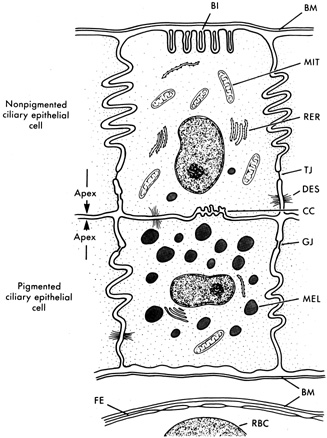
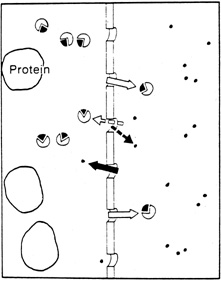
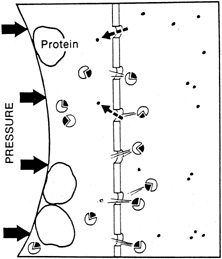
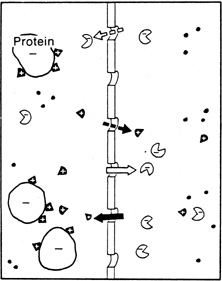
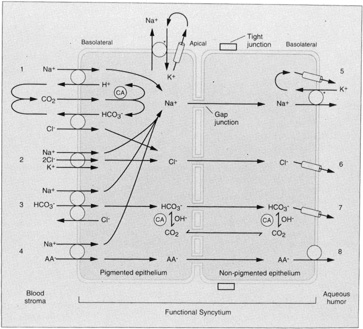
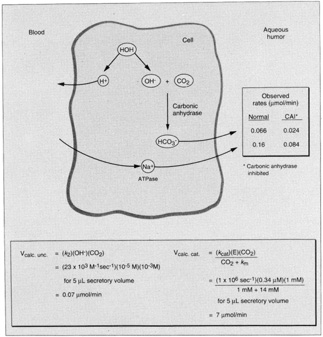
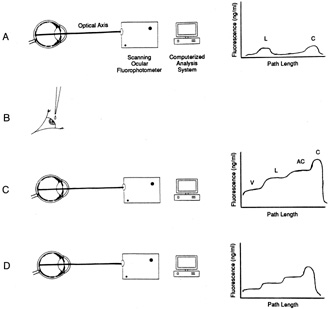
1. Davson H: The aqueous humor and the intraocular pressure. In Davson H, ed.: , ed.: Physiology of the Eye. New York: Pergamon Press, 1990:3–95 2. Freddo TF: Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res 73:581, 2001 3. Brubaker RF: Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci 32:3145, 1991 4. Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human Eye. An Atlas and Textbook. Philadelphia: WB Saunders Co., 1971:136–153, 260–319 5. Cole DF: Aqueous humor formation. Doc Ophthalmol 21:116, 1966 6. Alm A, Bill A: Ocular and optic nerve blood flow at normal and increased intraocular pressure
in monkeys (Macaca Irus): A study with radioactively
labelled microspheres, including flow determinatins in brain and some
other tissues. Exp Eye Res 15:15, 1973 7. Morrison JC, Van Buskirk EM: Anterior collateral circulation in the primate eye. Ophthalmology 90:707, 1983 8. Morrison JC, Van Buskirk EM: Ciliary process microvasculature. Am J Ophthalmol 97:372, 1984 9. Morrison JC, van Buskirk EM: Sequential microdissection and scanning electron microscopy of ciliary
microvascular castings. Scan Electron Microsc 2:857, 1984 10. Caprioli J, Sears ML, Mead A: Ocular blood flow in phakic and aphakic monkey eyes. Exp Eye Res 39:1, 1984 11. Morrison JC, de Frank MP, van Buskirk EM: Comparative microvascular anatomy of mammalian ciliary processes. Invest Ophthalmol Vis Sci 28:1325, 1987 12. Morrison JC, de Frank MP, van Buskirk EM: Regional microvascular anatomy of the rabbit ciliary body. Invest Ophthalmol Vis Sci 28:1314, 1987 13. Funk R, Rohen JW: Scanning electron microscopic study on the vasculature of the human anterior
eye segment, especially with respect to the ciliary processes. Exp Eye Res 51:651, 1990 14. Bill A: Blood circulation and fluid dynamics in the eye. Physiol Rev 55:383, 1975 15. Seidël E: Weitre experimentelle Untersuchungen über die Quelle und den Verlauf
der introkulären Saftsrömung. XII. Metteilung. Uber den manometrischen
Nachweis des physiologischen Druckgefalles zwishen Vorderkammer
und Schlemmshen Kanal. Graefe's Arch Clin Exp Ophthalmol 107:101, 1921 16. Seidël E: Weitre experimentelle Untersuchungen über die Quelle und den Verlauf
der introkulären Saftströmung. IX. Uber der Abfluss des Kammerwassers
aus der vorderen Augenkammer. Graefe's Arch Clin Exp Ophthalmol 104:357, 1921 17. Knutson SL, Sears ML: Herman Boerhaave and the history of vessels carrying aqueous humor from
the eye. Am J Ophthalmol 76:648, 1973 18. Ascher KW: Aqueous veins: Preliminary note. Am J Ophthalmol 25:31, 1942 19. Goldmann H: Enhalten die Kammervasservener Kammervasser? Ophthalmologica 117:240, 1949 20. Ashton N: Anatomical study of Schlemm's canal and aqueous veins by means of
neoprene casts: Part I. Aqueous veins. Br J Ophthalmol 35:291, 1951 21. Bill A: The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca
irius) with evidence for unconventional routes. Invest Ophthalmol 4:911, 1965 22. Bill A: The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp Eye Res 16:287, 1973 23. Brodwell J, Fischbarg J: The hydraulic conductivity of rabbit ciliary epithelium in vitro. Exp Eye Res 34:121, 1982 24. Caprioli J: The ciliary epithelia and aqueous humor. In Hart WM, ed.: Adler's Physiology of the Eye: Clinical Application, 9th ed. St. Louis: CV Mosby, 1992:228–247 25. Cole DF: Effects of some metabolic inhibitors upon the formation of the aqueous
humor in rabbits. Br J Ophthalmol 44:739, 1960 26. Cole DF: Secretion of the aqueous humor. Exp Eye Res 25(suppl):161, 1977 27. Green K, Pederson JE: Contribution of secretion and filtration to aqueous humor formation. Am J Physiol 222:1218, 1972 28. Maren TH: HCO3-formation in aqueous humor: Mechanism and relation to the treatment
of glaucoma. Invest Ophthalmol 13:479, 1974 29. Pederson JE: Fluid permeability of monkey ciliary epithelium in vivo. Invest Ophthalmol Vis Sci 23:176, 1982 30. Duke-Elder S: The aqueous humor. In Duke-Elder S, ed.: The Physiology of the Eye and of Vision System of Ophthalmology, Vol. 4. St. Louis: CV Mosby, 1968:104–200 31. Saier MH: Neurotransmission. In Zubay G, ed.: , ed.: Biochemistry. Menlo Park, CA: Addison-Wesley, 1983:1147–1167 32. Chattoraj SC, Watts NB: Endocrinology. In Tietz NW, ed.: Textbook of Clinical Chemistry. Philidelphia: WB Saunders, 1986:1116–1117 33. Weinbaum S, Langham ME, Goldgraben JR, et al: The role of secretion and pressure-dependent slow in aqueous humor
formation. Exp Eye Res 13:266, 1972 34. Green K, Pederson JE: Aqueous humor formation. Exp Eye Res 16:273, 1973 35. Bonting SL, Becker B: Studies on sodium-potassium activated adenosinetriphosphatase: XIV. Inhibition
of enzyme activity and aqueous humor flow in the rabbit
eye after intravitreal injection of ouabain. Invest Ophthalmol 3:523, 1964 36. Becker B: Ouabain and aqueous humor dynamics in the rabbit eye. Invest Ophthalmol 2:325, 1963 37. Becker B: Vanadate and aqueous humor dynamics. Proctor Lecture. Invest Ophthalmol Vis Sci 19:1156, 1980 38. Krupin T, Becker B, Podos SM: Topical vanadate lowers intraocular pressure in rabbits. Invest Ophthalmol Vis Sci 19:1360, 1980 39. Podos SM, Lee PY, Severin C, et al: The effect of vanadate on aqueous humor dynamics in cynomolgus monkeys. Invest Ophthalmol Vis Sci 25:359, 1984 40. Shahidullah M, Wilson WS, Yap M, et al: Effects of ion transport and channel-blocking drugs on aqueous humor
formation in isolated bovine eye. Invest Ophthalmol Vis Sci 44:1185, 2003 41. Hochgesand DH, Dunn JJ, Crook RB: Catecholaminergic regulation of Na-K-Cl contransport in pigmented
ciliary epithelium: Difference between PE and NPE. Exp Eye Res 72:1, 2001 42. Friedland BR, Maren TH: Carbonic anhydrase: Pharmacology of inhibitors and treatment of glaucoma. In Sears ML, ed.: , ed.: Pharmacology of the Eye. Berlin: Springer-Verlag, 1984:279–309 43. Brechue WF, Maren TH: A comparison between the effect of topical and systemic carbonic anhydrase
inhibotors on aqueous humor secretion. Exp Eye Res 57:67, 1993 44. Pierce WMJ, Sharir M, Waite KJ: Topically active ocular carbonic anhydrase inhibitors-novel biscarbonylamidothiadiazole
sulfonamides as ocular hypotensive agents. Proc Soc Exp Biol Med 203:360, 1993 45. Becker B: Hypothermia and aqueous humor dynamics of the rabbit eye. Trans Am Ophthalmol Soc 58:337, 1960 46. Bonting SL, Simon KA, Hawkins NM: Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative
distribution in several tissues of the cat. Arch Biochem 95:416, 1961 47. Riley MV: The sodium-potassium-stimulated adensosine triphosphatase
of rabbit ciliary epithelium. Exp Eye Res 3:76, 1964 48. Riley MV, Kishida K: ATPases of ciliary epithelium: Cellular and subcellular distribution and
probable role in secretion of aqueous humor. Exp Eye Res 42:559, 1986 49. Coca-Prados M, Lopez-Briones LG: Evidence that the a and the a(+) isoforms of the catalytic
subunit of (Na+, K+)-ATPase reside in distinct
ciliary epithelial cells of the mammalian eye. Biochem Biophys Res Commun 145:460, 1987 50. Flügel C, Lütjen-Drecoll E: Presence and distribution of Na+/K+-ATPase in the
ciliary epithelium of the rabbit. Histochemistry 88:613, 1988 51. Usukura J, Fain GL, Bok D: [3H]Ouabain localization of Na-K ATPase in the epithelium
of the rabbit ciliary body pars plicata. Invest Ophthalmol Vis Sci 29:606, 1988 52. Holland MG, Gipson CC: Chloride ion transport in the isolated ciliary body. Invest Ophthalmol Vis Sci 9:20, 1970 53. Cole DF: Electrochemical changes associated with the formation of the aqueous humor. Br J Ophthalmol 45:202, 1961 54. Cole DF: Evidence for active transport of chloride in ciliary epithelium of the
rabbit. Exp Eye Res 8:5, 1969 55. Kinsey VE, Reddy DVN: Chemistry and dynamics of aqueous humor. In Prince JH, ed.: , ed.: The Rabbit in Eye Research. Springfield, IL: Charles C. Thomas, 1964:218–319 56. Krupin T, Reinach PS, Candia OA, et al: Transepithelial electrical measurements on the isolated rabbit irisciliary
body. Exp Eye Res 38:115, 1984 57. Do CW, To CH: Chloride secretion by bovine ciliary epithelium: A model of aqueous humor
formation. Invest Ophthalmol Vis Sci 41:1853, 2000 58. Verkman AS: Role of aquaporin water channels in eye function. Exp Eye Res 76:137, 2003 59. Patil RV, Han Z, Yiming M, et al: Fluid transport by human nonpigmented ciliary epithelial layers in culture: A
homeostatic role for aquaporin-1. Am J Physiol 281:1139, 2001 60. Frigeri A, Gropper M, Turck CW, et al: Immunolocalization of the mercurial-insensitive water channel and
glycerol intrinsic protein in epithelial cell plasma membranes. Proc Nat Acad Sci 92:4328, 1995 61. Wistrand PJ: Carbonic anhydrase in the anterior uvea of the rabbit. Acta Physiol Scand 24:144, 1951 62. Ballintine EJ, Maren TH: Carbonic anhydrase activity and the distribution of Diamox in the rabbit
eye. Am J Ophthalmol 40:148, 1955 63. Bhattacherjee P: Distribution of carbonic anhydrase in the rabbit eye as demonstrated histochemically. Exp Eye Res 12:356, 1971 64. Tsukahara S, Maezara N: Cytochemical localization of adenyl cyclase in the rabbit ciliary body. Exp Eye Res 26:99, 1978 65. Mishima H, Sears M, Bausher L, et al: Ultracytochemistry of cholera toxin binding sites in ciliary processes. Cell Tissue Res 223:241, 1982 66. White A, Handler P, Smith EL: Introduction to metabolism. In White A, Handler P, Smith EL, eds.: Principles of Biochemistry, 5th ed. New York: McGraw-Hill, 1973:279–310 67. Mudge GH, Weiner IM: Agents affecting volume and composition of body fluids. In Goodman A, Rall TW, Nies AS, Taylor P, eds.: The Pharmacological Basis of Therapeutics, 8th ed. New York: McGraw-Hill, 1990:682–731 68. Muther TF, Friedland BR: Autoradiographic localization of carbonic anhydrase in the rabbit ciliary
body. J Histochem Cytochem 28:1119, 1980 69. Lütjen-Drecoll E, Lonnerholm G: Carbonic anhydrase distribution in the rabbit eye by light and electron
microscopy. Invest Ophthalmol Vis Sci 21:782, 1981 70. Lütjen-Drecoll E, Lonnerholm G, Eichhorn M: Carbonic anhydrase distribution in the human and monkey eye by light and
electron microscopy. Graefe's Arch Clin Exp Ophthalmol 220:285, 1983 71. Maren TH, Mayer E, Wadsworth BC: Carbonic anhydrase inhibition. I. The pharmacology of Diamox (1-acetylamino 1,3,4-thiadiazole-5-sulfonamide). Bull Johns Hopkins Hosp 95:199, 1954 72. Maren TH: Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol Rev 47:595, 1967 73. Maren TH: The development of ideas concerning the role of carbonic anhydrase in the
secretion of aqueous humor: Relations to the treatment of glaucoma. In Drance SM, Neufeld AH, eds.: Glaucoma Applied Pharmacology in Medical Treatment. Orlando: Grune & Stratton; 1984:325–555 74. Eller MG, Schoenwald RD, Dixson JA: Topical carbonic anhydrase inhibitors, III. Optimization model for corneal
penetration of ethoxzolamide analogues. J Pharm Sci 74:155, 1985 75. Maren TH, Jankowska L, Sanyal G, et al: The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors
and their effect on aqueous humor secretion. Exp Eye Res 36:457, 1983 76. Schoenwald RD, Eller MG, Dixson JA, et al: Topical carbonic anhydrase inhibitors. J Med Chem 27:810, 1984 77. Lippa EA, Carlson LE, Ehinger B, et al: Dose response and duration of action of dorzolamide, a topical carbonic
anhydrase inhibitor. Arch Ophthalmol 110:495, 1992 78. Wilkerson M, Cyrlin M, Lippa EA, et al: Four-week safety and efficacy study of dorzolamide, a novel, active
topical carbonic anhydrase inhibitor. Arch Ophthalmol 111:1343, 1993 79. Gunning FP, Greve EL, Bron AM, et al: Two topical carbonic anhydrase inhibitors sezolamide and dorzolamide in
Gelrite vehicle: A multiple-dose efficacy study. Graefes Arch Clin Exp Ophthalmol 231:384, 1993 80. Herkel U, Pfeiffer N: Update on topical carbonic anhydrase inhibitors. Curr Opin Ophthalmol 12:88, 2001 81. Cvetkovic RS, Perry CM: Brinzolamide: A review of its use in the management of primary open-angle
glaucoma and ocular hypertension. Drugs Aging 20:919, 2003 82. DeSantis L: Preclinical overview of brinzolamide. Surv Ophthalmol 44:S119, 2000 83. Wistrand P: Local action of the carbonic anhydrase inhibitor, acetazolamide, on the
intraocular pressure in cats. Acta Pharm Toxicol 14:27, 1957 84. Macri FJ: The constrictive action of acetazolamide on the arteries of the cat. Arch Ophthalmol 66:570, 1961 85. Macri FJ, Cevario SJ: A possible vascular mechanism for the inhibition of aqueous humor formation
by ouabain and acetazolamide. Exp Eye Res 20:563, 1975 86. Bill A: Effects of acetazolamide and carotid occlusion on the ocular blood flow
in unanesthetized rabbits. Invest Ophthalmol 13:954, 1974 87. Maren TH: Biochemistry of aqueous humor inflow. In Kaufman PL, Mittag TW, eds.: Glaucoma. London: Mosby-Year Book Europe Ltd., 1994:1.35–1.46 88. Maren TH: The rates of movement of Na+, Cl−, and HCO3− from plasma to posterior chamber: Effect of acetazolamide and relation
to the treatment of glaucoma. Invest Ophthalmol 15:356, 1976 89. Becker B: The mechanism of the fall in intraocular pressure induced by the carbonic
anhydrase inhibitor Diamox. Am J Ophthalmol 39:177, 1955 90. Kaufman PL, Mittag TW: Medical therapy of glaucoma. In Kaufman PL, Mittag TW, eds.: Glaucoma. London: Mosby-Year Book Europe Ltd., 1994:9.7–9.30 91. Sugrue MF, Mallorga P, Schwamm H: A comparison of L-671,152 and MK927, two topically effective ocular
hypotensive carbonic anhydrase inhibitors, in experimental animals. Curr Eye Res 9:607, 1990 92. Wang RF, Serle JB, Podos SM, et al: MK-507 (L 671, 152), a topically active carbonic anhydrase
inhibitor, reduces aqueous humor production in monkeys. Arch Ophthalmol 109:1297, 1991 93. Maren TH, Bar-Ilan A, Conroy CW, et al: Chemical and pharmacological properties of MK-927, a sulfonamide
carbonic anhydrase inhibitor tha lowers intraocular pressure by the topical
route. Exp Eye Res 50:27, 1990 94. Maus TL, Larsson LI, McLaren JW, et al: Comparison of dorzolamide and acetazolamide as suppressors of aqueous humor
flow in humans. Arch Ophthalmol 115:45, 1997 95. Michaud JE, Friren B: Comparison of topical brinzolamide 1% and dorzolamide 2% eye
drops given twice daily in addition to timolol 0.5% in patients
with primary open-angle glaucoma or ocular hypertension. Am J Ophthalmol 132:235, 2001 96. Seong GJ, Lee SC, Lee JH, et al: Comparisons of intraocular-pressure-lowering efficacy and
side effects of 2% dorzolamide and 1% brinzolamide. Ophthalmologica 215:188, 2001 97. Krause U, Raunio V: Proteins of the normal human aqueous humor. Ophthalmologica 159:178, 1969 98. Krause U, Raunio V: The proteins of the normal aqueous humor. Ophthalmologica 160:280, 1970 99. Rodriguez-Peralta L: The blood-aqueous barrier in five species. Am J Ophthalmol 80:713, 1975 100. Bill A: The drainage of albumin from the uvea. Exp Eye Res 3:179, 1964 101. Bill A: Capillary permeability to and extravascular dynamics of myoglobin, albumin, and
gammaglobulin in the uvea. Acta Physiol Scand. 73:204, 1968 102. Vegge T: An epithelial blood-aqueous barrier to horseradish peroxidase in
the ciliary processes of the vervet monkey (Cercopithecus aethiops). Zeitschr Zellforsch Mikrosk Anat 114:309, 1971 103. Shiose Y: Electron microscopic studies on blood-retinal and blood-aqueous
barriers. Jpn J Ophthalmol 14:73, 1970 104. Smith RS: Ultrastructural studies of the blood-aqueous barrier. 1. Transport
of an electron dense tracer in the iris and ciliary body of the mouse. Am J Ophthalmol 71:1066, 1971 105. Uusitalo R, Palkama A, Stjernschantz J: An electron microscopic study of the blood-aqueous barrier in the
ciliary body and iris of the rabbit. Exp Eye Res 17:49, 1973 106. Uusitalo R, Stjernschantz J, Palkama A: Studies on the ultrastructure of the blood-aqueous barrier in the
rabbit. Acta Ophthalmol 123:61, 1974 107. Freddo T, Raviola G: The homogeneous structure of blood vessels in the vascular tree in Macaca
mulatta iris. Invest Ophthalmol Vis Sci 22:279, 1982 108. Hirsch M, Montcourrier P, Arguillere P, et al: The structure of tight junctions in the ciliary epithelium. Curr Eye Res 4:493, 1995 109. Shabo AL, Maxwell DS: The blood-aqueous barrier to tracer protein. A light and electron
microscopic study of the primate ciliary process. Microvasc Res 4:142, 1972 110. Smith RS, Rudt LA: Ultrastructural studies of the blood-aqueous barrier 2. The barrier
to horseradish peroxidase in primates. Am J Ophthalmol 76:937, 1973 111. Vegge T: An electron microscopic study of the permeability of iris capillaries to
horseradish peroxidase in the vervet monkey. Z Zellforsch. 121:74, 1971 112. Davson H, Duke-Elder WS, Maurice DM: Changes in ionic distribution following dialysis of aqueous humor against
plasma. J Physiol (Lond) 109:32, 1949 113. Ross EJ: The transfer of non-electrolytes across the blood-aqueous
barrier. J Physiol (Lond) 112:229, 1951 114. Davson H, Matchett PA: The kinetics of penetration of the blood-aqueous barrier. J Physiol (Lond) 122:11, 1953 115. Hart WM: Intraocular pressure. In Hart WM, ed.: , ed.: Adler's Physiology of the Eye, 9th ed. St. Louis: CV Mosby, 1992:248–267 116. Bairati AJ, Orzalesi N: The ultrastructure of the epithelium of the ciliary body: A study of the
junctional complexes and of the changes associated with the production
of plasmoid aqueous humor. Z Zellforsch Milrosk Anat 69:635, 1966 117. Okisaka S: Effects of paracentesis on the blood-aqueous barrier: A light and
electron microscopic study on cynomolgus monkey. Invest Ophthalmol 15:824, 1976 118. Al-Ghadyan A, Mead A, Sears ML: Increased pressure after paracentesis of the rabbit eye is completely accounted
for by prostaglandin synthesis and release plus pupillary block. Invest Ophthalmol Vis Sci 18:361, 1979 119. Bartels SP, Pederson JE, Gaasterland DE, et al: Sites of breakdown of the blood-aqueous barrier after paracentesis
of the rhesus monkey eye. Invest Ophthalmol Vis Sci 18:1050, 1979 120. Ohnishi Y, Tanaka M: Effects of pilocarpine and paracentesis on occluding junctions between
the nonpigmented ciliary epithelial cells. Exp Eye Res 32:635, 1981 121. Gaasterland DF, Barranger JA, Rapoport SI: Long-term ocular effects of osmotic modification of the blood-brain
barrier in monkeys. 1. Clinical examination, aqueous ascorbate
and protein. Invest Ophthalmol Vis Sci 24:153, 1983 122. Miyake Y, Asakura M, Maekubo K: Consensual reactions of human blood-aqueous barrier to implant operations. Arch Ophthalmol 102:558, 1984 123. Kondo K, Coca-Prados M, Sears ML: Human ciliary epithelia in monolayer culture. Exp Eye Res 102:423, 1984 124. Jampol L, Neufeld A, Sears M: Pathways for the response of the eye to injury. Invest Ophthalmol Vis Sci 14:184, 1975 125. Shabo AL, Maxwell DS, Kreiger AE: Structural alterations in the ciliary process and the blood-aqueous
barrier of the monkey after systemic urea injections. Am J Ophthalmol 81:162, 1976 126. Raviola G: Blood-aqueous barrier can be circumvented by lowering intraocular
pressure. Proc Natl Acad Sci USA 73:638, 1976 127. Raviola G: Effects of paracentesis on the blood-aqueous barrier: An electron
microscopic study on Macaca mullata using horseradish peroxidase as
a tracer. Invest Ophthalmol 13:828, 1974 128. Whitelocke RA, Eakins KE: Vascular changes in the anterior uvea of the rabbit produced by prostaglandins. Arch Ophthalmol 89:495, 1973 129. Whitelocke RAF, Eakins KE, Bennett A: Acute anterior uveitis and prostaglandins. Proc R Soc Med 66:429, 1973 130. Green K, Kim K: Pattern of ocular response to topical and systemic prostaglandin. Invest Ophthalmol 14:36, 1975 131. Laties AM, Neufeld AH, Vegge T, et al: Differential reactivity of rabbit iris and ciliary process to topically
applied prostaglandin-E2 (dinoprostone). Arch Ophthalmol 94:1966, 1976 132. Crawford K, Kaufman PL, True-Gabelt B: Prostaglandins and aqueous humor dynamics. In Shields MB, Pollack IP, Kolker AE, eds.: Perspectives in Glaucoma Transactions of the First Scientific Meeting of
the American Glaucoma Society. Thorofare: Slack, Inc., 1988:259–267 133. Kaufman PL, Crawford K, Gabelt BT: The effects of prostaglandins on aqueous humor dynamics. In Kooner KS, Zimmerman TJ, eds.: New Ophthalmic Drugs. Philadelphia: WB Saunders, 1989:141–150 134. Kaufman PL, Rohen JW, Gabelt BT, et al: Parasympathetic denervation of the ciliary muscle following panretinal
photocoagulation. Curr Eye Res 10:437, 1991 135. Green K: Permeability properties of the ciliary epithelium in response to prostaglandins. Invest Ophthalmol 12:752, 1973 136. Neufeld AH, Sears ML: Prostaglandin and eye. Prostaglandins 4:157, 1973 137. Neufeld AH, Jampol LM, Sears ML: Aspirin prevents the disruption of the blood-aqueous barrier in
the rabbit eye. Nature 238:158, 1972 138. Bhattacherjee P: Autoradiographic localization of intravitreally or intracamerally injected [3H]-prostaglandins. Exp Eye Res 18:181, 1974 139. Bhattacherjee P, Hammond BR: Inhibition of increased permeability of the blood-aqueous barrier
by nonsteroidal anti-inflammatory compounds as demonstrated by
fluorescein angiography. Exp Eye Res 21:499, 1975 140. Bengtsson E: The effect of imidazole on the disruption of the blood-aqueous barrier
in the rabbit eye. Invest Ophthalmol 15:315, 1976 141. Chavis RM, Vygantis CM, Vygantis A: Experimental inhibition of prostaglandin-like inflammatory response
after cryotherapy. Am J Ophthalmol 82:310, 1976 142. Giuffré G: The effects of prostaglandin F2a in the human eye. Graefe's Arch Clin Exp Ophthalmol 222:139, 1985 143. Gabelt BT, Kaufman PL: Prostaglandin F2a increases uveoscleral outflow in the cynomolgus monkey. Exp Eye Res 49:389, 1989 144. Kaufman PL, Crawford K: Aqueous humor dynamics: How PGF2α lowers intraocular pressure. Prog Clin Biol Res 312:387, 1989 145. Nilsson SFE, Samuelsson M, Bill A, et al: Increased uveoscleral outflow as a possible mechanism of ocular hypotension
caused by prostaglandin F2a-1–isopropylester in the cynomolgus monkey. Exp Eye Res 48:707, 1989 146. Villumsen J, Alm A: Prostaglandin F2a-isopropylester eye drops: Effects in normal human eyes. Br J Ophthalmol 73:419, 1989 147. Camras CB, Siebold EC, Lustgarten JS, et al: Maintained reduction of intraocular pressure by prostaglandin F2a-1–isopropylester applied in multiple doses in ocular hypertensive
and glaucoma patients. Ophthalmology 96:1329, 1989 148. Kobayashi H, Kobayashi K, Okinami S: A comparison of intraocular pressure-lowering effect of prostaglandin
F2-alpha analogues, latanoprost and unoprotone isopropyl. J Glaucoma 10:487, 2001 149. Ziai N, Dolan JW, Kacere RD, et al: The effects on aqueous dynamics of PhXA41, a new prostaglandin F2a Analogue, after
topical application in normal and ocular hypertensive human
eyes. Arch Ophthalmol 111:1351, 1993 150. Alm A, Villumsen J, Tornquist P, et al: Intraocular-pressure-reducing effect of PhXA41 in patients
with increased eye pressure. A one-month study. Ophthalmology 100:1312, 1993 151. Alm A, Widengard I, Kjellgren D, et al: Latanoprost administered once daily caused a maintained reduction of intraocular
pressure in glaucoma patients treated concomitantly with timolol. Br J Ophthalmol 79:12, 1995 152. Toris CB, Camras CB, Yablonski ME: Effects of PhXA41, a new prostaglandin F2 alpha, on aqueous humor dynamics
in human eyes. Ophthalmology 100:1297, 1993 153. Racz P, Ruzsonyi MR, Nagy ZT, et al: Maintained intraocular pressure reduction with once-a-day
application of a new prostaglandin-F2-alpha analogue (PhXA41)—An
in-hospital, placebo-controlled
study. Arch Ophthalmol 111:657, 1993 154. Cantor LB: Bimatoprost: A member of a new class of agents, the prostamides, for glaucoma
management. Expert Opin Invest Drugs. 10:721, 2001 155. Brubaker RF: Mechanism of action of bimatoprost (Lumigan). Surv Ophthalmol 45(suppl)S347, 2001 156. Jaffe NS, Jaffe MS, Jaffe GF: Cataract surgery and its complications, 5th ed. St. Louis: CV Mosby; 1990:148 157. Benham GH, Duke-Elder WS, Hodgson TH: The osmotic pressure of the aqueous humor in the normal and glaucomatous
eye. J Physiol (Lond) 92:355, 1938 158. Roepke RR, Hetherington WA: Osmotic relation between aqueous humor and blood plasma. Am J Physiol 130:340, 1940 159. Kinsey VE: The chemical composition and the osmotic pressure of the aqueous humor
and plasma of the rabbit. J Gen Physiol 34:389, 1950 160. Cole DF: Electrolyte composition of anterior and posterior aqueous humor in the
sheep. Ophthalmic Res 4:1, 1973 161. Stjernschantz J, Uusitalo R, Palkama A: The aqueous proteins of the rat in the normal eye and after aqueous withdrawal. Exp Eye Res 16:215, 1973 162. Ghose T, Quigley JH, Landrigan PL, et al: Immunoglobulins in aqueous humor and iris from patients with endogenous
uveitis and patients with cataract. Br J Ophthalmol 57:897, 1973 163. Sen DK, Saren GS, Saha K: Immunoglobulins in human aqueous humor. Br J Ophthalmol 61:216, 1977 164. Fielder AR, Rahi AHS: Immunoglobulins of normal aqueous humor. Trans Ophthalmol Soc UK 99:120, 1979 165. Mondino BJ, Rao H: Complement levels in normal and inflamed aqueous humor. Invest Ophthalmol Vis Sci 24:380, 1983 166. Pandolfi M, Nilsson IM, Martinsson G: Coagulation and fibrinolytic components in primary and plasmoid aqueous
humor. Acta Ophthalmol 42:820, 1964 167. Sandberg HO, Class O: The alpha and gamma crystallin content in aqueous humor of eyes with clear
lens and with cataracts. Exp Eye Res 28:601, 1979 168. Fujiwara H: Lens crystallin reactive protein in the aqueous humor of cataract patients. Jpn J Ophthalmol 33:418, 1989 169. Kolodny NH, Freddo TF, Lawrence B, et al: Contrast-enhanced MRI confirmation of an anterior protein pathway
in normal rabbit eyes. Invest Ophthalmol Vis Sci 37:1602, 1996 170. Bert R, Freddo T, Caruthers SD, et al: Confirmation of anterior large-molecule diffusion pathway in the
normal human eye. Invest Ophthalmol Vis Sci 40(suppl):S198, 1999 171. Freddo TF, Bartels SP, Barsotti MF, et al: The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci 31:125, 1990 172. Barsotti M, Bartels SP, Barsotti MF, et al: The source of proteins in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci 33:581, 1992 173. Kee C, Gabelt BT, Gange SJ, et al: Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest Ophthalmol Vis Sci 37:1840, 1996 174. Johnson M, Gong H, Freddo TF, et al: Serum proteins and aqueous outflow resistance in bovine eyes. Invest Ophthalmol Vis Sci 34:3549, 1993 175. Sit AJ, Gong H, Ritter N, et al: The role of soluble proteins in generating aqueous outflow resistance in
the bovine and human eye. Exp Eye Res 64:813, 1997 176. Reddy DVN, Kinsey VE: Chemistry and dynamics of aqueous humor. In Prince JH, ed.: The Rabbit in Eye Research: Springfield: CC Thomas, 1966:218 177. Bito LZ, Davson H, Levin E: The relationship between the concentrations of amino acids in the ocular
fluids and blood plasma of dogs. Exp Eye Res 4:374, 1965 178. Ehlers N, Kristensen K, Schonheyder F: Amino acid transport in human ciliary epithelium. Acta Ophthalmol 56:777, 1978 179. Kinsey VE: Further study of the distribution of chloride between lasma and intraocular
fluids in the rabbit eye. Invest Ophthalmol 6:395, 1967 180. de Berardinis E, Tieri O, Polzella A, et al: The chemical composition of the human aqueous humor in normal and pathological
conditions. Exp Eye Res 4:179, 1965 181. DiMatteo J: Active transport of ascorbic acid into lens epithelium of the rat. Exp Eye Res 49:873, 1989 182. Reddy VN, Giblin FJ, Lin LR, et al: The effect of aqueous humor ascorbate on ultraviolet-B–induced
DNA damage in lens epithelium. Invest Ophthalmol Vis Sci 39:344, 1998 183. Ringvold A: Aqueous humor and ultraviolet radiation. Acta Ophthalmol 58:69, 1980 184. Ringvold A: The significance of ascorbate in the aqueous humour protection against
UV-A and UV-B. Exp Eye Res 62:261, 1996 185. Koskela TK, Reiss GR, Brubaker RF, et al: Is the high concentration of ascorbic acid in the eye an adaptation to
intense solar radiation? Invest Ophthalmol Vis Sci 31:2265, 1989 186. Rose RC, Richer SP, Bode AM: Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 217:397, 1998 187. Spector A, Garner WH: Hydrogen peroxide and human cataract. Exp Eye Res 33:673, 1981 188. Kahn MG, Giblin FJ, Epstein DL: Glutathione in calf trabecular meshwork and its relation to aqueous humor
outflow facility. Invest Ophthalmol Vis Sci 24:1283, 1983 189. Zhou L, Li Y, Yue BYJT: Oxidative stress affects cytoskeletal structure and cell-matrix
interactions in cells from a ocular tissue: The trabecular meshwork. J Cell Physiol 180:182, 1999 190. Alvarado J, Murphy C, Polansky J, et al: Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci 21:714, 1981 191. Grierson I, Wang Q, McMenanin PG, et al: The effects of age and antiglaucoma drugs on the meshwork cell population. Res Clin Forums. 4:69, 1982 192. Grierson I, Howes RC: Age-related depletion of the cell population in thhe human trabecular
meshwork. Eye 1:204, 1987 193. Alvarado J, Murphy C, Juster R: Trabecular meshwork cellularity in primary open-angle glaucoma and
nonglaucomatous normals. Ophthalmology 91:564, 1984 194. Riley MV: Intraocular dynamics of lactic acid in the rabbit. Invest Ophthalmol Vis Sci 11:600, 1972 195. Sato T, Roy S: Effect of high glucose on fibronectin expression and cell proliferation
in trabecular meshwrok cells. Invest Ophthalmol Vis Sci 43:170, 2002 196. Hogg P, Calthorpe M, Batterbury M, et al: Aqueous humor stimulates the migration of human trabecular meshwork cells
in vitro. Invest Ophthalmol Vis Sci 41:1091, 2000 197. Stefansson E, Wolbarsht ML, Landers MB: The corneal contact lens and aqueous humor hypoxia in cats. Invest Ophthalmol Vis Sci 24:1052, 1983 198. Stefansson E, Robinson D, Wolbarsht ML: Effects of epinephrine on PO2 in anterior chamber. Arch Ophthalmol 101:636, 1983 199. Heald K, Langham ME: Permeability of the cornea and the blood-aqueous barrier to oxygen. Br J Ophthalmol 40:705, 1956 200. Kleinstein RN, Kwan M, Fatt I, et al: In vivo aqueous humor oxygen tension-as estimated from measurements
on bare stroma. Invest Ophthalmol Vis Sci 21:415, 1981 201. Khodadoust AA, Stark WJ, Bill WR: Coagulation properties of intraocular humors and cerebrospinal fluid. Invest Ophthalmol Vis Sci 24:1616, 1983 202. Berzelius JJCJ: The ciliary epithelia and aqueous humor. In Hart WMJ, ed.: Adler's Physiology of the Eye, 9th ed. Philadelphia: CV Mosby, 1992:241 203. Varma SD, Reddy DVN: Phospholipid composition of aqueous humor plasma and lens in normal and
alloxan diabetic rabbits. Exp Eye Res 13:120, 1972 204. Kim JO, Cotlier E: Phospholipid distributions and fatty acid composition of lysophosphatidylcholine
in rabbit aqueous humor, lens and vitreous. Exp Eye Res 22:569, 1976 205. Obenberger J, Starka L, Hampl R: Quantitative determination of endogenous corticosteroids in the rabbit
plasma and aqueous humor. Graefe's Arch Clin Exp Ophthalmol 183:203, 1971 206. Andersson H: Monoamine metabolites in aqueous humor. J Pharmacol 24:998, 1972 207. Farkas TG, Plusec J: The occurrence of trivalent chromium in the aqueous and lens of rats. Invest Ophthalmol 5:398, 1966 208. Ainley RG, Phillips CI, Gibbs A: Aqueous humor vitamin B12 and intramuscular cobalamins. Br J Ophthalmol 53:854, 1969 209. Falbe-Hansen I, Degn JK: Sialic acid in the aqueous humor and the vitreous of normal human eyees
and of eyes with malignant melanoma of the choroid. Acta Ophthalmol 47:972, 1969 210. Laurent UBG: Hyaluronate in aqueous humor. Exp Eye Res 33:147, 1981 211. Lütjen-Drecoll E: Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res 18:91, 1998 212. Knepper PA, Mayanil CSK, Goossens W, et al: Aqueous humor in primary open-angle glaucoma contains an increased
level of CD44S. Invest Ophthalmol Vis Sci 43:133, 2002 213. Rauz S, Walker EA, Shackleton CHL, et al: Expression and putative role of 11β-hydroxysteroid dehydrogenase
isozymes within the human eye. Invest Ophthalmol Vis Sci 42:2037, 2001 214. Rauz S, Cheung CMG, Wood PJ, et al: Inhibition of 11β-hydroxysteroid dehydrogenase type 1 lowers
intraocular pressure in patients with ocular hypertension. Q J Med 96:481, 2003 215. Stokes J, Noble J, Brett L, et al: Distribution of glucocorticoid and mineralocorticoid receptors and 11β-hydroxysteroid
dehydrogenases in human and rat ocular tissues. Invest Ophthalmol Vis Sci 41:1629, 2000 216. Whorwood CB, Ricketts ML, Stewart PM: Regulation of sodium-potassium adenosine triphosphate subunit gene
expression by corticosteroids and 11β-hydroxysteroid dehydrogenase. Endocrinology 135:901, 1994 217. Ewart HS, Klip A: Hormonal regulation of Na+K+ATPase: Mechanisms underlying rapid
and sustained changes in pump activity. Am J Physiol 38:C295, 1995 218. Walker EA, Stewart PM: 11β-Hydroxysteroid dehydrogenase: Unexpected connections. Trends Endocrinol Metab 14:334, 2003 219. Cousins SW, McCabe MM, Danielpour D, et al: Identification of transforming growth factor-beta as an immunosuppressive
factor in aqueous humor. Invest Ophthalmol Vis Sci 32:2201, 1991 220. Granstein RD, Staszewski R, Knisely TL, et al: Aqueous humor contains transforming growth factor b and a small (<3500 dalton) inhibitor of thymocyte proliferation. J Immunol 144:3021, 1990 221. Jampel HD, Roche N, Stark WJ, et al: Transforming growth factor-beta in human aqueous humor. Curr Eye Res 9:963, 1990 222. Tripathi RC, Li J, Chan WF, et al: Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res 59:723, 1994 223. Wilbanks GA, Mammolenti M, Streilein JW: Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular
transforming growth factor. Eur J Immunol 22:165, 1992 224. Green H, Mann MJ, Paul SD: Elaboration of bicarbonate in intraocular fluids: V. Contribution of the
lens to chemistry of aqueous humor of the rabbit eye. Arch Ophthalmol 59:590, 1958 225. Bryson JM, Wolter JR, O'Keefe NT: Ganglion cells in the human ciliary body. Arch Ophthalmol 75:57, 1966 226. Becker B: Iodide transport by the rabbit eye. Am J Physiol 200:804, 1961 227. Bito LZ: Species differences in the response of the eye to irritation and trauma: A
hypothesis of divergence in ocular defense mechanisms, and the choice
of experimental animals for eye research. Exp Eye Res 39:807, 1984 228. Bito LZ: Prostaglandins: Old concepts and new perspectives. Arch Ophthalmol 105:1036, 1987 229. Bárány EH: Inhibition by hippurate and probenecid of in vitro uptake of iodipamide
and o-iodohippurate-composite uptake system for iodipamide
in choroid plexus, kidney cortex, and anterior uvea of several species. Acta Physiol Scand 86:12, 1972 230. Bárány EH: In vitro uptake of bile acids by choroid plexus, kidney cortex, and anterior
uvea: I. The iodipamide sensitive transprot systems in the rabbit. Acta Physiol Scand 93:250, 1975 231. Bárány EH: The liver-like anion transport system in rabbit kidney, uvea, and
choroid plexus: I. Selectivity of some inhibitors, dirction of transport, possible
physiological substrates. Acta Physiol Scand 88:412, 1973 232. Bárány EH: Organic cation uptake in vitro by the rabbit iris-ciliary body, renal
cortex, and choroid plexus. Invest Ophthalmol 15:341, 1976 233. Bárány EH: Elimination of organic cations from the eye. Klinische Monatsblatter fur Augenheilkunde 184:290, 1984 234. Goldmann H: Abflussdruck, minutemvolumen und Wider-stand der Kammerwasser-Stromung
des Menschen. Doc Ophthalmol 5-6:278, 1951 235. Langham ME, Taylor CB: The influence of superior cervical ganglionectomy on intraocular dynamics. J Physiol (Lond) 152:447, 1960 236. Langham ME, Rosenthal AR: The role of cervical sympathetic nerve in regulation of the intraocular
pressure and circulation. Am J Physiol 210:786, 1966 237. Bill A, Bárány EH: Gross facility, facility of conventional routes, and pseudofacility of
aqueous humor outflow in the cynomolgus monkey. Arch Ophthalmol 75:665, 1966 238. Bill A: Effects of longstanding stepwise increments in eye pressure on the rate
of aqueous humor formation in a primate (Cercopithecus ethiops). Exp Eye Res 12:184, 1971 239. Brubaker RF, Riley FCJ: The filtration coefficient of the blood-aqueous barrier. Invest Ophthalmol 11:752, 1972 240. Brubaker RF, Worthen DM: The filtration coefficient of the intraocular vasculature as measured by
low-pressure perfusion in a primate eye. Invest Ophthalmol 12:321, 1973 241. Kaufman PL: Aqueous humor dynamics following total iridectomy in the cynomolgus monkey. Invest Ophthalmol Vis Sci 18:870, 1979 242. Bito LZ: Accumulation and apparent active transport of prostaglandins by some rabbit
tissues in vitro. J Physiol 221:371, 1972 243. Grant WM: Tonographic method for measuring the facility and rate of aqueous flow
in human eyes. Arch Ophthalmol 44:204, 1950 244. Holm O, Krakau CET: Measurements of the flow of aqueous humor according to a new principle. Experientia 22:773, 1966 245. Holm O, Krakau CET: A method of measuring pupillary aqueous flow. Acta Ophthalmol 46:558, 1968 246. O'Rourke J, Macri FJ: Studies in uveal physiology: II. Clinical studies of the anterior chamber
clearance of isotopic tracers. Arch Ophthalmol 84:415, 1970 247. Nagataki S: Aqueous humor dynamics of human eyes as studied using fluorescein. Jpn J Ophthalmol 19:235, 1975 248. Jones RF, Maurice DM: New methods of measuring the rate of aqueous flow in man with fluorescein. Exp Eye Res 5:208, 1966 249. Araie M, Sawa M, Nagataki S, et al: Aqueous humor dynamics in man as studied by oral fluorescein. Jpn J Ophthalmol 24:346, 1980 250. Bárány EH, Kinsey VE: The rate of flow of aqueous humor: I. The rate of disappearance of para-aminohippuric
acid, radioactive rayopake, and radioactive diodrast
from the aqueous humor of rabbits. Am J Ophthalmol 32:177, 1949 251. Linnér E, Friedenwald JS: The appearance time of fluorescein as an index of aqueous flow. Am J Ophthalmol 44:225, 1957 252. Starr PA: Changes in aqueous flow determined by fluorophtometry. Trans Ophthalmol Soc UK 86:639, 1966 253. Bloom JN, Levene RZ, Thomas G, et al: Fluorophotometry and the rate of aqueous flow in man. I. Instrumentation
and normal values. Arch Ophthalmol 94:435, 1976 254. Langley D, MacDonald RK: Clinical method for observing changes in the rate of flow of aqueous humor
in the human eye: I. Normal eyes. Br J Ophthalmol 36:432, 1952 255. McLaren JW, Brubaker RF: A scanning ocular spectrofluorophotometer. Invest Ophthalmol Vis Sci 29:1285, 1988 256. Langham ME, Taylor CB: Fluorophotometric apparatus for the objective determination of fluorescence
in teh anterior chamber of the living eye. Br J Ophthalmol 38:52, 1954 257. Klein R, Ernest JT, Engerman RL: Fluorophotometry. I. Technique. Arch Ophthalmol 98:2231, 1980 258. Kinsey VE, Bárány EH: The rate of flow of aqueous humor: II. Derivation of rate of flow and its
physiological significance. Am J Ophthalmol 32:189, 1949 259. Goldmann H: Uber Fluorescein in der menschlichen Vord-erkammer. Das Kammerwasser-Minutenvolumen
des Menschen. Ophthalmologica 119:65, 1950 260. Hayashi M, Yablonski ME, Mindel JS: Methods for assisting the effects of pharmacologic agents on aqueous humor
dynamics. In Tasman W, Jaeger EA, eds.: Biomedical Foundations of Ophthalmology: Philadelphia: JB Lippincott, 1990:1–9 261. McLaren JW, Trocme SD, Relf S, et al: Rate of flow aqueous humor determined from measurements of aqueous flare. Invest Ophthalmol Vis Sci 31:339, 1990 262. Maurice DM: Theory and methodology of vitreous fluorophotometry. Jpn J Ophthalmol 29:119, 1985 263. Gaul GR, Brubaker RF: Measurement of aqueous flow in rabits with corneal and vitreous depots
of fluorescent dye. Invest Ophthalmol Vis Sci 27:1331, 1986 264. Becker B: The measurement of rate of aqueous flow with iodide. Invest Ophthalmol 1:52, 1962 265. Nagataki S, Brubaker RF: The effect of pilocarpine on aqueous humor formation in human beings. Arch Ophthalmol 100:818, 1982 266. Brubaker RF: Measurement with fluorophotometry: I. Plasma binding. II. Anterior segment, and
III. Aqueous humor flow. Graefes Arch Clin Exp Ophthalmol 222:190, 1985 267. Bárány EH: Simultaneous measurements of changing intraocular pressure and outflow
facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol 3:135, 1964 268. Bill A: Conventional and uveoscleral drainage of aqueous humor in the cynomolgus
monkey (Macaca irus) at normal and high intraocular pressu Res Exp Eye Res 5:45, 1966 269. Gabelt BT, Kaufman PL: The effect of prostaglandin F2a on trabecular outflow facility in cynomolgus monkeys. Exp Eye Res 51:87, 1990 270. Bill A: Circulation in the eye. In Renkin EM, Michel CC, eds.: The Cardiovascular System IV. Washington, DC: American Physiological Society, 1984:1001 271. Galin MA, Baras I, Mandell GKL: Measurement of aqueous flow utilizing the perilimbal suction cup. Arch Ophthalmol 66:65, 1961 272. Maus TL, McLaren JW, Shepard JWJ, et al: The effects of sleep on circulating catecholamines and aqueous flow in
human subjects. Exp Eye Res 62:351, 1996 273. Wetzel RK, Eldred WD: Specialized neuropeptide Y- and glucagon-like immunoractive
amacrine cells in the peripheral retina of the turtle. Vis NeuroSci 14:867, 1997 274. Wilson WS, Shahidullah M, Millar C: The bovine arterially-perfused eye: An in vitro method for the study of drug mechanisms on IOP, aqueous humour formation
and uveal vasculature. Curr Eye Res 12:609, 1993 275. Shahidullah M, Wilson WS, Millar C: Effects of timolol, terbutaline and forskolin on IOP, aqueous humour formation
and ciliary cyclic AMP levels in the bovine eye. Curr Eye Res 14:519, 1995 276. Bill A: Early effects of epinephrine on aqueous humor dynamics in vervet monkeys (Cercopithecus ethiops). Exp Eye Res 8:35, 1969 277. Bill A: Effects of norepinephrine, isoproterenol and sympathetic stimulation on
aqueous humour dynamics in vervet monkeys. Exp Eye Res 10:31, 1970 278. Townsend DJ, Brubaker RF: Immediate effect of epinephrine on aqueous formation in the normal human
eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci 19:256, 1980 279. Topper JE, Brubaker RF: Effects of timolol, epinephrine, and acetazolamide on aqueous flow during
sleep. Invest Ophthalmol Vis Sci 26:1315, 1985 280. Vareilles P, Silverstone D, Plazonnet B, et al: Comparison of the effects of timolol and other adrenergic agents on intraocular
pressure in the rabbit. Invest Ophthalmol 16:987, 1977 281. Gregory D, Sears M, Bausher L: Intraocular pressure and aqueous flow are decreased by cholera toxin. Invest Ophthalmol Vis Sci 20:371, 1981 282. Schmitt CJ, Lotti VJ, Varailles P, et al: β-Adrenergic blockers: Lack of relationship between antagonism
of isoproterenol and lowering of intraocular pressure in rabbits. In Sears M, ed.: New Directions in Ophthalmic Research. New Haven: Yale University Press, 1981:147–162 283. Caprioli J, Sears ML: Forskolin lowers intraocular pressure in rabbits, monkeys, and man. Lancet 1:958, 1983 284. Smith BR, Gaster RN, Leopold IH, et al: Forskolin, a potent adenylate cyclase activator, lowers rabbit intraocular
pressure. Arch Ophthalmol 102:146, 1984 285. Bartels SP, Lee SR, Neufeld AH: The effects of forskolin on cyclic AMP, intraocular pressure and aqueous
humor formation in rabbits. Curr Eye Res 6:307, 1987 286. Shibata T, Mishima H, Kurokawa T: Ocular pigmentation and intraocular pressure response to forskolin. Curr Eye Res 7:667, 1988 287. Elena PP, Fredj-Reygrobellet D, Moulin G, et al: Pharmacological characteristics of β-adrenergic adenylate cyclase
in nonpigmented and in pigmented cells of bovine ciliary processes. Curr Eye Res 3:1383, 1984 288. Nathanson JA: Adrenergic regulation of intraocular pressure: Identification of beta 2-adrenergic-stimulated adenylate cyclase in the ciliary
process epithelium. Proc Natl Acad Sci USA 77:7420, 1980 289. Nathanson JA: Effects of a potent and specific β2-adrenoceptor antagonist on intraocular pressure. Br J Ophthalmol 73:97, 1981 290. Nathanson JA: Differential inhibition of β-adrenergic receptors in human and
rabbit ciliary processes and heart. J Pharmacol Exp Ther 232:119, 1985 291. Nathanson JA: Biochemical and physiological effects of S-32-468, a new β-adrenoceptor
antagonist with possible oculoselectivity. Curr Eye Res 4:191, 1985 292. Nathanson JA: Atriopeptin-activated guanylate cyclase in the anterior segment. Identification, localization and effects of atriopeptins on IOP. Invest Ophthalmol Vis Sci 28:1357, 1987 293. Nathanson JA: Stereospecificity of beta adrenergic antagonists: R-enantiomers
show increased selectivity for beta-2 receptors in ciliary process. J Pharmacol Exp Ther 245:94, 1988 294. Nathanson JA: Direct application of a guanylate cyclase activator lowers intraocular
pressure. Eur J Pharmacol 147:155, 1988 295. Wax MB, Molinoff PB: Distribution and properties of β-adrenergic receptors in human
iris-ciliary body. Invest Ophthalmol Vis Sci 28:420, 1987 296. Millar C, Wilson WS: Comparison of the effects of vasodilator drugs on intraocular pressure
and vascular relaxation. Br J Pharmacol 104 (suppl):55, 1991 297. Burke JA, Potter DE: Ocular effects of a relatively selective α2-agonist (UK14304-18) in cats, rabbits, and monkeys. Curr Eye Res 5:665, 1986 298. Serle JB, Steidl S, Wang R-F, et al: Selective α2-adrenergic agonists B-HT 920 and UK14304-18. Effects
on aqueous humor dynamics in monkeys. Arch Ophthalmol 109:1158, 1991 299. Gabelt BT, Robinson JC, Hubbard WC, et al: Apraclonidine and brimonidine effects of anterior ocular and cardiovascular
physiology in normal and sympathectomized monkeys. Exp Eye Res 59:633, 1994 300. Gharagozloo NZ, Relf SJ, Brubaker RF: Aqueous flow is reduced by the alpha-adrenergic agonist, apraclonidine
hydrochloride (ALO 2145). Ophthalmology 95:1217, 1988 301. Burke J, Kharlamb A, Shan T, et al: Adrenergic and imidazoline receptor-mediated responses to UK-14,304-18 (brimonidine) in rabbits and monkeys. A
species difference. Ann NY Acad Sci 763:78, 1995 302. Allen RC, Langham ME: The intraocular pressure response of conscious rabbits to clonidine. Invest Ophthalmol 15:815, 1976 303. Harvey SC: Hypnotics and sedatives. In Gilman AG, Goodman LS, Rall TW, Murad R, eds.: , eds.: The Pharmacological Basis of Therapeutics, 7th ed. New York: Macmillan, 1985:339–371 304. Marshall BE, Longnecker DE: General anesthetics. In Gilman AG, Rall TW, Nies AS, Taylor P, eds.: The Pharmacological Basis of Therapeutics, 8th ed. New York: McGraw-Hill, Inc., 1993:285–310 305. Robinson JC, Kaufman PL: Dose-dependent suppression of aqueous humor formation by timolol
in the cynomolgus monkey. J Glaucoma. 2:251, 1993 306. Nilsson SFE, Maepea O, Samuelsson M, et al: Effects of timolol on terbutaline- and VIP-stimulated aqueous
humor flow in the cynomolgus monkey. Curr Eye Res 9:863, 1990 307. Gartner S: Blood vessels of the conjunctiva. Arch Ophthalmol 32:464, 1944 308. Wilke K: Early effects of epinephrine and pilocarpine on the intraocular pressure
and the episcleral venous pressure in the normal human eye. Acta Ophthalmol 52:231, 1974 309. Alm A, Bill A, Young FA: The effects of pilocarpine and neostigmine on the blood flow through the
anterior uvea in monkeys. A study with radioactively labelled microspheres. Res Exp Eye Res 15:31, 1973 310. James RG, Calkins JP: Effect of certain drugs on iris vessels. Arch Ophthalmol 57:414, 1957 311. Kolker AE, Hetherington JJ: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas, 4th ed. St. Louis: CV Mosby, 1976:325–334 312. Swan K, Hart W: A comparative study of the effects of mecholyl, doryl, pilocarpine, atropine, and
epinephrine on the blood-aqueous barrier. Am J Ophthalmol 23:1311, 1940 313. Bito LZ, Davson H, Snider N: The effects of autonomic drugs on mitosis and DNA synthesis in the lens
epithelium and on the composition of the aqueous humor. Exp Eye Res 4:54, 1965 314. Wålinder P-E: Influence of pilocarpine on iodopyracet and iodide accumulation by rabbit
ciliary body-iris preparations. Invest Ophthalmol 5:378, 1966 315. Wålinder P-E, Bill A: Influence of intraocular pressure and some drugs on aqueous flow and entry
of cycloleucine into the aqueous humor of vervet monkeys (Cercopithecus
ethiops). Invest Ophthalmol 8:446, 1969 316. Bárány EH: A mathematical formulation of intraocular pressure as dependent on secretion, ultrafiltration, bulk outflow, and osmotic reabsorption of fluid. Invest Ophthalmol 2:584, 1963 317. Gaasterland D, Kupfer C, Ross K: Studies of aqueous humor dynamics in man: IV. Effects of pilocarpine upon
measurements in young normal volunteers. Invest Ophthalmol 14:848, 1975 318. Kupfer C: Clinical significance of pseudofacility. Sanford R. Gifford Memorial Lecture. Am J Ophthalmol 75:193, 1973 319. Wålinder P-E, Bill A: Aqueous flow and entry of cycloleucine into the aqueous humor of vervet
monkeys (Cercopithecus ethiops). Invest Ophthalmol 8:434, 1969 320. Macri FJ, Cevario SJ: The induction of aqueous humor formation by the use of Ach + eserine. Invest Ophthalmol 12:910, 1973 321. Macri FJ, Cevario SJ: The dual nature of pilocarpine to stimulate or inhibit the formation of
aqueous humor. Invest Ophthalmol 13:617, 1974 322. Bill A: Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus
monkeys (macaca irus). Exp Eye Res 6:120, 1967 323. Bill A, Walinder P-E: The effects of pilocarpine on the dynamics of aqueous humor in a primate (macaca
irus). Invest Ophthalmol 5:170, 1966 324. Miichi H, Nagataki S: Effects of pilocarpine, salbutamol, and timolol on aqueous humor formation
in cynomolgus monkeys. Invest Ophthalmol Vis Sci 24:1269, 1983 325. Polansky JR, Wood IS, Maglio MT, et al: Trabecular meshwork cell culture in glaucoma research: Evaluation of biological
activity and structural properties of human trabecular cells
in vitro. Ophthalmology 91:580, 1984 326. Polansky JR, Bloom E, Konami D, et al: Cultured human trabecular cells: Evaluation of hormonal and pharmacological
responses in vitro. In Ticho U, David R, eds.: Recent Advances in Glaucoma. Amsterdam: Excerpta Medica, 1984:201–206 327. Carlson KH, Bourne WM, McLaren JW, et al: Variations in human corneal endothelial cell morphology and permeability
to fluorescein with age. Exp Eye Res 47:27, 1988 328. Lütjen-Drecoll E: Functional morphology of the ciliary epithelium. In Lütjen-Drecoll E, ed.: Basic Aspects of Glaucoma Research. Stuttgart: Schattauer, 1982:69–87 329. Bárány EH: Pseudofacility and uveoscleral outflow routes: Some nontechnical difficulties
in the determination of outflow facility rate and rate of formation
of aqueous humor. In Leydhecker W, ed.: Glaucoma Symposium. Tutzing Castle. Basel: Karger, 1966:27–51 330. Bill A: Further studies on the influence of the intraocular presssure on aqueous
humor dynamics in cynomolgus monkeys. Invest Ophthalmol 6:364, 1967 331. Goldmann H: On pseudofacility. Bibl Ophthalmol 76:1, 1968 332. Moses RA: Intraocular pressure. In Moses RA, ed.: Adler's Physiology of the Eye: Clinical Application. St Louis: CV Mosby, 1981:227 333. Kaufman PL, Wiedman T, Robinson JR: Cholinergics. In Sears ML, ed.: Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, 1984:149–191 334. Bill A: The effect of ocular hypertension caused by red cells on the rate of formation
of aqueous humor. Invest Ophthalmol 7:162, 1968 335. Mäepea O, Nilsson SF: Suppression of VIP- and terbutaline stimulated aqueous humor flow
by increased intraocular pressure in the cynomolgus monkey. Curr Eye Res 10:703, 1991 336. Householder JR, Clausen DF, Harris JE: The IOP sensitivity of the anterior chamber appearance time of intraarterially
injected fluorescein. Exp Eye Res 4:83, 1965 337. Hoskins HD, Jr, Kass MA: Medical treatment. In Hoskins HD, Jr, Kass MA, eds.: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas, 6th ed. ed. St. Louis: CV Mosby, 1989:405–493 338. Bill A: Aspects on suppressability of aqueous humor formation. Doc Ophthalmol 26:73, 1969 339. Kaufman PL, Bill A, Bárány EH: Formation and drainage of aqueous humor following total iris removal and
ciliary muscle disinsertion in the cynomolgus monkey. Invest Ophthalmol Vis Sci 16:226, 1977 340. Toris CB, Pederson JE: Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci 28:477, 1987 341. Toris CB, Gregerson DS, Pederson JE: Uveoscleral outflow using different-sized fluorescent tracers in
normal and inflamed eyes. Exp Eye Res 45:525, 1987 342. Cole DF: Reduction in aqueous humor formation as caused by iodate, spironolactone
and polyphloretin phosphate. Br J Ophthalmol 46:291, 1962 343. Maus TL, Young WFJ, Brubaker RF: Aqueous flow in humans after adrenalectomy. Invest Ophthalmol Vis Sci 35:3325, 1994 344. Gharagozloo NZ, Brubaker RF: The correlation between serum progesterone and aqeuous dynamics during
the menstrual cycle. Acta Ophthalmol 69:791, 1991 345. Horven I, Lilleaasen P, Aasen A: Intraocular pressure before, during, and after extracorporeal circulation
in pigs. Scand J Thorac Cardiovasc Surg 15:269, 1981 346. van Loenen AC, Van Bijsterveld OP, Nijkamp F: Some aspects of waterloading in rabbits. Doc Ophthalmol 56:345, 1984 347. Kolker AE: Hyperosmotic agents in glaucoma. Invest Ophthalmol Vis Sci 9:418, 1970 348. Lorimer DW, Hakanson NE, Pion PD, et al: The effect of intravenous mannitol or oral glycerol on intraocular pressure
in dogs. Cornell Vet 79:249, 1989 349. Gafter U, Pinkas M, Hirsch J: Intraocular pressure in uremic patients on chronic hemodialysis. Nephron 40:74, 1985 350. Zimmerman TJ: Topical ophthalmic beta blockers. A comparative review. J Ocular Pharmacol 9:373, 1993 351. Coakes RL, Brubaker RF: The mechanism of timolol in lowering intraocular pressure. Arch Ophthalmol 96:2045, 1978 352. Yablonski ME, Zimmerman TJ, Waltman SR: A fluorophotometric study of the effect of topical timolol on aqueous humor
dynamics. Exp Eye Res 27:135, 1978 353. Schenker JI, Yablonski ME, Podos SM, et al: Fluorophotometric study of epinephine and timolol in human subjects. Arch Ophthalmol 99:1212, 1981 354. Stewart RH, Kimbrough RL, Ward RL: Betaxolol vs. timolol. A six-month double-blind comparison. Arch Ophthalmol 104:46, 1986 355. Araie M, Takase M: Effects of S-596 and carteolol, new β-adrenergic blockers, and
flurbiprofen on the human eye: A fluorometric study. Graefes Arch Clin Exp Ophthalmol 222:259, 1985 356. Yablonski ME, Novack GD, Burke PJ: The effect of levobunolol on aqueous humor dynamics. Exp Eye Res 44:49, 1987 357. Mills KB, Wright G: A blind randomized cross-over trial comparing metipranolol 0.3% with
timolol 0.25% in open-angle glaucoma: A pilot
study. Br J Ophthalmol 70:39, 1986 358. Main BG, Tucker H: Recent advances in beta-adrenergic blocking agents. In Ellis GP, West GB, eds.: Progress in Medicinal Chemistry. Amsterdam: Elsevier, 1985:121–164 359. Novack GD: Ophthalmic β-blockers since timolol. Surv Ophthalmol 31:307, 1987 360. Bonomi L, Steindler P: Effect of pindolol on intraocular pressure. Br J Ophthalmol 59:301, 1975 361. Murray DL, Podos SM, Wei C, et al: Ocular effects in normal rabbits of topically applied labetalol, a combined α- and β-adrenergic antagonist. Arch Ophthalmol 97:723, 1979 362. Bouzoubaa M, Leclerc G, Decker N, et al: Synthesis and β-adrenergic blocking activity of new aliphatic
and alicyclic oxime ethers. J Med Chem 27:1291, 1984 363. Sugrue MF, Gautheron P, Grove J: MK-927: A topically active ocular hypotensive carbonic anhydrase
inhibitor. J Ocul Pharmacol 6:9, 1990 364. Becker B: Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, Diamox. Am J Ophthalmol 37:13, 1954 365. Hoskins HD Jr, Kass MA: Aqueous humor formation. In Klein EA, ed.: Becker-Shaffers's Diagnosis and Therapy of the Glaucomas, 6th ed. St. Louis: CV Mosby, 1989:18–40 366. Woltersdorf OWJ, Schwam H, Bicking JB: Topically active carbonic anhydrase inhibitors. 1. O-acyl derivatives
of 6-hydroxybenzothiazole-2-sulfonamide. J Med Chem 32:2486, 1989 367. Sugrue MF, Viader MP: Synthetic atrial natriuretic factor lowers rabbit intraocular pressure. Eur J Pharmacol 130:349, 1986 368. Millar JC, Carr RD, Humphries RG, et al: Drug effects on intraocular pressure and vascular flow in the bovine perfused
eye using radiolabelled microsphere. Res J Ocular Pharmacol Ther 11:11, 1995 369. Mittag TW, Tormay A, Ortega M, et al: Atrial natriuretic peptide (ANP), guanylate cyclase, and intraocular
pressure in the rabbit eye. Curr Eye Res 6:1189, 1987 370. Korenfeld MS, Becker B: Atrial natriuretic peptides: Effects on intraocular pressure, cGMP, and
aqueous flow. Invest Ophthalmol Vis Sci 30:2385, 1989 371. Karnezis TA, Murphy MB: Dopamine receptors and intraocular pressure. Trends Pharmacol Sci 9:389, 1988 372. Krupin T, Weiss A, Becker B, et al: Increased intraocular pressure following topical azide or nitroprusside. Invest Ophthalmol Vis Sci 16:1002, 1977 373. Wiederholt M, Sturm A, Lepple-Wienhues A: Relaxation of trabecular meshwork and ciliary muscle by release of nitric
oxide. Invest Ophthalmol Vis Sci 35:2515, 1994 374. Nathanson JA, McKee M: Identification of an extensive system of nitric oxide-producing
cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci 36:1765, 1995 375. Rasmussen CA, Gabelt BT, Kaufman PL: Effect of nitric oxide compounds on ciliary muscle in vitro [Abstract 5026]. Invest Ophthalmol Vis Sci 45:S5026, 2004 376. Kee C, Kaufman PL, Gabelt BT: Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci 35:2769, 1994 377. Becker B: Topical 8-bromo-cyclic BMP lowers intraocular pressure in
rabbits. Invest Ophthalmol Vis Sci 31:1647, 1990 378. Payne LJ, Slagle TM, Cheeks LT, et al: Effect of calcium channel blockers on intraocular pressure. Ophthalmic Res 22:337, 1990 379. Beatty JF, Krupin T, Nichols PF, et al: Elevation of intraocular pressure by calcium channel blockers. Arch Ophthalmol 102:1072, 1984 380. Chang FW, Burke JA, Potter DE: Mechanism of the ocular hypotensive action of ketanserin. J Ocular Pharmacol 1:137, 1985 381. Krootila K, Palkama A, Uusitalo H: Effect of serotonin and its antagonist (ketanserin) on intraocular
pressure in the rabbit. J Ocular Pharmacol 3:279, 1987 382. Barnett NL, Osborne NN: The presence of serotonin (5-HT1) receptors negatively
coupled to adenylate cyclase in rabbit and human ciliary processes. Exp Eye Res 57:209, 1993 383. Meyer-Bothling U, Bron AJ, Osborne NN: Topical application of serotonin or the 5-HT1a-agonist 5-CT
increases intraocular pressure in rabbits. Invest Ophthalmol Vis Sci 34:3035, 1993 384. Chidlow G, Cupido A, Melena J, et al: Flesinoxan, a 5-HT1A receptor agonist/alpha 1-adrenoceptor
antagonist, lowers intraocular pressure in NZW rabbits. Curr Eye Res 23:144, 2001 385. Gabelt BT, Millar CJ, Kiland JA, et al: Effects of serotonergic compounds on aqueous humor dynamics in monkeys. Curr Eye Res 23:120, 2001 386. May JC, McLaughlin MA, Sharif NA, et al: Evaluation of the ocular hypotensive response of serotonin 5-HT1A
and 5-HT-2 receptor ligands in conscious ocular hypertensive
cynomolgus monkeys. J Pharmacol Exp Ther 306:301, 2003 387. May JA, Chen HH, Rusinko A, et al: A novel and selective 5-HT2 receptor agonist with ocular hypotensive
activity: (S)-(+)-1-(2-aminopropyl)-8,9-dihydropyrano[3,2-e]indole. J Med Chem 46:4188, 2003 388. Mastropasqua L, Costagliola C, Ciancaglinin M, et al: Ocular hypotensive effect of ketanserin in patients with primary open angle
glaucoma. Acta Ophthalmol Scand (Suppl) 224:24, 1997 389. Takenaka H, Mano T, Maeno T, et al: The effect of anplag (sarpogrelate HCl), novel selective 5-HT2 antagonist
on intraocular pressure in glaucoma patients [Abstract 3390]. Invest Ophthalmol Vis Sci 36:S734, 1995 390. Okka M, Gabelt BT, Dean TR, et al: Aqueous humor dynamics in monkeys after topical R(+)-DOI. Invest Ophthalmol Vis Sci 45:5034, 2004 391. Crawford KS, Kaufman PL: Dose-related effects of prostaglandin F2a isopropylester on intraocular pressure, refraction and pupil diameter
in monkeys. Invest Ophthalmol Vis Sci 32:510, 1991 392. Sharif NA, Kelly CR, Crider JY, et al: Human ciliary muscle and trabecular meshwork cells express functional serotonin-2 (5HT2) receptors coupled to phosphoinositide
turnover and intracellular Ca2+ mobilization [Abstract 2084]. Invest Ophthalmol Vis Sci 44:S2084, 2003 393. Mallorga P, Sugrue MF: Characterization of serotonin receptors in the iris+ciliary body of
the albino rabbit. Curr Eye Res 6:527, 1987 394. De Vries GW, Mobasser A, Wheeler LA: Stimulation of endogenous cyclic AMP levels in ciliary body by SK&F28526, a
novel dopamine receptor agonist. Curr Eye Res 5:449, 1986 395. Chu E, Chu TC, Potter DE: Potential sites of action of TNPA: A dopamine-2 agonist. Exp Eye Res 69:611, 1999 396. Chu E, Chu TC, Potter DE: Mechanisms and sites of ocular action of 7-hydroxy-2-dipropylaminotetralin: A dopamine(3) receptor agonist. J Pharmacol Exp Ther 293:710, 2000 397. Leopold IH, Duzman E: Observations on the pharmacology of glaucoma. Ann Rev Pharmacol Toxicol 26:401, 1986 398. Chu E, Socci R, Chu TC: PD128,907 induces ocular hypotension in rabbits: Involvement of D2/D3 dopamine
receptors and brain natriuretic peptide. J Ocul Pharmacol Ther 20:15, 2004 399. Potter DE, Burke JA: Effects of ergoline derivatives on intraocular pressure and iris function
in rabbits and monkeys. Curr Eye Res 2:281, 1982 400. Potter DE, Burke JA, Chang FW: Ocular hypotensive action of ergoline derivatives in rabbits: Effects of
sympathectomy and domperidone pretreatment. Curr Eye Res 3:307, 1984 401. Langer SZ: Presynaptic regulation of the release of catecholamines. Phamacol Rev 32:337, 1980 402. Siegel MJ, Lee PY, Podos SM, et al: Effect of topical pergolide on aqueous dynamics in noral and glaucomatous
monkeys. Exp Eye Res 44:227, 1987 403. Mekki QA, Hassan SM, Turner P: Bromocriptine lowers intraocular pressure without affecting blood pressure. Lancet 1:1250, 1983 404. Mekki QA, Warrington SJ, Turner P: Bromocriptine eye-drops lower intraocular pressure without affecting
prolactin levels. Lancet 1:287, 1984 405. Geyer O, Robinson D, Lazar M: Hypotensive effect of bromocriptine in normal eyes. J Ocul Pharmacol 3:291, 1987 406. Chiou GCY: Treatment of ocular hypertension and glaucoma with dopamine antagonists. Ophthalmic Res 16:129, 1984 407. Chiou GCY: Ocular hypotensive actions of haloperidol, a dopaminergic antagonist. Arch Ophthalmol 102:143, 1984 408. Krupin T, Feitl M, Becker B: Effect of prazosin on aqueous humor dynamics in rabbits. Arch Ophthalmol 98:1639, 1980 409. Mittag TW: Ocular effects of selective alpha-adrenergic agents: A new drug
paradox? Ann Ophthalmol 15:201, 1983 410. Mittag TW, Tormay A, Severin C, et al: Alpha-adrenergic antagonists: Correlation of the effect on intraocular
pressure and on α2-adrenergic receptor binding specificity
in the rabbit eye. Exp Eye Res 40:591, 1985 411. Hedler L, Stamm G, Weitzell R, et al: Functional characterization of central α-adrenoceptors by yohimbine
diastereoisomers. Eur J Pharmacol 70:43, 1981 412. Serle JB, Stein AJ, Podos SM, et al: Coryanthine and aqueous humor dynamics in rabbits and monkeys. Arch Ophthalmol 102:1385, 1984 413. Jampel HD, Robin AL, Quigley HA, et al: Apraclonidine. A one-week dose-response study. Arch Ophthalmol 106:1069, 1988 414. Robin AL: Short-term effects of unilateral 1% apraclonidine therapy. Arch Ophthalmol 106:912, 1988 415. Abrams DA, Robin AL, Pollack IP, et al: The safety and efficacy of topical 1% ALO 2145 (p-aminoclonidine
hydrochloride) in normal volunteers. Arch Ophthalmol 105:1205, 1987 416. Coleman AL, Robin AL, Pollack IP, et al: Cardiovascular and intraocular pressure effects and plasma concentrations
of apraclonidine. Arch Ophthalmol 108:1264, 1990 417. Robin AL, Coleman AL: Apraclonidine hydrochloride: An evaluation of plasma concentrations, and
a comparison of its intraocular pressure lowering and cardiovascular
effects to timolol maleate. Trans Am Ophthalmol Soc 88:159, 1990 418. McCannel C, Koskela T, Brubaker RF: Topical flurbiprofen pretreatment does not block apraclonidine's effect
on aqueous flow in humans. Arch Ophthalmol 109:810, 1991 419. Schadlu R, Maus TL, Nau CB, et al: Comparison of the eficacy of apraclonidine and brimonidine as aqueous suppressants
in humans. Arch Ophthalmol 116:1441, 1998 420. Barnebey HS, Robin AL, Zimmerman TJ, et al: The efficacy of brimonidine in decreaseing elevations in intaocular pressure
after laser trabeculoplasty. Ophthalmology 100:1083, 1993 421. Igic R, Kojovic V: Angiotensin I converting enzyme (kininase II) in ocular tissues. Exp Eye Res 30:299, 1980 422. Vita JB, Anderson JA, Hulem CD, et al: Angiotensin-converting enzyme activity in ocular fluids. Invest Ophthalmol Vis Sci 20:255, 1981 423. Weinreb RN, Sandman R, Ryder MI, et al: Angiotensin-converting enzyme activity in human aqueous humor. Arch Ophthalmol 103:34, 1985 424. Ferrari-Dileo G, Ryan JW, Rockwook EJ, et al: Angiotensin-converting enzyme in bovine, feline, and human ocular
tissues. Invest Ophthalmol Vis Sci 29:876, 1988 425. Sramek SJ, Wallow IH, Tewksbury DA, et al: An ocular renin-angiotensin system. Immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci 33:1627, 1992 426. Wallow IH, Sramek SJ, Bindley CD, et al: Ocular renin angiotensin: EM immunocytochemical localization of prorenin. Curr Eye Res 12:945, 1993 427. Sramek SJ, Wallow IHL, Day RP, et al: Ocular renin-angiotensin: Immunohistochemical evidence for the presence
of prorenin in eye tissue. Invest Ophthalmol Vis Sci 29:1749, 1988 428. Watkins RW, Baum T, Cedeno K: Topical ocular hypotensive effects of the novel angiotensin converting
enzyme inhibitor SCH 33861 in conscious rabbits. J Ocul Pharmacol 3:295, 1987 429. Constad WH, Fiore P, Samson C, et al: Use of an angiotensin converting enzyme inhibitor in ocular hypertension
and primary open-angle glaucoma. Am J Ophthalmol 105:674, 1988 430. Krupin T, Siverstein B, Feitl M: The effect of H1-blocking antihistamines on intraocular pressure
in rabbits. Ophthalmology. 87:1167, 1980 431. Purnell WD, Gregg JM: Δ9-tetrahydrocannabinol, euphoria and intraocular pressure in man. Ann Ophthalmol 7:921, 1975 432. Green K, Pederson JE: Effect of Δ1 -tetrahydrocannabinol on aqueous dynamics and ciliary body permeability
in the rabbit. Exp Eye Res 15:499, 1973 433. Green K, Roth M: Ocular effects of topical administration of Δ9-tetrahydrocannabinol in man. Arch Ophthalmol 100:265, 1982 434. Jay WM, Green K: Multiple-drop study of topically applied 1% Δ9-tetrahydrocannabinol in human eyes. Arch Ophthalmol 101:591, 1983 435. Seeman JL, Gabelt BT, Kiland JA, et al: HU-210 and WIN 55,212,2 effects on IOP, pupil and refraction in
cynomolgus monkeys [Abstract 4501]. Invest Ophthalmol Vis Sci 42:S839, 2001 436. Chien FY, Wang RF, Mittag TW, et al: Effect of WIN 55212-2, a cannabinoid receptor agonist, on aqueous
humor dynamics in monkeys. Arch Ophthalmol 121:87, 2003 437. Oltmanns MH, Lattanzio FA, Allen RC, et al: Topical WIN 55 212-2 reduces intraocular pressure in ocular hypertensive
rats [Abstract 2093]. Invest Ophthalmol Vis Sci 45(suppl):S2093, 2004 438. Laine K, Järvinen K, Mechoulam R, et al: Comparison of the enzymatic stability and intraocular pressure effects
of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest Ophthalmol Vis Sci 43:3216, 2002 439. Song A-H, Slowey C-A: Involvement of cannabinoid receptors in the intraocular pressure-lowering
effects of WIN55212-2. J Pharmacol Exp Ther 292:136, 2000 440. Porcella A, Chiara M, Gessa GL, et al: The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure
in human glaucoma resistant to conventional therapies. Eur J Neurosci 13:409, 2001 441. Stamer WD, Golightly SF, Hosohata Y, et al: Cannabinoid CB(1) receptor expression, activation and detection
of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol 431:277, 2001 442. Straiker AJ, Maguire G, Mackei K, et al: Localization of cannabinoid CB1 receptors in the human anterior eye and
retina. Invest Ophthalmol Vis Sci 40:2442, 1999 443. Russell KR, Wang DR, Potter DE: Modulation of ocular hydrodynamics and iris function by bremeazocine, a
kappa opioid receptor agonist. Exp Eye Res 70:675, 2000 444. Ruskell KR, Potter DE: Dynorphin modulates ocular hydrodynamics and releases atrial natriuretic
peptide via activation of kappa-Opioid receptors. Exp Eye Res 75:259, 2002 445. Russell KR, Moore TT, Potter DE: Elevation of atrial natriuretic peptide levels in aqueous humor of the
rabbit by kappa opioid receptor agonists. Neuropeptides 35:232, 2001 446. Potter DE, Russell KR, Manhiani M: Bremazocine increases C-type natriuretic peptide levels in aqueous humor
and enhances outflow facility. J Pharmacol Exp Ther 309:548, 2004 447. Tisha T, Potter DE: Kappa opioid agonist-induced changes in IOP: Correlation with 3H-NE
release and cAMP accumulation. Exp Eye Res 73:167, 2001 448. Rasmussen CA, Gabelt BT, Russell KR, et al: Blood pressure effects on bremazocine IOP resp outflow facility in cynomolgus
monkeys [Abstract 3436]. Invest Ophthalmol Vis Sci 44:S3436, 2003 449. Anderson L, Wilson WS: Inhibition by indomethacin of the increased facility of outflow induced
by adrenaline. Exp Eye Res 50:119, 1990 450. Coakes RL, Siah PB: Effects of adrenergic drugs on aqueous humor dynamics in the normal human
eye. I. Salbutamol. Br J Ophthalmol 68:393, 1984 451. Larson RS, Brubaker RF: Isoproterenol stimulates aqueous flow in humans with Horner's syndrome. Invest Ophthalmol Vis Sci 29:621, 1988 452. Gharagozloo NZ, Larson RS, Kullerstrand W: Terbutaline stimulates aqueous humor flow in humans during sleep. Arch Ophthalmol 106:1218, 1988 453. Lee DA, Topper JE, Brubaker RF: Effect of clonidine on aqueous humor flow in normal human eyes. Exp Eye Res 38:239, 1984 454. Fechtner RD: Beta blockers. In: Glaucoma Medical Therapy Principles and Management. San Francisco: The
Foundation of the American Academy of Ophthalm 1999:25–39 455. Becker B: Does hyposecretion of aqueous humor damage the trabecular meshwork? J Glaucoma. 4:303, 1995 456. Lütjen-Drecoll E, Kaufman PL: Long-term timolol and epinephrine in monkeys. II. Morphological
alterations in trabecular meshwork and ciliary muscle. Trans Ophthalmol Soc UK 105:196, 1986 457. Hoskins HD, Kass MA: Aqueous humor outflow. In Hoskins HD, Kass MA, eds.: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. St. Louis: CV Mosby, 1989:41–66 458. Hoskins HD, Kass MA: Secondary open-angle glaucoma. In Hoskins HD, Kass MA, eds.: Becker-Shaffer's Diagnosis and Therapy of the glaucomas. St. Louis: CV Mosby, 1989:308–350 459. Toris CB, Yablonski ME, Wang YL, et al: Aqueous humor hynamics in the aging human eye. Am J Ophthalmol 127:407, 1999 460. Sperber GO, Bill A: A method for near-continuous determination of aqueous humor flow: Effects
of anaesthetics, temperature and indomethacin. Exp Eye Res 39:435, 1984 461. Suguro K, Toris CB, Pederson JE: Uveoscleral outflow following cyclodialysis in the monkey eye using a fluorescent
tracer. Invest Ophthalmol Vis Sci 26:810, 1985 462. Friedenwald JS: Contribution to the theory and practice of tonometry. Am J Ophthalmol 20:985, 1937 463. Friedenwald JS: Tonometer calibration: An attempt to remove discrepancies found in the 1954 calibration
scale for Schiotz tonometers. Trans Am Acad Ophthalmol Otolaryngol 61:108, 1957 464. Prijot E, Weekers R: Mesure de la resistance a l'ecoulement de l'humeur aqueuse au
moyen du tonometre electonique. Ophthalmologica 123:1, 1952 465. Topper JE, McLaren J, Brubaker RF: Measurement of aqueous humor flow with scanning ocular fluorophotometers. Curr Eye Res 3:1391–1395,1984 466. Rosengren B: A method for producing intraocular rise of tension. Acta Ophthalmol 12:403, 1934 467. Itoi M: Pv tonography: Tonometers for Pv tonography. Nippon Ganka Gakkai Zasshi 76:97, 1972 468. Yablonski ME, Cook DJ, Gray J: A fluorophotometric study of the effect of argon laser trabeculoplasty
on aqueous humor dynamics. Am J Ophthalmol 99:579, 1985 469. Toris CB, Gleason ML, Camras CB, et al: Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol 113:1514, 1995 470. Toris CB, Tafoya ME, Camras CB, et al: Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology. 102:456–461,1995 471. Toris CB, Zhan GL, Camras CB, McLaughlin MA: Effects of travoprost on aqueous humor dynamics in monkeys. J Glaucoma 14:70, 2005 472. McLaughlin MA, Toris CB, Brooks DE, et al: Effects of Rho kinase inhibitors on intraocular pressure (IOP) and
aqueous humor dynamics in rabbits and monkeys [Abstract 3534]. Invest Ophthalmol Vis Sci 45(suppl):S3534, 2004 473. Tafoya ME, Toris CB, Camras CB, et al: Effects of apraclonidine on aqueous humor dynamics in human eyes. Invest Ophthalmol Vis Sci 34:929, 1993 474. Toris CB, Yablonski ME, Wang YL, Camras CB: Aqueous humor dynamics in the aging human eye. Am J Ophthalmol 127:407, 1999 475. Toris CB, Zhan GL, Zhao J, et al: Potential mechanism for the additivity of pilocarpine and latanoprost. Am J Ophthalmol 131:722, 2001 476. Toris CB, Camras CB, Yablonski ME: Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma 11:253, 2002 477. Ashton N, Brini A, Smith R: Anatomical studies of the trabecular meshwork of the normal human eye. Br J Ophthalmol 40:257, 1956 478. Rohen JW, Lütjen E, Bárány E: The relation between the ciliary muscle and the trabecular meshwork and
its importance for the effect of miotics on aqueous outflow resistance. Albrecht von Graefes Arch Klin Exp Ophthalmol 172:23, 1967 479. Rohen JW, Futa R, Lütjen-Drecoll E: The fine structure of the cribriform meshwork in normal and glaucomatous
eyes as seen in tangential sections. Invest Ophthalmol Vis Sci 21:574, 1981 480. Vegge T: Ultrastructure of normal human trabecular endothelium. Acta Ophthalmol 41:193, 1963 481. Holmberg AS: Schlemm's canal and the trabecular meshwork. An electron microscopic
study of the normal structure in man and monkey (Cercopithecus ethiops). Doc Ophthalmol 19:339, 1965 482. Spencer WH, Alvarado J, Hayes TL: Scanning electron microscopy of human ocular tissues: The trabecular meshwork. Invest Ophthalmol 7:651, 1968 483. Peterson WS, Jocson VL: Hyaluronidase effects on aqueous outflow resistance: Quantitative and localizing
studies in the rhesus monkey eye. Am J Ophthalmol 77:573, 1974 484. Grant WM: Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 69:783, 1963 485. Ellingsen BA, Grant WM: Influence of intraocular pressure and trabeculotomy on aqueous outflow
in enucleated monkey eyes. Invest Ophthalmol 10:705, 1971 486. Ellingsen BA, Grant WM: Trabeculotomy and sinusotomy in enucleated human eyes. Invest Ophthalmol 11:21, 1972 487. Erickson-Lamy K, Rohen JW, Grant WM: Outflow facility studies in the perfused human ocular anterior segment. Exp Eye Res 52:723, 1991 488. Bahler CK, Fautsch MP, Hann CR, et al: Factors influencing intraocular pressure in cultured human anterior segments. Invest Ophthalmol Vis Sci 45:3137, 2004 489. Bahler CK, Hann CR, Fautsch MP, et al: Pharmacologic disruption of Schlemm's canal cells and outflow facility
in anterior segments of human eyes. Invest Ophthalmol Vis Sci 45:2246, 2004 490. Rosenquist R, Epstein DL, Melamed S, et al: Outflow resistance of enucleated human eyes perfused at two different perfusion
pressures and different extents of trabeculotomy. Curr Eye Res 8:1233, 1989 491. Inomata H, Bill A, Smelser GK: Aqueous humor pathways through the trabecular meshwork and into Schlemm's
canal in the cynomolgus monkey (Macaca irus): An electron
microscopic study. Am J Ophthalmol 73:760, 1972 492. Raviola G, Raviola E: Paracellular route of aqeuous outflow in the trabecular meshwork and canal
of Schlemm. A freeze-fracture study of the endothelial junctions
in the sclerocorneal angle of the macaque monkey eye. Invest Ophthalmol 21:52, 1981 493. Alvarado JA, Yun AJ, Murphy CG: Juxtacanalicular tissue in primary open-angle glaucoma and in nonglaucomatous
normals. Arch Ophthalmol 104:1517, 1986 494. Murphy CG, Johnson M, Alvarado JA: Juxtacanalicular tissue in pigmentary and primary open-angle glaucoma. The
hydrodynamic role of pigment and other constituents. Arch Ophthalmol 110:1779, 1992 495. Hamard P, Valtot F, Sourdille P, et al: Confocal microscopic examination of trabecular meshwork removed during
ab externo trabeculectomy. Br J Ophthalmol 86:1046, 2002 496. Gong H, Ruberti J, Overby D, et al: A new view of the human trabecular meshwork using quick-freeze, deep-etch
electron microscopy. Exp Eye Res 75:347, 2002 497. Sabanay I, Gabelt BT, Tian B, et al: H-7 effects on structure and fluid conductance of monkey trabecular
meshwork. Arch Ophthalmol 118:955, 2000 498. Sabanay I, Tian B, Gabelt BT, et al: Functional and structural reversibility of H-7 effects on the conventional
aqueous outflow pathway in monkeys. Exp Eye Res 78:137, 2004 499. Overby D, Gong H, Qiu G, et al: The mechanism of increasing outflow facility during washout in the bovine
eye. Invest Ophthalmol Vis Sci 42:3455, 2002 500. Homberg AS: Our present knowledge of the structure of the trabecular meshwork. In Leydhecker W, ed.: Glaucoma Twentieth International Congress of Ophthalmology, Tutzing Symposium. Basel: S. Karger, 1966 501. Johnstone MA: The morphology of the aqueous outflow system. In Drance SM, Neufeld AH, eds.: Glaucoma: Applied Pharmacology. New York: Grune & Stratton, 1984:87–109 502. Feeney L: Outflow studies using an electron dense tracer. Trans Am Acad Ophthalmol Otolaryngol 70:792, 1966 503. Anderson DR: Scanning electron microscopy of primate trabecular meshwork. Am J Ophthalmol 71:90, 1971 504. Kayes J: Pressure gradient changes on the trabecular meshwork of monkeys. Am J Ophthalmol 79:549, 1975 505. Shabo AL, Reese TS, Gaasterland D: Postmortem formation of giant endothelial vacuoles in Schlemm's canal
of monkey. Am J Ophthalmol 76:896, 1973 506. van Buskirk EM, Grant WM: Lens depression and aqueous outflow in enucleated primate eyes. Am J Ophthalmol 76:632, 1973 507. Tripathi RC: The functional morphology of the outflow systems of ocular and cerebrospinal
fluids. Exp Eye Res 25(suppl):65, 1977 508. Tripathi RC: Mechanism of the aqueous outflow across the trabecular wall of Schlemm's
canal. Exp Eye Res 11:116, 1971 509. Tripathi RC: Aqueous outflow pathway in normal and glaucomatous eyes. Br J Ophthalmol 56:157, 1972 510. Cole DF, Tripathi RC: Theoretical considerations on the mechanism of the aqueous outflow. Exp Eye Res 12:25, 1971 511. Epstein DL, Rohen JW: Morphology of the trabecular meshwork and inner wall endothelium after
cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci 32:160, 1991 512. Grierson I, Lee WR: Pressure-induced changes in the ultrastructure of the endothelium
lining of Schlemm's canal. Am J Ophthalmol 80:863, 1975 513. Brilakis HS, Johnson DH: Giant vacuole survival time and implications for aqeuous humor outflow. J Glaucoma 10:277, 2001 514. Bill A, Svedbergh B: Scanning electron microscopic studies of the trabecular meshwork and the
canal of Schlemm: An attempt to localize the main resistance to outflow
of aqueous humor in man. Acta Ophthalmol 50:295, 1972 515. Johnson M, Shapiro A, Ethier CR, et al: Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci 33:1670, 1992 516. Johnson M, Chan D, Read AT, et al: The pore density in the inner wall endothelium of Schlemm's canal
of glaucomatous eyes. Invest Ophthalmol Vis Sci 43:2950, 2002 517. Pollack IP, Becker B, Constant MA: The effect of hypothermia on aqueous humor dynamics: I. Intraocular pressure
and outflow facility of the rabbit eye. Am J Ophthalmol 49:1126, 1960 518. Stamer WD, Snyder RW, Smith BL, et al: Localization of aquaporin CHIP in the human eye: Implications in the pathogenesis
of glaucoma and other cisorders of ocular fluid balance. Invest Ophthalmol Vis Sci 35:3867, 1994 519. Hamann S, Zeuthern T, La Cour M, et al: Aquaporins in complex tissues: Distribution of aquaporins 1-5 in
human and rat eye. Am J Physiol 274:C1332, 1998 520. Stamer WD, Seftor RE, Snyder RW, et al: Cultured human trabecular meshwork cells express aquaporin-1 water
channels. Curr Eye Res 14:1095, 1995 521. Stamer WD, Peppel K, O'Donnell ME, et al: Expression of aquaporin-1 in human trabecular meshwork cells: Role
in resting cell volume. Invest Ophthalmol Vis Sci 42:1803, 2001 522. Brandt JD, O'Donnell ME: How does the trabecular meshwork regulate outflow? Clues from the vascular
endothelium [Review]. J Glaucoma 8:328, 1999 523. O'Donnell ME, Brandt JD, Curry F-RE: Na-K-Cl cotransport regulates intracellular volume and monolayer
permeablility of trabecular meshwork cells. Am J Physiol 268:C1067, 1995 524. Al-Aswad LA, Gong H, Lee D, et al: Effects of Na-K-2Cl cotransport regulators on outflow facility
in calf and human eyes in vitro. Invest Ophthalmol Vis Sci 40:1695, 1999 525. Gabelt BT, Wiederholt M, Clark AF, et al: Anterior segment physiology following bumetanide inhibition of Na-K-Cl
cotransport. Invest Ophthalmol Vis Sci 38:1700, 1997 526. Putney LK, Brandt JD, O'Donnell ME: Na-K-Cl cotransport in normal and glaucomatous human trabecular
meshwork cells. Invest Ophthalmol Vis Sci 40:425, 1999 527. Mitchell CH, Fleischhauer JC, Stamer WD, et al: Human trabecular meshwork cell volume regulation. Am J Physiol Cell Physiol. 283:C315, 2002 528. Johnstone MA: The aqueous outflow system as a mechanical pump. Evidence from examination
of tissue and aqueous movement in human and non-human primates. J Glaucoma 13:421, 2004 529. Tamm ER, Flügel C, Stefani FH, et al: Nerve endings with structural characteristics of mechanoreceptors in the
human scleral spur. Invest Ophthalmol Vis Sci 35:1157, 1994 530. Ruskell GL: Trigeminal innervation of the scleral spur in cynomolgus monkeys. J Anat 184:511, 1994 531. Tamm ER, Koch TA, Mayer B, et al: Innervation of myofibroblast-like scleral spur cells in human and
monkey eyes. Invest Ophthalmol Vis Sci 36:1633, 1995 532. Holland M, Sallman LV, Collins E: A study of the innervation of the chamber angle. Am J Ophthalmol 42:148, 1956 533. Stone RA, McGlinn AM: Calcitonin gene-related peptide immunoreactive nerves in human and
rhesus monkey eyes. Invest Ophthalmol Vis Sci 29:305, 1988 534. Laties AM, Stone RA, Brecha NC: Substance P-like immunoreactive nerve fibers in the trabecular meshwork. Invest Ophthalmol Vis Sci 21:484, 1981 535. Stone RA, Laties AM, Brecha NC: Substance P-like immunoreactive nerves in the anterior segment of
the rabbit, cat, and monkey eye. Neuroscience 7:2459, 1982 536. Stone RA, Laties AM, Emson PC: Neuropeptide Y and the ocular innervation of rat, guinea pig, cat, and
monkey. Neuroscience. 17:1207, 1986 537. Selbach JM, Gottanka J, Wittmann M, et al: Efferent and afferent innervation of primate trabecular meshwork and scleral
spur. Invest Ophthalmol Vis Sci 41:2184, 2000 538. Gabelt BT, Robinson JC, Gange SJ, et al: Superior cervical ganglionectomy in monkeys: Aqueous humor dynamics and
their responses to drugs. Exp Eye Res 60:575, 1995 539. Wentworth WO, Brubaker RF: Aqueous humor dynamics in a series of patients with third neuron Horner's
syndrome. Am J Ophthalmol 92:407, 1981 540. Hubbard WC, Robinson JC, Schmidt K, et al: Superior cervical ganglionectomy in monkeys: Effects on refraction and
intraocular pressure. Exp Eye Res 68:637, 1999 541. Marshall GE, Konstas AG, Lee WR: Immunogold localization of type IV collagen and laminin in the aging human
outflow system. Exp Eye Res 51:691, 1990 542. Marshall GE, Konstas AG, Lee WR: Immunogold ultrastructural localization of collagens in the aged human
outflow system. Ophthalmology. 98:692, 1991 543. Hann CR, Springett MJ, Wang X, et al: Ultrastructural localization of collagen IV, fibronectin, and laminin in
the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res 33:314, 2001 544. Ueda J, Wentz-Hunter K, Yue BYJT: Distribution of myocilin and extracellular matrix components in the juxtacanalicular
tissue of human eyes. Invest Ophthalmol Vis Sci 43:1068, 2002 545. Bhatt K, Gong H, Freddo TF: Freeze-fracture studies of interendothelial junctions in the angle
of the human eye. Invest Ophthalmol Vis Sci 36:1379, 1995 546. Ye W, Gong H, Sit A, et al: Interendothelial junctions in normal human schlemm's canal respond
to changes in pressure. Invest Ophthalmol Vis Sci 38:2460, 1997 547. Alvarado JA, Murphy CG: Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol 110:1769, 1992 548. Shirato S, Murphy CG, Bloom E, et al: Kinetics of phagocytosis in trabecular meshwork cells: Flow cytometry and
morphometry. Invest Ophthalmol Vis Sci 30:2499, 1989 549. Zhou L, Fukuchi T, Kawa JE, et al: Loss of cell-matrix cohesiveness after phagocytosis by trabecular
meshwork cells. Invest Ophthalmol Vis Sci 36:787, 1995 550. Kaufman PL, Gabelt BT: Presbyopia, prostaglandins and primary open angle glaucoma. In Krieglstein GK, ed.: Glaucoma Update V Proceedings of the Symposium of the Glaucoma Society
of the International Congress of Ophthalmology in Quebec City, June 1994. New York-Berlin: Springer-Verlag, 1995:224–241 551. Funk RH, Rohen JW: Scanning electron microscopic study of episcleral arterioveous anastomoses
in the owl and cynomolgus monkey. Curr Eye Res 15:321, 1996 552. Ascher KW: Aqueous veins: Their status eleven years after their detection. Arch Ophthalmol 49:438, 1953 553. Sears ML: The mechanism of action of adrenergic drugs in glaucoma. Invest Ophthalmol 5:115, 1966 554. Krasnov MM: Microsurgery of glaucoma. Indications and choice of techniques. Am J Ophthalmol 67:857, 1969 555. Ellingsen BA, Grant WM: The relationship of pressure and aqueous outflow in enucleated human eyes. Invest Ophthalmol 10:430, 1971 556. Lütjen-Drecoll E, Futa R, Rohen JW: Ultrahistochemical studies on tangential sections of the trabecular meshwork
in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 21:563, 1981 557. Rohen JW: The evolution of the primate eye in relation to the problem of glaucoma. In Lutjen-Drecoll E, ed.: Basic Aspects of Glaucoma Research. Stuttgart: Schattauer, 1982:3–33 558. Rohen JW: Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical
considerations. Ophthalmology 90:758, 1983 559. Lütjen-Drecoll E, Rohen JW: Morphologic basis for drug action on aqueous outflow. In Drance SM, ed.: Applied Pharmacology in the Medical Treatment of Glaucomas. New York: Grune & Stratton, 1984:459–475 560. Tamm E, Flügel C, Stefani FH, et al: Contractile cells in the human scleral spur. Exp Eye Res 54:531, 1992 561. Kaufman PL, Bárány EH: Residual pilocarpine effects on outflow facility after ciliary muscle disinsertion
in the cynomolgus monkey. Invest Ophthalmol 15:558, 1976 562. Kaufman PL, Bárány EH: Loss of acute pilocarpine effect on outflow facility following surgical
disinsertion and retrodisplacement of the ciliary muscle from the scleral
spur in the cynomolgus monkey. Invest Ophthalmol 15:793, 1976 563. Kaufman PL, Bárány EH: Recent observations concerning the effects of cholinergic drugs on outflow
facility in monkeys. In Bito LZ, Davson H, Fenstermacher JD, eds.: The Ocular and Cerebrospinal Fluids. Fogarty International Center Symposium, 1977:415–418 564. James RJ, Croft MA, Rasmussen CA, et al: Effect of centrally stimulated ciliary muscle contraction on outflow facility
in young rhesus monkeys [Abstract 4669]. Invest Ophthalmol Vis Sci 45(suppl):S4669, 2004 565. Armaly MF, Burian HM: Changes in the tonogram during accommodation. Arch Ophthalmol 60:60, 1958 566. Flügel C, Bárány EH, Lütjen-Drecoll E: Histochemical differences within the ciliary muscle and its function in
accommodation. Exp Eye Res 50:219, 1990 567. Erickson-Lamy KA, Kaufman PL: Effect of cholinergic drugs on outflow facility after ciliary ganglionectomy. Invest Ophthalmol Vis Sci 29:491, 1988 568. Erickson-Lamy K, Schroeder A: Dissociation between the effect of aceclidine on outflow facility and accommodation. Exp Eye Res 50:143, 1990 569. Gabelt BT, Kaufman PL: Inhibition of outflow facility, accommodative, and miotic responses to
pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J Pharmacol Exp Ther 263:1133, 1992 570. Gabelt BT, Kaufman PL: Inhibition of aceclidine-stimulated outflow facility, accommodation
and miosis by muscarinic receptor subtype antagonists in rhesus monkeys. Exp Eye Res 58:623, 1994 571. Poyer JF, Kaufman PL, Flügel C: Age does not affect contractile responses of the isolated rhesus monkey
ciliary muscle to muscarinic agonists. Curr Eye Res 12:413, 1993 572. Poyer JF, Gabelt BT, Kaufman PL: The effect of muscarinic agonists and selective receptor subtype antagonists
on the contractile response of the isolated rhesus monkeys ciliary
muscle. Exp Eye Res 59:729, 1994 573. Ehinger B: Adrenergic nerves to the eye and to related structures in man and in the
cynomolgus monkey (Macaca irus). Invest Ophthalmol 5:42, 1966 574. van Alphen GWHM, Robinette SL, Macri FJ: Drug effects on ciliary muscle and choroid preparations in vitro. Arch Ophthalmol 68:111, 1962 575. van Alphen GWHM, Kern R, Robinette SL: Adrenergic receptors of the intraocular muscles. Comparison to cat rabbit, and
monkey. Arch Ophthalmol 74:253, 1965 576. Törnqvist G: Effect of cervical sympathetic stimulation on accommodation in monkeys. An
example of a beta-adrenergic inhibitory effect. Acta Physiol Scand. 67:363, 1966 577. Kaufman PL: The effects of drugs on the outflow of aqueous humor. In Drance SM, Neufeld AH, eds.: Glaucoma: Applied Pharmacology in Medical Treatment. Orlando: Grune & Stratton, 1984:429–458 578. Kaufman PL, Bárány EH: Adrenergic drug effects on aqueous outflow facility following ciliary muscle
retrodisplacement in the cynomolgus monkey. Invest Ophthalmol Vis Sci 20:644, 1981 579. Nilsson SFE, Bill A: Physiology and neurophysiology of aqueous humor inflow and outflow. In Kaufman PL, Mittag TW, eds.: Glaucoma. London: Mosby-Yearbook Europe Ltd., 1994:1.17–1.34 580. Wax MB, Coca-Prados M: Receptor-mediated phosphoinositide hydrolysis in human ocular ciliary
epithelial cells. Invest Ophthalmol Vis Sci 30:1675, 1989 581. Alvarado JA, Franse-Carman L, McHolm G, et al: Epinephrine effects on major cell types of the aqueous outflow pathway: In
vitro studies/clinical implications. Trans Am Ophthalmol Soc 88:267, 1990 582. Sears ML, Neufeld AH: Adrenergic modulation of the outflow of aqueous humor. Invest Ophthalmol 14:83, 1975 583. Thomas JV, Epstein DL: Timolol and epinephrine in primary open angle glaucoma. Transient additive
effect. Arch Ophthalmol 99:91, 1981 584. Cyrlin MN, Thomas JV, Epstein DL: Additive effect of epinephrine to timolol therapy in primary open-angle
glaucoma. Arch Ophthalmol 100:414, 1982 585. Allen RC, Epstein DL: Additive effects of betaxolol and epinephrine in primary open angle glaucoma. Arch Ophthalmol 104:1178, 1986 586. Robinson JC, Kaufman PL: Effects and interactions of epinephrine, norepinephrine, timolol and betaxolol
on outflow facility in the cynomolgus monkey. Am J Ophthalmol 109:189, 1990 587. Busch MJWM, van Oosterhout JGM, Hoyng PFJ: Effects of cyclic nucleotide analogs on intraocular pressure and trauma-induced
inflammation in the rabbit eye. Curr Eye Res 11:5, 1992 588. Neufeld AH, Dueker DK, Vegge T, et al: Adenosine 3',5'-monophosphate increases the outflow of
aqueous humor from the rabit eye. Invest Ophthalmol Vis Sci 14:40, 1975 589. Kaufman PL: Adenosine 3',5' cyclic monophosphate and outflow facility in
monkey eyes with intact and retrodisplaced ciliary muscle. Exp Eye Res 44:415, 1987 590. Erickson-Lamy KA, Nathanson JA: Epinephrine increases facility of outflow and cyclic AMP content in the
human eye in vitro. Invest Ophthalmol Vis Sci 33:2672, 1992 591. Crawford KS, Robinson JC, Heideman W, et al: Indomethacin and epinephrine effects on outflow facility and cAMP formation
in monkeys. Invest Ophthalmol Vis Sci 37:1348, 1996 592. Erickson K, Liang L, Shum P, et al: Adrenergic regulation of aqueous outflow. J Ocular Pharmacol 10:241, 1994 593. Alvarado JA, Murphy CG, Franse-Carman L, et al: Effect of β-adrenergic agonists on paracellular width and fluid
flow across outflow pathway cells. Invest Ophthalmol Vis Sci 39:1813, 1998 594. Wiederholt M: Direct involvement of trabecular meshwork in the regulation of aqueous
humor outflow. Curr Opin Ophthalmol. 9:46, 1998 595. Robinson JC, Kaufman PL: Cytochalasin B potentiates epinephrine's outflow facility increasing
effect. Invest Ophthalmol Vis Sci 32:1614, 1991 596. Robinson JC, Kaufman PL: Phalloidin inhibits epinephrine's and cytochalsin B's facilitation
of aqueous outflow. Arch Ophthalmol 112:1610, 1994 597. Tripathi BJ, Tripathi RC: Effect of epinephrine in vitro on the morphology, phagocytosis, and mitotic
activity of human trabecular endothelium. Exp Eye Res 39:731, 1984 598. Lütjen-Drecoll E, Shimuzu R, Rohrbach M, et al: Quantitative analysis of "plaque material" in the inner and outer wall
of Schlemm's canal in normal and glaucomatous eyes. Exp Eye Res 42:443, 1986 599. Rodriques M, Spaeth G, Weinreb S, et al: Spectrum of trabecular pigmentation in open-angle glaucoma: A clinicopathologi
study. Trans Am Acad Ophthalmol Otolaryngol 81:258, 1976 600. Alvarado J, Murphy C, Juster R, et al: Studies on the pathogenesis of primary open-angle glaucoma: Regional
analysis of trabecular meshwork cellularity and dense collagen. In Ticho U, David R, eds.: Recent Advances in Glaucoma. New York: Elsevier Science Publishing, 1984:3–8 601. Grierson I: What is open angle glaucoma? Eye 1:15, 1987 602. Rohen JW, Lutjen-Drecoll E: Age changes of the trabecular meshwork in human and monkey eyes. A light
and electron microscopic study. Altern Entwickl Aging Dev 1:1, 1971 603. Gabelt BT, Kaufman PL: Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res May 23:[Epub ahead of print], 2005 604. Tian B, Geiger B, Epstein DL, et al: Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci 41:619, 2000 605. Volberg T, Geiger B, Citi S, et al: Effect of protein kinase inhibitor H-7 on the contractility, integrity
and membrane anchorage of the microfilament system. Cell Motility Cytoskel 29:321, 1994 606. Birrell GB, Hedberg KK, Habliston DL, et al: Protein kinase C inhibitor H-7 alters the actin cytoskeleton of
cultured cells. J Cell Physiol 141:74, 1989 607. Liu X, S: C, Glasser A, et al. Effect of H-7 on cultured human trabecular meshwork cells. Mol Vis Jun 27;7:145-53, 2001 608. Yu JC, Gottlieb AI: Disruption of endothelial actin microfilaments by protein kinase C inhibitors. Microvasc Res 43:100, 1992 609. Bershadsky A, Chausovsky A, Becker E, et al: Involvement of microtubules in the control of adhesion-dependent
signal transduction. Curr Biol 6:1279, 1996 610. Gills JP, Roberts BC, Epstein DL: Microtubule disruption leads to cellular contraction in human trabecular
meshwork cells. Invest Ophthalmol Vis Sci 39:653, 1998 611. Spector I, Shochet NR, Kashman Y, et al: Latrunculins: Novel marine toxins that disrupt microfilament organization
in cultured cells. Science 219:493, 1983 612. Coué M, Brenner SL, Spector I, et al: Inhibition of actin polymerization by latrunculin A. FEBS Lett 213:316, 1987 613. Cai S, Liu X, Glasser A, et al: Effect of latrunculin-A on morphology and actin-associated
adhesions of cultured human trabecular meshwork cells. Mol Vis 6:132, 2000 614. Perkins TW, Alvarado JA, Polansky JR, et al: Trabecular meshwork cells grown on filters: Conductivity and cytochalasin
effects. Invest Ophthalmol Vis Sci 29:1836, 1988 615. Tian B, Kaufman PL, Volber T, et al: H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol 116:633, 1998 616. Tian B, Gabelt BT, Peterson JA, et al: H-7 increases trabecular facility and facility after ciliary muscle
disinsertion in monkeys. Invest Ophthalmol Vis Sci 40:239, 1999 617. Peterson JA, Tian B, Bershadsky AD, et al: Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci 40:931, 1999 618. Svedbergh B, Lütjen-Drecoll E, Ober M, et al: Cytochalasin B-induced structural changes in the anterior ocular
segment of the cynomolgus monkey. Invest Ophthalmol Vis Sci 17:718, 1978 619. Johnstone M, Tanner D, Chau B, et al: Concentration-dependent morphologic effects of cytochalasin B in
the aqueous outflow system. Invest Ophthalmol Vis Sci 19:835, 1980 620. Kaufman PL, Bárány EH: Cytochalasin B reversibly increases outflow facility in the eye of the
cynomolgus monkey. Invest Ophthalmol Vis Sci 16:47, 1977 621. Kaufman PL, Erickson KA: Cytochalasin B and D dose-outflow facility response relationships
in the cynomolgus monkey. Invest Ophthalmol Vis Sci 23:646, 1982 622. Kaufman PL: Non-additivity of maximal pilocarpine and cytochalasin effects on
outflow facility. Exp Eye Res 44:283, 1987 623. Bill A: Effects of Na2EDTA and alpha-chymotrypsin on aqueous humor outflow conductance
in monkey eyes. Uppsala J Med Sci 85:311, 1980 624. Bill A, Lutjen-Drecoll E, Svedbergh B: Effects of intracameral Na2EDTA and EGTA on aqueous outflow routes in the monkey eye. Invest Ophthalmol Vis Sci 19:492, 1980 625. Hamanaka T, Bill A: Morphological and functional effects of Na2EDTA on the outflow routes for
aqueous humor in monkeys. Exp Eye Res 44:171, 1987 626. Tian B, Gabelt BT, Kaufman PL: Effect of staurosporine on outflow facility in monkeys. Invest Ophthalmol Vis Sci 40:1009, 1999 627. Tian B, Brumback LC, Kaufman PL: ML-7, chelerythrine and phorbol ester increase outflow facility
in the monkey eye. Exp Eye Res 71:551, 2000 628. Tian B, Kiland JA, Kaufman PL: Effects of the marine macrolides swinholide A and jasplakinolide on outflow
facility in monkeys. Invest Ophthalmol Vis Sci 42:3187, 2001 629. Peterson JA, Tian B, McLaren JW, et al: Latrunculin's effects on intraocular pressure, aqueous humor flow
and corneal endothelium. Invest Ophthalmol Vis Sci 41:1749, 2000 630. Peterson JA, Tian B, Geiger B, et al: Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res 70:307, 2000 631. Tian B, Kaufman PL: Effects of the Rho kinase inhibitor Y-27632 and the phosphatase
inhibitor calyculin on outflow facility in monkeys. Invest Exp Eye Res 80:215, 2005 632. Epstein DL, Rowlette L-L, Roberts BC: Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci 40:74, 1999 633. Honjo M, Tankhara H, Inatani M, et al: Effects of rho-associated protein kinase inhibitor Y-27632 on
intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci 42:137, 2001 634. Honjo M, Inatani M, Kido N, et al: A myosin light chain kinase inhibitor, ML-9, lowers the intraocular
pressure in rabbit eyes. Exp Eye Res 75:135, 2002 635. Waki M, Yoshida Y, Oka T, et al: Reduction of intraocular pressure by topical administration of an inhibitor
of the rho-associated protein kinase. Curr Eye Res 22:470, 2001 636. Rao PV, Deng P-F, Kumar J, et al: Modulation of aqueous humor outflow facility by the rho kinase-specific
inhibitor Y-27632. Invest Ophthalmol Vis Sci 42:1029, 2001 637. Sward K, Dreja K, Susnjar M, et al: Inhibition of Rho-associated kinase blocks agonist-induced
Ca2+ sensitization of myosin phosphorylation and force in guinea-pig
ileum. J Physiol 522:33, 2000 638. Burridge K, Chrzanowska-Wodnicka M: Focal adhesions, contractility, and signaling. Ann Rev Cell Devel Biol 12:463, 1996 639. Tian B, Wang R-F, Podos SM, et al: Effects of topical H-7 on outflow facility, intraocular pressure
and corneal thickness in monkeys. Arch Ophthalmol 122:1171, 2004 640. Tian B, Sabanay I, Gabelt BT, et al: Latrunculin B effects on aqueous outflow and trabecular meshwork and corneal
endothelium structure in the monkey eye [Abstract 2092]. Invest Ophthalmol Vis Sci 45:S2092, 2004 641. Kaufman PL: Aqueous humor outflow. In Zadunaisky JA, Davson H, eds.: Current Topics in Eye Research. New York: Academic Press, 1984:97–138 642. Sawaguchi S, Yue BY, Yeh P, et al: Effects of intracameral injection of chondroitinase ABC in vivo. Arch Ophthalmol 110:110, 1992 643. Bárány EH, Scotchbrook S: Influence of testicular hyaluronidase on the resistance to flow through
the angle of the anterior chamber. Acta Physiol Scand. 30:240, 1954 644. Van Buskirk EM, Brett J: The canine eye: In vitro dissolution of the barriers to aqueous outflow. Invest Ophthalmol Vis Sci 17:258, 1978 645. Knepper PA, Farbman AI, Tesler AG: Exogenous hyaluronidases and degradation of hyaluronic acid in the rabbit
eye. Invest Ophthalmol Vis Sci 25:286, 1984 646. Kaufman PL, Svedbergh B, Lütjen-Drecoll E: Medical trabeculocanalotomy in monkeys with cytochalasin B or EDTA [Editorial]. Ann Ophthalmol 11:795, 1979 647. Gabelt BT, Crawford K, Kaufman PL: Outflow facility and its response to pilocarpine decline in aging rhesus
monkeys. Arch Ophthalmol 109:879, 1991 648. Liu X, Filla MS, Brandt CR, et al: Exoenzyme C3 transferase gene transfer to cultured human trabecular meshwork
cells and ciliary muscle cells—Towards glaucoma gene therapy [Abstract 4563]. Invest Ophthalmol Vis Sci 45(suppl):S4563, 2004 649. Vittitow JL, Garg R, Rowlette LL, et al: Gene transfer of dominant-negative RhoA increases outflow facility
in perfused human anterior segment cultuRes Mol Vis. 8:32, 2002 650. Kee C, Sohn S, Hwang J-M: Stromelysin gene transfer into cultured human trabecular cells and rat
trabecular meshwork in vivo. Invest Ophthalmol Vis Sci 42:2856, 2001 651. Santas AJ, Bahler CK, Peterson JA, et al: Effect of heparin II domain of fibronectin on aqueous outflow in cultured
anterior segments of human eyes. Invest Ophthalmol Vis Sci 44:4796, 2003 652. Becker B, Hahn K: Topical corticosteroids and heredity in primary open-angle glaucoma. Am J Ophthalmol 57:543, 1964 653. Becker B: Intraocular pressure response to topical corticosteroids. Invest Ophthalmol Vis Sci 4:198, 1965 654. Kolker AE, Becker B: Topical corticosteroids and glaucoma: Current status. In Bellows JG, ed.: Contemporary Ophthalmology. Baltimore: Williams & Wilkins, 1972:161–167 655. Woods DC, Contaxis I, Sweet D, et al: Response of rabbits to corticosteroids. I. Influence on growth, introacular
pressure and lens transparency. Am J Ophthalmol 63:841, 1967 656. Bonomi L, Perfetti S, Noya E, et al: Experimental corticosteroid ocular hypertension in the rabbit. Albrcht Von Graefes Arch Klin Exp Ophthalmol 209:73, 1978 657. Francois J: Corticosteroid glaucoma. Ophthalmologica 188:76, 1984 658. Green K, Phillips CI, Gore SM, et al: Ocular fluid dynamics in response to topical RU486, a steroid blocker. Curr Eye Res 4:605, 1985 659. Tchernitchin A, Wenk EJ, Southren AL, et al: Radioautography of dexamethasone in rabbit gingiva and buccal mucosa. J Dental Res 59:2100, 1980 660. Weinreb RN, Bloom E, Baxter JD: Detection of glucocorticoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci 21:403, 1981 661. Hernandez MR, Wenk EJ, Weinstein BI, et al: Glucocorticoid target cells in human outflow pathway: Autopsy and surgical
specimens. Invest Ophthalmol Vis Sci 24:1612, 1983 662. Armaly MF: Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The
effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol 70:492, 1963 663. Fingert JH, Clark AF, Craig JE, et al: Evaluation of the myocilin (MYOC) glaucoma gene in monkey and
human steroid-induced ocular hypertension. Invest Ophthalmol Vis Sci 42:145, 2001 664. Armaly MF: Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The
effect of dexamethasone in the nromal eye. Arch Ophthalmol 70:482, 1963 665. Levi L, Schwartz B: Decrease of ocular pressure with oral metyrapone. A double masked crossover
trial. Arch Ophthalmol 105:777, 1987 666. Polansky JR, Palmberg P, Matulich D: Cellular sensitivity to glucocorticoids in patients with POAG. Steroid
receptors and responses in cultured skin fibroblasts. Invest Ophthalmol Vis Sci 26:805, 1985 667. Schwartz B, Levene RZ: Plasma cortisol differences between normal and glaucomatous patients: Before
and after dexamethasone suppression. Arch Ophthalmol 87:369, 1972 668. Schwartz B, McCarty G, Rosner B: Increased plasma free cortisol in ocular hypertension and open-angle
glaucoma. Arch Ophthalmol 105:1060, 1987 669. Weinreb RN, Polansky JR, Kramer SG, et al: Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci 26:170, 1985 670. Southren AL, Gordon GG, Munnangi PR, et al: Altered cortisol metabolism in cells cultured from trabecular meshwork
specimen obtained from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 26:1413, 1983 671. Weinstein BI, Munnangi P, Gordon GG, et al: Defects in cortisol-metabolizing enzymes in primary open-angle
glaucoma. Invest Ophthalmol Vis Sci 26:890, 1985 672. Southren AL, Wandel T, Gordon GG, et al: Treatment of glaucoma with 3a,5b-tetrahydrocortisol: A new therapeutic
modality. J Ocular Pharmacol 10:385, 1994 673. Clark AF, Lane D, Wilson K, et al: Inhibition of dexamethasone-induced cytoskeletal changes in cultured
human trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci 37:805, 1996 674. Rohen JW, Linner E, Witmer R: Electron microscopic studies on the trabecular meshwork in two cases of
corticosteroid glaucoma. Exp Eye Res 17:19, 1973 675. Yun AJ, Murphy CG, Polansky JR, et al: Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Invest Ophthalmol Vis Sci 30:2012, 1989 676. Steely HT, Browder S, Julian MB: The effects of dexamethasone on fibronectin expression in cultured trabecular
meshwork cells. Invest Ophthalmol Vis Sci 33:2242, 1992 677. Johnson DH, Bradley JM, Acott TS: The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork
in perfusion organ culture. Invest Ophthalmol Vis Sci 31:2568, 1990 678. Zhou L, Li Y, Yue BYJT: Glucocorticoid effects on extracellular matrix proteins and integrins in
bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med 1:339, 1998 679. Dickerson JEJ, Steely HTJ, English-Wright SL, et al: The effect of dexamethasone on integrin and laminin expression in cultured
human trabecular meshwork cells. Exp Eye Res 66:731, 1998 680. Snyder RW, Stamer WD, Kramer TR, et al: Corticosteroid treatment and trabecular meshwork proteases in cell and
organ culture supernatants. Exp Eye Res 57:461, 1993 681. Samples JR, Alexander JP, Acott TS: Regulation of the levels of human trabecular matrix metalloproteinases
and inhibitor by interleukin-1 and dexamethasone. Invest Ophthalmol Vis Sci 34:3386, 1993 682. Clark AF, Wilson K, McCartney MD, et al: Glucocorticoid-induced formation of cross-linked actin networks
in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 35:281, 1994 683. Wilson K, McCartney MD, Miggans ST, et al: Dexamethasone induced ultrastructural changes in cultured human trabecular
meshwork cells. Exp Eye Res 12:783, 1993 684. Tripathi BJ, Tripathi RC, Swift HH: Hydrocortisone-induced DNA endoreplication in human trabecular cells
in vitro. Exp Eye Res 49:259, 1989 685. Matsumoto Y, Johnson DH: Dexamethasone decreases phagocytosis by human trabecular meshwork cells
in situ. Invest Ophthalmol Vis Sci 38:1902, 1997 686. Partridge CA, Weinstein BI, Southren AL: Dexamethasone induces specific proteins in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 30:1843, 1989 687. Polansky JR, Kurtz RM, Alvarado JA, et al: Eicosanoid production and glucocorticoid regulatory mechanisms in cultured
human trabecular meshwork cells. Prog Clin Biol Res 312:113, 1989 688. Nguyen TD, Huang WD, Bloom E, et al: Glucocorticoid (GC) effects on HTM cells: Molecular biology approaches. In Lütjen-Drecoll E, ed.: Basic Aspects of Glaucoma Research III. Stuttgart: Schattauer, 1993:331–343 689. Ueda J, Wentz-Hunter K, Cheng EL, et al: Ultrastructural localization of myocilin in human trabecular meshwork cells
and tissues. J Histochem Cytochem 48:1321, 2000 690. Filla MS, Liu X, Nguyen TD, et al: In vitro localization of TIGR/MYOC in trabecular meshwork extracellular
matrix and binding to fibronectin. Invest Ophthalmol Vis Sci 43:151, 2002 691. Mertts M, Garfield S, Tanemoto K, et al: Identification of the region in the N-terminal domain responsible
for the cytoplasmic localization of MYOC/TIGR and its association
with microtubules. Lab Invest 79:1237, 1999 692. Wentz-Hunter K, Ueda J, Shimizu N, et al: Myocilin is associated with mitochondria in human trabecular meshwork cells. J Cell Physiol 190:46, 2002 693. Stone EM, Fingert JH, Alward WLM, et al: Identification of a gene that causes primary open angle glaucoma. Science 275:668, 1997 694. Alward WL, Fingert JH, Coote MA, et al: Clinical features associated with mutations in the chromosome 1 open-angle
glaucoma gene. N Engl J Med 338:1022, 1998 695. Tamm ER: Myocilin and glaucoma: Facts and ideas. Prog Retin Eye Res 21:395, 2002 696. Polansky JR, Fauss DJ, Chen P, et al: Cellular pharmacology and molecular biology of the trabecular meshwork
inducible glucocorticoid response gene product. Ophthalmologica 211:126, 1997 697. Nguyen TD, Chen P, Huang WD, et al: Gene structure and properties of TIGR, an olfactomedin-related glycoprotein
cloned from glucocorticoid-induced trabecular meshwork
cells. J Biol Chem 273:6341, 1988 698. Wentz-Hunter K, Kubota R, Shen X, et al: Extracellular myocilin affects activity of human trabecular meshwork cells. J Cell Physiol. 200:45, 2004 699. Caballero M, Rowlette LL, Borrás T: Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Bioch Biophys Acta 1502:447, 2000 700. Jacobson N, Andrews M, Shepard AR, et al: Non-secretion of mutant proteins of the glaucoma gene myocilin in
cultured trabeuclar meshwork cells and in aqueous humor. Hum Mol Genet 10:117, 2001 701. Lo WR, Rowlette LL, Caballero M, et al: tissue differential microarray analysis of dexamethasone induction reveals
potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci 44:473, 2003 702. Ishihashi T, Takagi Y, Mori K, et al: cDNA microarray analysis of gene expression changes induced by dexamethasone
in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 43:3691, 2002 703. Underwood JL, Murphy CG, Chen J, et al: Glucocorticoids regulate transendothelial fluid flow resistance and formation
of intercellular junctions. Am J Physiol 277:C330, 1999 704. Rohen JW, Lütjen-Drecoll E: Morphology of aqueous outflow pathways in normal and glaucomatous eyes. In Ritch R, Shields MB, Krupin T, eds.: The Glaucomas. St. Louis: CV Mosby, 1989:41 705. Lütjen-Drecoll E, Rohen JW: Pathology of the trabecular meshwork in primary open angle glaucoma. In Kaufman PL, Mittag TW, eds.: Glaucoma. Textbook of Ophthalmology Series. New York: CV Mosby, 1994 706. Johnson D, Gottanka J, Flügel C, et al: Ultrastructural changes in the trabecular meshwork of human eyes treated
with corticosteroids. Arch Ophthalmol 115:375, 1997 707. Weinreb RN, Mitchell MD, Alvarado JA, et al: Glucocorticoid regulation of eicosandoid biosynthesis in cultured human
trabecular cells. In Ticho U, David R, eds.: Recent Advances in Glaucoma. New York: Elsevier Sciences Publishers B.V., 1984:213–218 708. Polansky JR, Kurtz RM, Fauss DJ: In vitro correlates of glucocorticoid effects on intraocular pressure. In Krieglestein GK, ed.: Glaucoma Update IV. Berlin: Springer-Verlag, 1991:20–29 709. Polansky JR, Alvarado JA: Cellular mechanisms influencing the aqeuous humor outflow pathway. In Albert DM, Jakobiec FA, eds.: Principles and Practice of Ophthalmology: Basic Sciences. Philadelphia: WB Saunders, 1994:26 710. Polansky JR: HTM cell culture model for steroid effects on intraocular pressure: Overview. In Lütjen-Drecoll E, ed.: Basic Aspects of Glaucoma Research III. Stuttgart: Schattauer, 1993:307–318 711. Weinreb RN, Polansky JR, Alvarado JA, et al: Arachidonic acid metabolism in human trabecular meshwork cells. Invest Ophthal Visual Sci 29:1708, 1988 712. Lee PY, Podos SM, Severin C: Effect of prostaglandin F2α on aqueous humor dynamics of rabbit, cat, and monkey. Invest Ophthalmol Vis Sci 25:1087, 1984 713. Alm A, Villumsen J: PhXA34, a new potent ocular hypotensive drug. A study on dose-response
relationship and on aqueous humor dynamics in healthy volunteers. Arch Ophthalmol 109:1564, 1991 714. Millard CB, Tripathi BJ, Tripathi RC: Age-related changes in protein profiles of the normal human trabecular
meshwork. Exp Eye Res 45:623, 1987 715. Flügel C, Tamm E, Lütjen-Drecoll E, et al: Age-related loss of a-smooth muscle actin in normal and glaucomatous
human trabecular meshwork of different age groups. J Glaucoma 1:165, 1992 716. Spiro RG: Nature of the glycoprotein components of basement membranes. Ann NY Acad Sci 312:106, 1978 717. Bornstein P, Duksin D, Balian G, et al: Organization of extracellular proteins on the connective tissue cell surface: Relevance
to cell-matrix interactions in vitro and in vivo. Ann NY Acad Sci 312:93, 1978 718. Babizhayev MA, Brodskaya MW: Fibronectin detection in drainage outflow system of human eyes in ageing
and progression of open angle glaucoma. Mech Ageing Dev 47:145, 1989 719. Bjorksten J: The crosslinkage theory of aging. J Am Geriatr Soc 16:408, 1968 720. Babizhayev MA, Bunin AY: Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol 67:371, 1989 721. Green K: Free radicals and aging of anterior segment tissues of the eye: A hypothesis. Ophthalmic Res 27(suppl):143, 1995 722. Ochiai Y, Ochiai H: Higher concentration of transforming growth factor-beta in aqueous
humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol 46:249, 2002 723. Picht G, Welge-Luessen U, Grehn F, et al: Transforming growth factor beta 2 levels in the aqeuous humor in different
types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol 239:199, 2001 724. Fuchshofer R, Welge-Lüssen U, Lütjen-Drecoll E: The effect of TGF-β2 on human trabecular meshwork extracellular
proteolytic system. Exp Eye Res 77:757, 2003 725. Borisuth NS, Tripathi BJ, Tripathi RC: Identification and partial characterization of TGF-beta 1 receptors
on trabecular cells. Invest Ophthalmol Vis Sci 33:596, 1992 726. Gottanka J, Chan D, Eichhorn M, et al: Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci 45:153, 2004 727. Lütjen-Drecoll E, Kaufman PL: Morphological changes in primate aqueous humor formation and drainage tissues
after long-term treatment with antiglaucomatous drugs. J Glaucoma 2:316, 1993 728. Kiland JA, Gabelt BT, Kaufman PL: Studies on the mechanism of action of timolol and on the effects of suppression
and redirection of aqueous flow on outflow facility. Exp Eye Res 78(Special Issue):639, 2004 729. Bill A, Phillips I: Uveoscleral drainage of aqueous humor in human eyes. Exp Eye Res 21:275, 1971 730. Bill A: Aqueous humor dynamics in monkeys (Macaca irus and Cercopithecus ethiops). Exp Eye Res 11:195, 1971 731. Gabelt BT, Gottanka J, Lütjen-Drecoll E, et al: Aqueous humor dynamics and trabecular meshwork and anterior ciliary muscle
morphologic changes with age in rhesus monkeys. Invest Ophthalmol Vis Sci 44:2118, 2003 732. Kaufman PL: Physiology and phamacology of aqueous humor outflow: Implications for treatment. In Caprioli J, ed.: Contemporary Issues in Glaucoma. Philadelphia: WB Saunders, 1991:781–802 733. Emi K, Pederson JE, Toris CB: Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci 30:233, 1989 734. Poyer JF, Millar C, Kaufman PL: Prostaglandin F2a effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci 36:2461, 1995 735. Sagara T, Gaton DD, Lindsey JD, et al: Reduction of collagen type I in the ciliary muscle of inflamed monkey eyes. Invest Ophthalmol Vis Sci 40:2568, 1999 736. Sagara T, Gaton DD, Lindsey JD, et al: Topical prostaglandin F2a treatment reduces collagen types I, III, and IV in the monkey uveoscleral
outflow pathway. Arch Ophthalmol 117:794, 1999 737. Lütjen-Drecoll E, Tamm E: Morphological study of the anterior segments of cynomolgus monkey eyes
following treatment with prostaglandin F2a. Exp Eye Res 47:761, 1988 738. Tamm E, Rittig M, Lütjen-Drecoll E: Elektronenmikroskopische und immunhistochemische Untersuchungen zur augendrucksenkenden
Wirkung von Prostaglandin F2a. Fortschr Ophthalmol 87:623, 1990 739. Gaton DD, Sagara T, Lindsey JD, et al: Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral
outflow pathway following topical prostaglandin F(2 alpha)-isopropyl
ester treatment. Arch Ophthalmol 119:1165, 2001 740. Lindsey JD, Kashiwagi K, Kashiwagi F, et al: Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism—Implications
for uveoscleral outflow. Surv Ophthalmol 41(Suppl 2):S53, 1997 741. Kim J-W, Lindsey JD, Wang N, et al: Increased human scleral permeability with prostaglandin exposure. Invest Ophthalmol Vis Sci 42:1514, 2001 742. Weinreb RN: Enhancement of scleral macromolecular permeability iwht prostaglandins. Trans Am Ophthalmol Soc 99:319, 2001 743. Stjernschantz J, Selén G, Ocklind A, et al: Effects of latanoprost and related prostaglandin analogues. In Alm A, Weinreb RN, eds.: Uveoscleral Outflow Biology and Clinical Aspects. London: Mosby-Wolfe Medical Communications, 1998:57–72 744. Lee P-Y, Shoo H, Xu L, et al: The effect of prostaglandin F2a on intraocular pressure in normotensive human subjects. Invest Ophthalmol Vis Sci 29:1474, 1988 745. Bito LZ, Stjernschantz J, Resul B, et al: The ocular effects of prostaglandins and the therapeutic potential of a
new PGF2α analog, PhXA41 (latanoprost), for glaucoma management. J Lipid Mediators 6:535, 1993 746. Camras CB: Comparison of latanoprost and timolol in patients with ocular hypertension
and glaucoma. A six-month, masked, multicenter trial in the
United States. Ophthalmology 103:138, 1996 747. Camras CB, Alm A, Watson P, et al: Latanoprost, a prostaglandin analog, for glaucoma therapy. Ophthalmol 103:1916, 1996 748. Parrish RK, Palmberg P, Sheu W-P, et al: A comparison of latanoprost, bimatoprost, and travoprost in patients with
elevated intraocular pressure: A 12-week, randomized, masked-evaluator
multicenter study. Am J Ophthalmol 135:688, 2003 749. Kurtz S, Shemesh G: The efficacy and safety of once-daily verss once-weekly latanoprost
treatment for increased intraocular pressure. J Ocul Pharmacol Ther 20:321, 2004 750. Eisenberg DL, Toris CB, Camras CB: Bimatoprost and travoprost: A review of recent studies of two new glaucoma
drugs. Surv Ophthalmol 47:S105, 2002 751. Noecker RS, Dirks MS, Choplin NT, et al: A six-month randomized clinical trial comparing the intraocular
prssure-lowering efficacy of bimatoprost and latanoprost in patients
with ocular hypertension or glaucoma. Am J Ophthalmol 135:55, 2003 752. Gandolfi SA, Cimino L: Effect of bimatoprost on patients with primary open-angle glaucoma
or ocular hypertension who are nonresponders to loatanoprost. Ophthalmology 110:609, 2003 753. Kaufman PL: The prostaglandin wars [Editorial]. Am J Ophthalmol 136:727, 2003 754. Brubaker RF, Schoff EO, Nau CB, et al: Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol 131:19, 2001 755. Christensen GA, Nau CB, McLaren JW, et al: Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in
patients with ocular hypertension or glaucoma. Ophthalmology 111:1658, 2004 756. Sharif NA, Kelly CR, Crider JY: Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and
other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci 44:715, 2003 757. Gagliuso DJ, Wang R-F, Mittag TW, et al: Additivity of bimatoprost or travoprost to latanoprost in glaucomatous
monkey eyes. Arch Ophthalmol 122:1342, 2004 758. Richter M, Krauss AH-P, Woodward DF, et al: Morphological changes in the anterior eye segment after long-term
treatment with different receptor selective prostaglandin agonists and
a prostamide. Invest Ophthalmol Vis Sci 44:4419, 2003 759. Toris CB, Camras CB, Yablonski ME: Acute versus chronic effects of brimonidine on aqueous humor dynamics in
ocular hypertensive patients. Am J Ophthalmol 128:8, 1999 760. Engstrom P, Dunham EW: Alpha-adrenergic stimulation of prostaglandin release from rabbit
iris-ciliary body in vitro. Invest Ophthalmol Vis Sci 22:757, 1982 761. Yousufzai SY, Abdel-Latif AA: Effects of norepinephrine and other pharmacological agents on prostaglandin
E2 release by rabbit and bovine irides. Exp Eye Res 37:279, 1983 762. Camras CB, Feldman SG, Podos SM, et al: Inhibition of the epinephrine-induced reduction of intraocular pressure
by systemic indomethacin in humans. Am J Ophthalmol 100:169, 1985 763. Bhattacherjee P, Hammond BR: Effect of indomethacin on the ocular hypotensive action of adrenaline in
the rabbit. Exp Eye Res 24:307, 1977 764. Lutjen-Drecoll E, Rohen JW: Pathology of the trabecular meshwork in primary open angle glaucoma. In Kaufman PL, Mittag TW, eds.: Glaucoma Textbook of Ophthalmology Series. New York: CV Mosby, 1994:1.1–1.16 765. Wiederholt M, Helbig H, Korbmacher C: Ion transport across the ciliary epithelium: Lessons from cultured cells
and proposed role of the carbonic anhydrase. In Botrè F, Gross G, eds.: Carbonic Anhydrase. Cambridge: Verlag-Chemie, 1991:232–244 766. Maren TH: The kinetics of HCO3− synthesis related to fluid secretion, pH control, and CO2 elimination. Ann Rev Physiol 50:695, 1988 767. Kanski JJ: Glaucoma. In Kanski JJ, ed.: Clinical Ophthalmology, 2nd ed. London: Butterworth-Heinemann, 1989:182–231 768. Shields MB: Aqueous humor dynamics. I. Anatomy and physiology. In Shield MB, ed.: Textbook of Glaucoma, 4th ed. Baltimore: Williams & Wilkins, 1998:5–34 769. Wiederholt M, Stumpff F: The trabecular meshwork and aqueous humor reabsorption. In Civan MM, ed.: Current Topics in Membranes. San Diego: Academic Press, 1998:163–202 770. Kolker AE, Hetherington JJ: Gonioscopic and microscopic anatomy of the angle of the anterior chamber
of the eye. In Kolker AE, Hetherington JJ, eds.: Becker-Schaffer's Diagnosis and Therapy of the Glaucomas, 4th ed. St Louis: Mosby-Year Book, 1976:21–41 771. Kaufman PL, Tian B, Gabelt BT, et al: Outflow enhancing drugs and gene therapy in glaucoma. In Weinreb R, Krieglstein G, Kitazawa Y, eds.: Glaucoma in the 21st Century. London: Harcourt-Mosby, 2000:117–128 |