sci 16:168, 1989 113. Zemon V, Pinkhasov E, Gordon J: Electrophysiological tests of neural models: Evidence for nonlinear binocular
interactions in humans. Proc Natl Acad Sci U S A 90:2975, 1993 114. Baitch LW, Levi DM: Evidence for nonlinear binocular interactions in human visual cortex. Vision Res 28:1139, 1988. 115. Stevens JL, Berman JL, Schmeisser ET, Baker RS: Dichoptic luminance beat visual evoked potentials in the assessment of
binocularity in children. J Pediatr Ophthalmol Strabismus 31:368, 1994 116. France TD, Ver Hoeve JN: VECP evidence for binocular function in infantile esotropia. J Pediatr Ophthalmol Strabismus 31:225, 1994 117. Odom JV, Chao GM: Models of binocular luminance interaction evaluated using visually evoked
potential and psychophysical measures: A tribute to M. Russell Harter. Int J Neurosci 80:255, 1995 118. Suter S, Suter PS, Perrier DT, et al: Differentiation of VEP intermodulation and second harmonic components by
dichoptic, monocular, and binocular stimulation. Vis Neurosci 13:1157, 1996 119. Baitch LW, Ridder WH 3rd, Harwerth RS, Smith EL 3rd: Binocular beat VEPs: Losses of cortical binocularity in monkeys reared
with abnormal visual experience. Invest Ophthalmol Vis Sci 32:3096, 1991 120. Odom JV, Brown RJ, Boothe RG: Maturation of binocular luminance interaction in normal young and adult
rhesus monkeys. Doc Ophthalmol 95:257, 1998 121. Porciatti V, Sartucci F: Normative data for onset VEPs to red-green and blue-yellow
chromatic contrast. Clin Neurophysiol 110:772, 1999 122. Porciatti V, Di Bartolo E, Nardi N, Fiorentini A: Responses to chromatic and luminance contrast in glaucoma: a psychophysical
and electrophysiological study. Vision Res 37:1975, 1997 123. Sartucci F, Murri L, Orsini C, Porciatti V: Equiluminant red-green and blue-yellow VEPs in multiple sclerosis. J Clin Neurophysiol 18:583, 2001 124. Spekreijse H, Dagnelie G, Maier J, Regan D: Flicker and movement constituents of the pattern reversal response. Vision Res 25:1297, 1985 125. Kubova Z, Kuba M, Hubacek J, Vit F: Properties of visual evoked potentials to onset of movement on a television
screen. Doc Ophthalmol 75:67, 1990 126. Kuba M, Kubova Z: Visual evoked potentials specific for motion onset. Doc Ophthalmol 80:83, 1992 127. Kubova Z, Kuba M: Clinical application of motion-onset visual evoked potentials. Doc Ophthalmol 81:209, 1992 128. Bach M, Ullrich D: Motion adaptation governs the shape of motion-evoked cortical potentials. Vision Res 34:1541, 1994 129. Odom JV, De Smedt E, Van Malderen L, Spileers W: Visually evoked potentials evoked by moving unidimensional noise stimuli: Effects
of contrast, spatial frequency, active electrode location, reference
electrode location, and stimulus type. Doc Ophthalmol 95:315, 1998 130. Zemon V, Gordon J, Welch J: Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked
cortical potentials. Vis Neurosci 1:145, 1988 131. Harter MR: Visually evoked cortical responses to the on- and off-set
of patterned light in humans. Vision Res 11:685, 1971 132. Regan D, Milner BA: Objective perimetry by evoked potential recording: Limitations. Electroencephalogr Clin Neurophysiol 44:393, 1978 133. Sutter RE: A practical nonstochastic approach to non-linear time-domain
analysis. In Marmarelis VZ (ed): Advanced Methods of Physiological System Modeling. Vol 1. Los Angeles, Biomedical Simulations Resource, 1987 134. Sutter EE: Field topography of the visual evoked response. Invest Ophthalmol Vis Sci 29(suppl):432, 1988 135. Sutter EE, Dodsworth-Feldman B, Haegerstrom-Portnoy G: Simultaneous multifocal ERGs in diseased retinas. Invest Ophthalmol Vis Sci 27(suppl):300, 1986 136. Odom JV: Kernel Analysis. In Heckenlively JR, Arden GB (eds): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 137. Hood DC: Assessing retinal function with the multifocal technique [Review]. Prog Retin Eye Res 19:607, 2000 138. Keating D, Parks S, Evans A: Technical aspects of multifocal ERG recording. Doc Ophthalmol 100:77, 2000 139. Hood DC, Wladis EJ, Shady S, et al: Multifocal rod electroretinograms. Invest Ophthalmol Vis Sci 39:1152, 1998 140. Kondo M, Miyake Y: Assessment of local cone on- and off-pathway function using
multifocal ERG technique. Doc Ophthalmol 108:139, 2000 141. Ogden TE, Larkin RM, Fender DF, et al: The use of non-linear analysis of the primate ERG to detect retinal
dysfunction. Exp Eye Res 31:381, 1980 142. Hood DC, Bearse MA Jr, Sutter EE, et al: The optic nerve head component of the monkey's (Macaca mulatta) multifocal
electroretinogram (mERG). Vision Res 41:2029, 2001 143. Hood DC, Bearse MA Jr, Sutter EE, et al: The optic nerve head component of the monkey's (Macaca mulatta) multifocal
electroretinogram (mERG). Vision Res 41:2029, 2001 144. Hood DC, Frishman LJ, Viswanathan S, et al: Evidence for a ganglion cell contribution to the primate electroretinogram (ERG): Effects
of TTX on the multifocal ERG in macaque. Vis Neurosci 16:411, 1999 145. Hood DC, Greenstein V, Frishman L, et al: Identifying inner retinal contributions to the human multifocal ERG. Vision Res 39:2285, 1999 146. Fortune B, Cull G, Wang L, et al: Factors affecting the use of multifocal electroretinography to monitor
function in primate model of glaucoma. Doc Ophthalmol 105:151, 2002 147. Hare WA, Ton H: Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal
and conventional methods in monkey. Doc Ophthalmol 105:189, 2002 148. Viswanathan S, Frishman LJ, Robson JG: Inner-retinal contributions to the photopic sinusoidal flicker electroretinogram
of macaques. Macaque photopic sinusoidal flicker ERG. Doc Ophthalmol 105:223, 2002 149. Priem HA, De Rouck AF, De Laey JJ, Bird AC: Electroretinographic studies in birdshot chorioretinopathy. Am J Ophthalmol 106:430, 1988 150. Leys M, Candaele CM, De Rouck AF, Odom JV: The detection of hidden visual loss in multiple sclerosis: A comparison
of pattern reversal VEPs and contrast sensitivity. Doc Ophthalmol 77:255, 1991 151. Sieving PA, Fishman GA, Jampol LM, Pugh D: Multiple evanescent white dot syndrome. II. Electrophysiology of the photoreceptors
during retinal pigment epithelial disease. Arch Ophthalmol 102:675, 1984 152. Leys A, Leys M, Jonckheere P, De Laey JJ: Multiple evanescent white dot syndrome (MEWDS). Bull Soc Belge Ophthalmol 236:97, 1990 153. Arden GB, Vaegan , Hogg CR: Clinical and experimental evidence that the pattern electroretinogram (PERG) is
generated in more proximal retinal layers than the focal electroretinogram. Ann NY Acad Sci 388:580, 1982 154. Schmeisser ET, Smith TJ: High frequency flicker visual evoked potential losses in glaucoma. Ophthalmology 96:620, 1989 155. Towle VL, Moskowitz A, Sokol S, Schwartz B: The visual evoked potential in glaucoma and ocular hypertension. Effects
of check size, field size and stimulation rate. Invest Ophthalmol Vis Sci 24:175, 1983 156. Trick GL: Pattern reversal retinal potentials in ocular hypertensives at high and
low risk of developing glaucoma. Doc Ophthalmol 65:79, 1987 157. Odom JV, Feghali JG, Jin JC, Weinstein GW: Visual function deficits in glaucoma: Electroretinogram pattern and luminance
nonlinearities. Arch Ophthalmol 108: 222, 1990 158. Millecchia LL, Nork TM, Lemley HL, Schochet TL: Regional damage to photoreceptors in human eyes with chronic glaucoma. Invest Ophthalmol Vis Sci 33:1093, 1992 159. Nork TM: Acquired color vision loss and a possible mechanism of ganglion cell death
in glaucoma. Trans Am Ophthalmol Soc 98:331, 2000 160. Holopigian K, Seiple W, Mayron C, et al: Electrophysiological and psychophysical flicker sensitivity in patients
with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 31:1863, 1990 161. Velten IM, Korth M, Horn FK: The a-wave of the dark-adapted electroretinogram: Are photoreceptors
affected. Br J Ophthalmol 85:397, 2001 162. Rosenshein JS, Cyrlin MN: Glaucoma multi-testing. Invest Ophthalmol Vis Sci 32:811, 1991 163. Bray LC, Mitchell KW, Howe JW, Gashau A: Visual function in glaucoma: a comparative evaluation of computerized static
perimetry and the pattern visual evoked potential. Clin Vis Sci 7:21, 1992 164. Klistorner AI, Graham SS, Martins A: Multifocal pattern electroretinogram does not demonstrate localized field
defects in glaucoma. Doc Ophthalmol 100:155, 2000 165. Hood DC, Zhang X: Multifocal ERG and VEP responses and visual fields: Comparing disease-related
changes. Doc Ophthalmol 100:115, 2000 166. Bodis-Wollner I, Atkin A, Raab E, Wolkstein M: Visual association cortex and vision in man: Pattern-evoked occipital
potentials in a blind boy. Science 198:629, 1977 167. Sutter EE, Tran D: The field topography of ERG components in man. I. The photopic luminance
response. Vision Res 32:433, 1992 168. Odom JV: Amblyopia and clinical electrophysiology. In Heckenlively JR, Arden GB (eds): Principles and Practice of Clinical Electrophysiology of Vision. St. Louis, Mosby–Year Book, 1991 169. Gottlob I, Fendick MG, Guo S, et al: Visual acuity measurements by swept spatial frequency visual-evoked-cortical
potentials (VECPs): Clinical application in children
with various visual disorders. J Pediatr Ophthalmol Strabismus 27:40, 1990 170. Charlier J, Bocquet X, Zanlonghi X, et al: Analyse en temps réel des potentiels évoqués stationnaires: principes
et methode. Revue Oto Neuro Ophthalmol 8:18, 1990 171. Weinstein GW, Odom JV, Hobson RR: Visual acuity and cataract. In Reinecke RD (ed): Ophthalmology Annual 1987. Norwalk, CT, Appleton-Century-Crofts, 1986 172. Odom JV, Chao GM, Weinstein GW: Preoperative prediction of postoperative visual acuity in patients with
cataracts: A quantitative review. Doc Ophthalmol 70:5, 1988 173. Odom JV, Chao GM, Hobson R, Weinstein GW: Prediction of post cataract extraction visual acuity: 10 Hz visually evoked
potentials. Ophthalmic Surg 19:212, 1988 174. Hutton WL, Fuller DG: Factors influencing final visual results in severely injured eyes. Am J Ophthalmol 97:715, 1984 175. Vadrevu VL, Cavender S, Odom JV: Predicting final visual acuity in diabetic eyes with vitreous hemorrhage: 10 Hz
flicker VEPs. Doc Ophthalmol 79:371, 1992 |