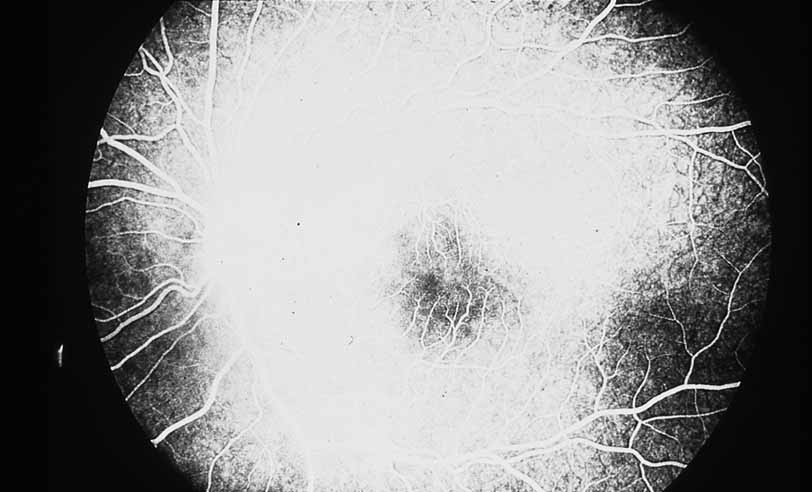
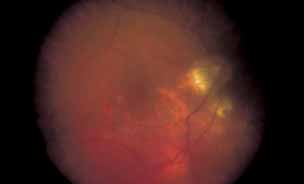
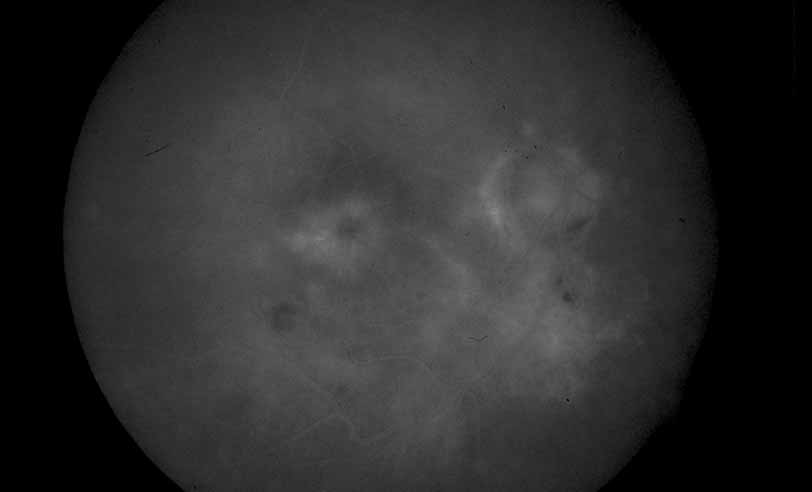
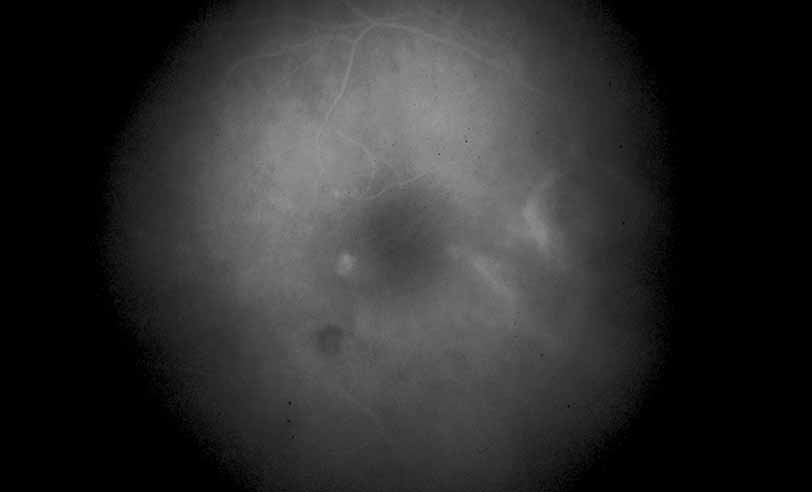
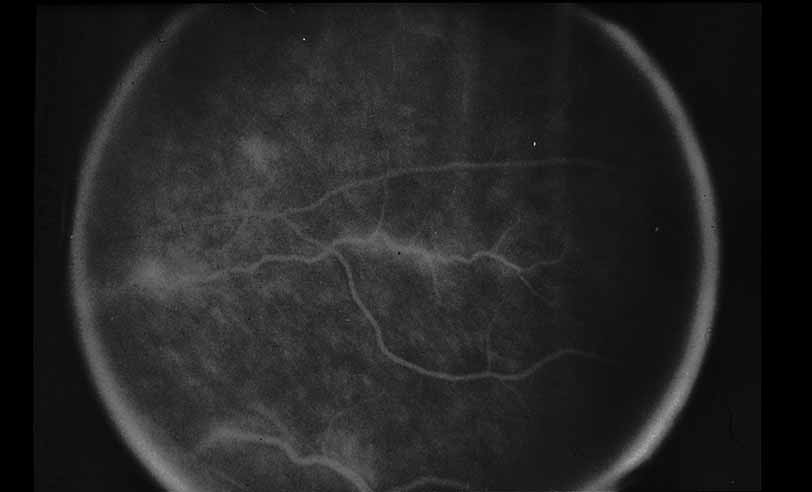
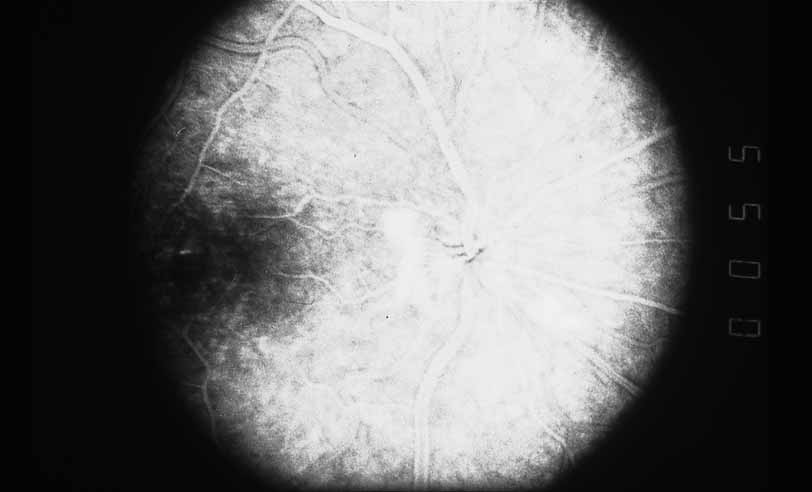
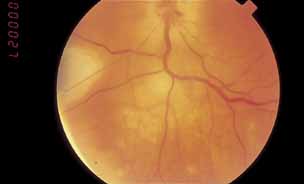
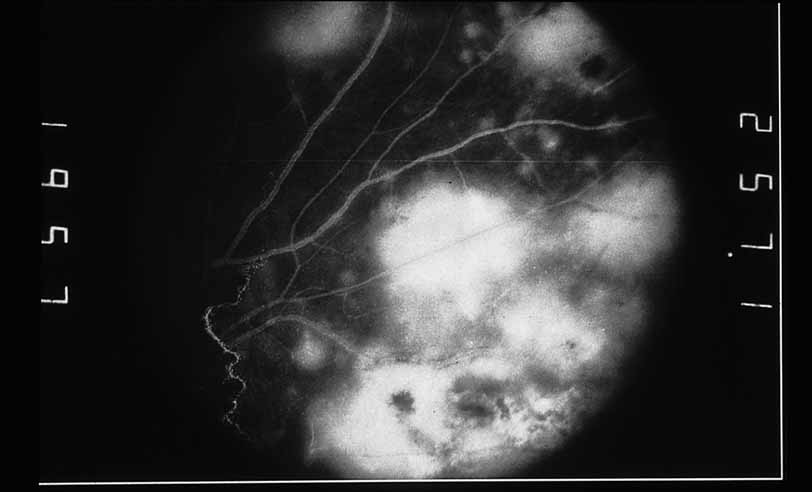
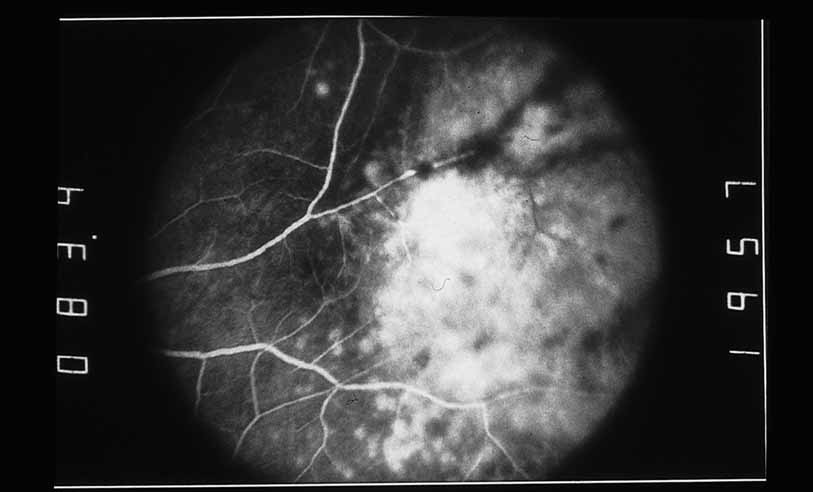
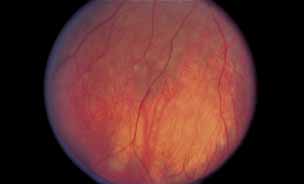
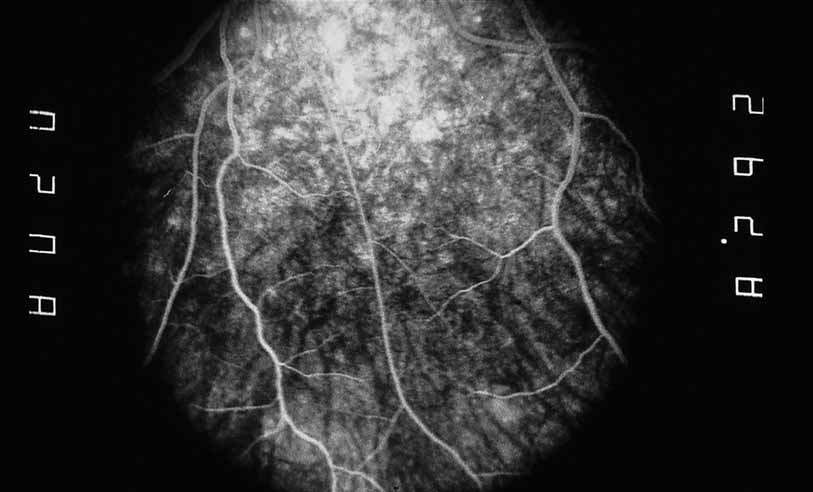
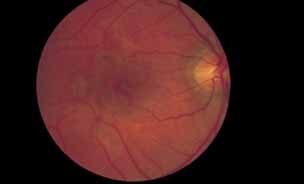
1. Gass JDM: Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, 3rd ed. St. Louis: CV Mosby, 1987 2. Orth D: Color and Fluorescein Angiographic Atlas of Retinal Vascular Disorders. Baltimore: Williams & Wilkins, 1984 3. Ryan SJ: Retina, Vol 2, Medical Retina. St. Louis: CV Mosby, 1989 4. Yannuzzi LA, Gitter KA, Schatz H: The Macula: A Comprehensive Text and Atlas. Baltimore: Williams & Wilkins, 1979 5. Obana A, Kusumi M, Miki T: Indocyanine Green Angiographic Aspects of Multiple Evanescent White Dot
Syndrome. Retina 16:97–104, 1996 6. Ie D, Glaser BM, Murphy RP, et al: Indocyanine Green Angiography in Multiple Evanescent White-Dot Syndrome. Am J Ophthalmol 117:7–12, 1994 7. Ikeda N, Ikeda T, Tano R, et al: Location of Lesions in Multiple Evanescent White Dot Syndrome and the Cause
of the Hypofluorescent Spots Observed by Indocyanine Green Angiography. Graefe's Arch Clin Exp Ophthalmol 239:242–247, 2001 8. Dhaliwal RS, Maguire AM, Flower RW, et al: Acute Posterior Multifocal Placoid Pigment Epitheliopathy. Retina 13:317–325, 1993 9. Vadala M, Lodato G, Cillino S: Multifocal Choroiditis: Indocyanine Green Angiographic Features. Ophthalmologica 215:16–21, 2001 10. Okada AA, Mizusawa T, Sakai J, et al: Videofundoscopy and Videoangiography Using the Scanning Laser Ophthalmoscope
in Vogt-Koyanagi-Harada Syndrome. Br J Ophthalmol 82:1175–1181, 1998 11. Oshima Y, Harino S, Hara Y, et al: Indocyanine Green Angiographic Findings in Vogt-Koyanagi-Harada
Disease. Am J Ophthalmol 122:58–66, 1996 12. Yuzawa M, Kawamura A, Matsui M: Indocyanine Green Video-Angiographic Findings in Harada's Disease. Jpn J Ophthalmol 37:456–466, 1993 13. Matsuo T, Itami M, Shiraga F: Choroidopathy in Patients with Sarcoidosis Observed by Simultaneous Indocyanine
Green and Fluorescein Angiography. Retina 20:16–21, 2000 14. Bozzoni-Pantaleoni F, Gharbiya M, Pirraglia M, et al: Indocyanine Green Angiographic Findings in Behcet Disease. Retina 21:230–236, 2001 15. Matsuo T, Sato Y, Shiraga F, et al: Choroidal Abnormalities in Behcet Disease Observed by Simultaneous Indocyanine
Green and Fluorescein Angiography with Scanning Laser Ophthalmoscopy. 16. Arruga J, Valentines J, Mauri F, et al: Neuroretinitis in acquired syphilis. Ophthalmology 92:262, 1985 17. Cunha De Souza E, Jalkh AE, Trempe CL, et al: Unusual central chorioretinitis as the first manifestation of early secondary
syphilis. Am J Ophthalmol 105:271, 1988 18. DeLuise VP, Clark SW, Smith JL: Syphilitic retinal detachment and uveal effusion. Am J Ophthalmol 94:757, 1982 19. Halperin LS, Lewis H, Blumenkranz MS, et al: Choroidal neovascular membrane and other chorioretinal complications of
acquired syphilis. Am J Ophthalmol 108:554, 1989 20. Mendelsohn AD, Jampol LM: Syphilitic retinitis: A cause of necrotizing retinitis. Retina 4:221, 1984 21. Morgan CM, Webb RM, O'Connor GR: Atypical syphilitic chorioretinitis and vasculitis. Retina 4:225, 1984 22. Blumenkranz MS, Gass JDM, Clarkson JG: Atypical serpiginous choroiditis. Arch Ophthalmol 100:1773, 1982 23. Broekhuyse RM, van Herck M, Pinckers AJ, et al: Immune responsiveness to retinal S-antigen and opsin in serpiginous
choroiditis and other retinal disease. Doc Ophthalmol 69:83, 1988 24. Exp Ophthalmol 219:131, 1982 25. Hardy RA, Schatz H: Macular geographic helicoid choroidopathy. Arch Ophthalmol 105:1237, 1987 26. Mansour AM, Jampol LM, Packo L, et al: Macular serpiginous choroiditis. Retina 8:125, 1988 27. Schatz H, Maumenee AE, Patz A: Geographic helicoid peripapillary choroidopathy: Clinical presentations
and fluorescein angiographic findings. Trans Am Acad Ophthalmol Otolaryngol 78:747, 1974 28. Wojno T, Meredith TA: Unusual findings in serpiginous choroiditis. Am J Ophthalmol 94:650, 1982 29. Wu JS, Lewis H, Fine SL, et al: Clinicopathologic findings in a patient with serpiginous choroiditis and
treated choroi-dal neovascularization. Retina 9:292, 1989 30. Friberg TR: Serpiginous choroiditis with branch vein occlusion and bilateral periphlebitis: Case
report. Arch Ophthalmol 106:585, 1988 31. Eichenbaum JW, Friedman AH, Mamelok AE: A clinical and histopathological review of intermediate uveitis (pars
planitis). Bull NY Acad Med 64:164, 1988 32. Henderly DE, Haymond RS, Rao NA, Smith RE: The significance of the pars plana exudate in pars planitis. Am J Ophthalmol 103:669, 1987 33. Wetzig RP, Chan CC, Nussenblatt RB, et al: Clinical and immunopathologic studies of pars planitis in a family. Br J Ophthalmol 72:5, 1988 34. Brod RD: Presumed sarcoid choroidopathy mimicking birdshot retinochoroidopathy. Am J Ophthalmol 109:357, 1990 35. Campo RV, Aaberg TM: Choroidal granuloma in sarcoidosis. Am J Ophthalmol 97:419, 1984 36. Duker JS, Brown GC, McNamara JA: Proliferative sarcoid retinopathy. Ophthalmology 95:1680, 1988 37. Franceschetti A, Babel J: La choriorétinité en taches de bougie: Manifestations de
la maladie de Besnier-Boech. Ophthalmologica 118:701, 1949 38. Marcus DF, Bovino JA, Burton TC: Sarcoid granuloma of the choroid. Ophthalmology 89:1326, 1982 39. Mizuno K, Takahashi J: Sarcoid cyclitis. Ophthalmology 93:511, 1986 40. Spalton DJ, Sanders MD: Fundus changes in histologically confirmed sarcoidosis. Br J Ophthalmol 65:348, 1981 41. Chan CC, Palestine AG, Kuwabara T, Nussenblatt RB: Immunopathologic study of Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 105:607, 1988 42. Albert DM, Diaz-Rohena R: A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol 34:1, 1989 43. Carney MD, Tessler HH, Peyman GA, et al: Sympathetic ophthalmia and subretinal neovascularization. Ann Ophthalmol 22:184, 1990 44. Jennings T, Tessler HH: Twenty cases of sympathetic ophthalmia. Br J Ophthalmol 73:140, 1989 45. Maisel JM, Vorwerk PA: Sympathetic uveitis after giant tear repair. Retina 9:122, 1989 46. Tessler HH, Jennings T: High-dose short-term chlorambucil for intractable sympathetic
ophthalmia and Behçet's disease. Br J Ophthalmol 74:353, 1990 47. Blumenkranz MS, Kaplan HJ, Clarkson JG, et al: Acute multifocal hemorrhagic retinal vasculitis. Ophthalmology 95:1663, 1988 48. Bertram B, Wolf S, Hof A, et al: Rheologic findings in patients with Eale's disease. Klin Monatsbl Augenheilkd 195:254, 1989 49. Gordon MF, Coyle PK, Golub B: Eale's disease presenting as stroke in the young adult. Ann Neurol 24:264, 1988 50. Magargal LE, Walsh AW, Magargal HO, et al: Treatment of Eale's disease with scatter laser photocoagulation. Ann Ophthalmol 21:300, 1989 51. Moyenin P, Bonnet M, Grange JD: Eale's syndrome and photocoagulation: A propos of 29 cases. Bull Soc Ophthalmol Fr 87:383, 1987 52. Riss I, Le-Rebeller MJ, Tapiero B: Extensive fibrovascular proliferations in Eale's disease. Bull Soc Ophthalmol Fr 86:1549, 1555, 1986 53. Blumenkranz M, Clarkson J, Culbertson WW, et al: Visual results and complications after retinal reattachment in the acute
retinal necrosis syndrome: The influence of operative technique. Retina 9:170, 1989 54. Blumenkranz MS, Clarkson J, Culbertson WW, et al: Vitrectomy for retinal detachment associated with acute retinal necrosis. Am J Ophthalmol 106:426, 1988 55. Browning DJ, Blumenkranz MS, Culbertson WW, et al: Association of varicella zoster dermatitis with acute retinal necrosis
syndrome. Ophthalmology 94:602, 1987 56. Clarkson JG, Blumenkranz MS, Culbertson WW, et al: Retinal detachment following the acute retinal necrosis syndrome. Ophthalmology 91:1665, 1984 57. Culbertson WW, Blumenkranz MS, Haines H, et al: The acute retinal necrosis syndrome: II. Histopathology and etiology. Ophthalmology 89:1317, 1982 58. Culbertson WW, Blumenkranz MS, Pepose JS, et al: Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology 93:559, 1986 59. Bisher JP, Lewis ML, Blumenkranz MS: The acute retinal necrosis syndrome: I. Clinical manifestations. Ophthalmology 89:1309, 1982 60. Han DP, Lewis H, Williams GA, et al: Laser photocoagulation in the acute retinal necrosis syndrome. Arch Ophthalmol 105:1051, 1987 61. Holland GN, Cornell PJ, Park MS, et al: An association between acute retinal necrosis syndrome and HLA-DQw7 and
phenotype Bw62, DR4. Am J Ophthalmol 108:370, 1989 62. Immunen I, Laatikainen L, Linnanvuori K: Acute retinal necrosis syndrome treated with vitrectomy and intravenous
acyclovir. Acta Ophthalmol 67:106, 1989 63. Lewis ML, Culbertson WW, Post JD, et al: Herpes simplex virus type I:. A cause of the acute retinal necrosis syndrome. Ophthalmology 96:875, 1989 64. Ludwig IH, Zegarra H, Zakov ZN: The acute retinal necrosis syndrome: Possible herpes simplex retinitis. Ophthalmology 91:1659, 1984 65. Matsuo T, Koyama M, Matsuo N: Acute retinal necrosis as a novel complication of chickenpox in adults. Br J Ophthalmol 74:443, 1990 66. Matsuo T, Ohno A, Matsuo N: Acute retinal necrosis syndrome following chickenpox in pregnant woman. Jpn J Ophthalmol 32:70, 1988 67. Saga U, Ozawa H, Soshi S, et al: Acute retinal necrosis (Kirisawa's uveitis). Jpn J Ophthalmol 27:353, 1983 68. Sergott RC, Belmont JB, Savino PJ, et al: Optic nerve involvement in the acute retinal necrosis syndrome. Arch Ophthalmol 103:1160, 1985 69. Sternberg P Jr , Know DL, Finkelstein D, et al: Acute retinal necrosis syndrome. Retina 2:145, 1982 70. Wang CL, Kaplan HJ, Waldrep JC, et al: Retinal neovascularization associated with acute retinal necrosis. Retina 3:249, 1983 71. Yeo JH, Pepose JS, Stewart JA: Acute retinal necrosis syndrome following herpes zoster dermatitis. Ophthalmology 93:1418, 1986 72. Schilling H, Bornfeld N, Windeck R, et al: Cystostatic and immunosuppressive treatment of ocular Behçet's
syndrome. Klin Monatsbl Augenheilkd 196:62, 1990 73. Sugihara I: Measurement of retinal circulation in uveitis of Behçet's and
Harada's disease. Nippon Geka Gakkai Zasshi 93:705, 1989 74. Wakefield D, McCluskey P: Behçet's syndrome: Ocular features in Australian population. Aust NZ J Ophthalmol 18:129, 1990 75. Parke DW, Font RL: Diffuse toxoplasmic retinochoroiditis in a patient with AIDS. Arch Ophthalmol 104:571, 1986 76. Brown GC, Brown RH, Brown MM: Peripheral proliferative retinopathies. Int Ophthalmol 11:41, 1987 77. James CR, Jacobs PM, Leaver PK, McLeod D: Closed microsurgery for the sequelae of neovascularization from veno-occlusive
retinopathies. Eye l(pt 1) :106, 1987 78. Regan CD, Foster CS: Retinal vascular diseases: Clinical presentation and diagnosis. Int Ophthalmol Clin. 26:25, 1986 79. Jampol LM, Sieving PA, Pugh D, et al: Multiple evanescent white dot syndrome. Arch Ophthalmol 102:671, 1984 80. Charteris DG, Khanna V, Dhillon B: Acute posterior multifocal placoid pigment epitheliopathy complicated by
central retinal vein occlusion. Br J Ophthalmol 73:765, 1989 81. Foulds WS, Damato BE: Investigation and prognosis in the retinal pigment epitheliopathies. Aust NZ J Ophthalmol 14:301, 1986 82. Gass JDM: Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 80:177, 1968 83. Isashiki M, Koide H, Yamashita T, Ohba N: Acute posterior multifocal placoid pigment epitheliopathy associated with
diffuse retinal vasculitis and late hemorrhagic macular detachment. Br J Ophthalmol 70:255, 1986 84. Kersten DH, Lessell S, Carlow TJ: Acute posterior multifocal placoid pigment epitheliopathy and late-onset
meningo-encephalitis. Ophthalmology 94:393, 1987 85. Williams DF, Mieler WF: Long-term follow-up of acute multifocal posterior placoid
pigment epitheliopathy. Br J Ophthalmol 73:985, 1989 86. Schlaegel TF: Presumed histoplastic choroiditis. Am J Ophthalmol 63:919, 1967 87. Pepose JS, Holland GN, Nestor MS, et al: Acquired immune deficiency syndrome: Pathogenic mechanisms of ocular disease. Ophthalmology 92:472, 1985 88. Schuman JS, Friedmari AH: Retinal manifestations of the acquired immune deficiency syndrome (AIDS): Cytomegalovirus, Candida albicans, Cryptococcus, toxoplasmosis, and Pneumocystis carinii. Trans Ophthalmol Soc UK 103(pt 2):177, 1983 89. Cantrill HL, Folk JC: Multifocal choroiditis associated with progressive subretinal fibrosis. Am J Ophthalmol 101:170, 1986 90. Dreyer RF, Gass JDM: Multifocal choroiditis and panuveitis: A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol 102:1776, 1984 |