
|
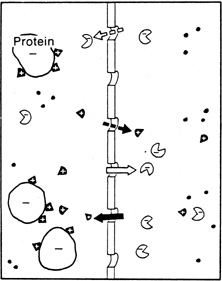
| Fig. 7. Gibbs-Donnan effect. Because protein molecules carry an overall electrical charge (usually negative) at physiological pH, the distribution of ions at equilibrium is altered slightly to reflect the tendency of the protein molecules to attract oppositely charged and repel like-charged ions. Therefore there is a higher concentration of cations on the protein side and a higher concentration of anions on the nonprotein side of the system. (Courtesy of RL Stamper, MD.) |