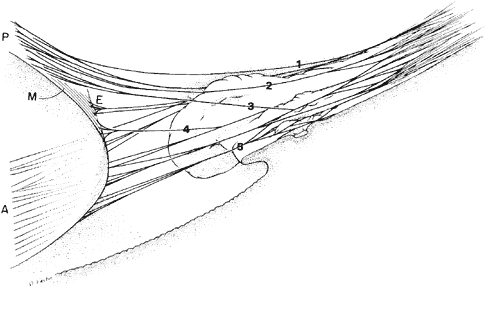
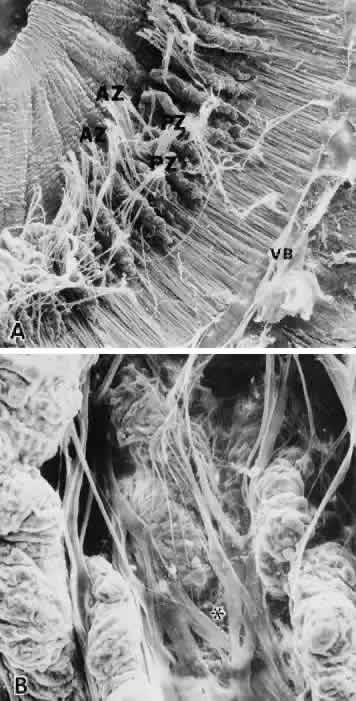
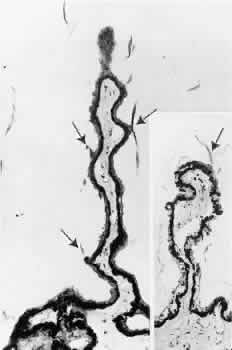
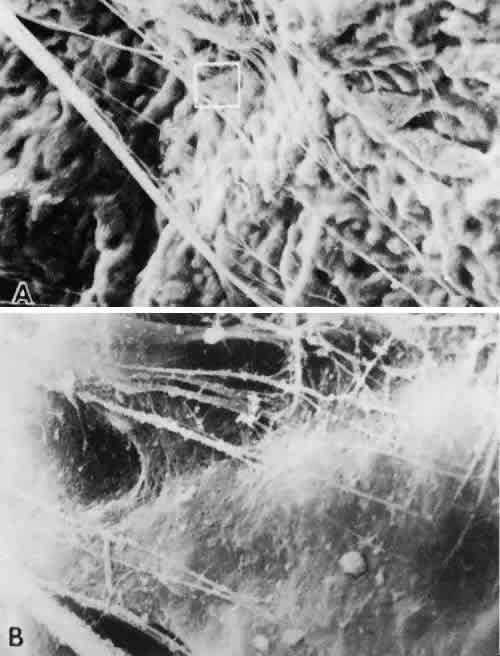
1. Vail D: The zonule of Zinn and ligament of Wieger. Trans Ophthalmol Soc UK 77:441, 1957 2. Barber AN: Embryology of the Human Eye, pp 154–157. St. Louis, CV
Mosby, 1955 3. Gloor BP: Zur Entwicklung des Glaskorpers und der Zonule: Vl. Autoradiographische
Untersuchungen zur Entwicklung der Zonule der Maus mit 3H-markeiten Aminoauren
und 3H-Glucose. Graefes Arch Clin Exp Ophthalmol 189:105–124, 1974 4. Rhodes RH, Mandelbaum SH, Minckler DS, Cleary PE: Tritrated fucose incorporation in the vitreous body, lens and zonules of
the pigmented rabbit. Exp Eye Res 34:921–931, 1982 5. Briglin CA, Liz-Y, Streeten BW: Cultured lens epithelial cells synthesize matrix immunopositive for elastic
microfibrillar proteins, including fibrillin and a zonular peptide. Invest Ophthalmol Vis Sci 32(suppl):777, 1991 6. Robb BW, Wachi H, Schaub T et al: Characterization of an in vitro model of elastic fiber assembly. Mol Biol Cell 10:3595–3605, 1999 7. Sakai LY, Keene DR, Engvall E: Fibrillin, a new 350-kd glycoprotein, is a component of extracellular microfibrils.J Cell Biol 103:2499–2509, 1986 8. Qi Y, Streeten BW, Wallace RN, Hoepner: Expression of fibrillin-1 mRNA in cells related to the zonular system of
the developing mouse eye by in situ hybridization. Invest Ophthalmol Vis Sci 37(suppl):90, 1996 9. Buddecke E, Wollensak J: Zur Biochemie der Zonulafaser des Rinderauges. Z Naturforsch 216:337–341, 1966 10. Dische Z, Murty VLN: An insoluble structural glycoprotein: A major constituent of the zonula
Zinni. Invest Ophthalmol Vis Sci 13:991–995, 1974 11. Streeten BW, Swann DA, Licari PA et al: The protein composition of the ocular zonules. Invest Ophthalmol Vis Sci 24:119–123, 1983 12. Streeten BW, Gibson SA: Identification of extractable proteins from the bovine ocular zonule: Major
zonular antigens of 32 kd and 250 kd. Curr Eye Res 7:139–146, 1988 13. Streeten BW, Gibson SA, Li Z-Y: Lectin binding topseudoexfoliative material and the ocular zonules. Invest Ophthalmol Vis Sci 27:1516–1521, 1986 14. Raviola G: The fine structure of the ciliary zonule and ciliary epithelium. With special
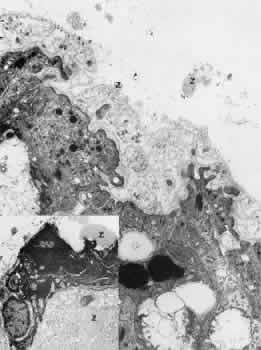
regard to the organization and insertion of the zonular fibrils. Invest Ophthalmol Vis Sci 10:851–869, 1971 15. Ross R, Bornstein P: The elastic fiber: 1. The separation and partial characterization of its
macromolecular components. J Cell Biol 40:366–381, 1969 16. Streeten BW, Licari PA, Marucci AA, Dougherty RM: Immunohistochemical comparison of ocular zonules and the microfibrils of
elastic tissue. Invest Ophthalmol Vis Sci 21:130–135, 1981 17. Lee B. Godfrey M, Vitale E et al: Linkage of Marfan syndrome and a phenotypically related disorder to two
different fibrillin genes. Nature 352:330–334, 1991 18. Magenis RE, Maslen CL, Smith L et al: Localization of the fibrillin (FBN) gene to chromosome 15 and band 21.1.Genomics 11:346–351, 1991 19. Streeten BW, Gibson SA, Dark AJ: Pseudoexfoliative material contains an elastic microfibrillar-associated
glycoprotein. Trans Am Ophthalmol Soc 84:304–320, 1986 20. Dietz HC, Cutting GR, Pyeritz RE et al: Marfan syndrome caused by a recurrent de novo missense mutation in the
fibrillin gene. Nature 352:337–339, 1991 21. Tsipouras P, DelMastro R, Safarazi M et al: Linkage analysis demonstrates that Marfan syndrome, ectopia lentis, and
congenital contractural arachnodactyly are linked to the fibrillin genes
on chromosomes 15 and 5. N Engl J Med 326:905–909, 1991 22. Horrigan S, Rich C, Streeten BW et al: Characterization of a microfibril-associated protein through recombinant
DNA techniques. J Biol Chem 267:10087–10095, 1992 23. Yeh H, Chow M, Abrams WR et al: Structure of the hu-man gene encoding the associated microfibrillar protein(MFAP-1) and
localization to chromosome 15q21. Genomics 23:443–449, 1994 24. Kielty CM, Shuttleworth CA: Microfibrillar elements of the dermal matrix. Microscopy Res Tech 38:413–427, 1997 25. cmReinhardt DP, Mechling DE, Boswell BA et al:Calcium determines the shape of fibrillin. J Biol Chem 272:7368–7373, 1997 26. Cardy CM, Handford PA: Metal ion dependency of microfibrils supports a rod-like conformation for
fibrillin-1 calcium-binding epidermal growth factor-like domains. J Mol Biol 276:855–860, 1998 27. Qian R-Q, Glanville RW: Alignment of fibrillar molecules in elastic microfibrils is defined by
transglutaminase-derived cross-links. Biochemistry 36:15841–15847, 1997 28. Fleischmajer R, Contard P, Schwartz et al: Elastin-associated microfibrils (10 nm) in a three-dimensional fibroblast
culture. J Invest Dermatol 97:639–643, 1991 29. Cotta-Pereira G, Rodrigo FG, Bittencourt-Sampaio S: Oxytalan, elaunin and elastic fibers in the human skin. J Invest Dermatol 66:143–148, 1976 30. Fullmer HM, Sheetz JH, Narkates AJ: Oxytalan connective tissue fibers: A review. J Oral Pathol 3:296–316, 1975 31. Wirtz MK, Samples JR, Kramer PL et al: Weill-Marchesani syndrome: possible linkage of the autosomal dominant form
to 15q21.1Am J Med Genetics 65:68–75, 1996 32. Schlötzer-Schrehardt U: Pseudoexfoliation syndrome: Ocular manifestation of a systemic disorder? Arch Ophthalmol 110:1752–1756, 1992 33. Streeten BW, Li ZY, Wallace RN et al: Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation
syndrome. Arch Ophthalmol 110:1757–1762, 1992 34. Canals M, Costa-Vila J, Potau JM et al: Scanning electron microsocopy of the human zonule of the lens (zonula ciliaris). Acta Anatomica 157:309–314, 1996 35. Wislocki GB: The anterior segment of the eye of the rhesus monkey investigated by histochemical
means. Am J Anat 91:233–255, 1952 36. Garzino A: Le fibre della zonula di Zinn studiate a fresco con il microscopio a contralto
di fase. Rass Ital Ottalmol 22:3, 1953 37. McCulloch C: The zonule of Zinn: Its origin, course and insertion, and
its relation to neighboring structures. Trans Am Ophthalmol Soc 52:525–585, 1954Üc 38. Kaczurowski Ml: I. Zonular fibers of the human eye. Am J Ophthalmol 58:1030–1047, 1964; II. The surface of the vitreous. Am J Ophthalmol 63:419–428, 1967 39. Wallace RN, Streeten BW, Hanna RB: Rotary shadowing of elastic system microfibrils in the ocular zonule, vitreous, and
ligamentum nuchae. Curr Eye Res 10:99–109, 1991 40. Ren ZX, Brewton RG, Mayne R: An analysis by rotary shadowing of the mammalian vitreous humor and zonular
apparatus. J Ultrastruct Biol 106:57–63, 1991 41. Keene DR, Maddox BK, Kuo H-J et al: Extraction of extendable beaded structures and their identification as
fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem 39:441–449, 1991 42. Wess TJ, Purslow PP, Kielty CM: Fibrillin-rich microfibrils: an x-ray diffraction study of the fundamental
axial periodicity. FEBS Letters 413:424–428, 1997 43. McConnell CJ, Demont ME, Wright GM: Microfibrils provide non-linear elastic behavior in the abdominal artery
of the lobster Homarus americanus. J Physiol 499:513–526, 1997 44. Reinhardt DP, Keene DR, Corson GM et al: Fibrillin-1: Organization in microfibrils and structural properties. J Mol Biol 258:104–116, 1996 45. Downing AK, Knott V, Werner JM et al: Solution structure of a pair of calcium-binding epidermal growth factor-like
domains: implications for the Marfan syndrome and other genetic
disorders. Cell 85:597–605, 1996 46. Roll P. Reich M, Hofmann H: Der Verlauf der Zonulafasern. Graefes Arch Clin Exp Ophthalmol 195:41–47, 1975 47. Rohen JW, Rentsch FJ: Der konstructive Bau des Zonnula Apparates beim Menschen und dessen functionelle
Bedeutung: Morphologische Grundlagen fur eine neue Akkomodationstheorie. Graefes Arch Clin Exp Ophthalmol 178:1–19, 1969 48. Bornfeld N, Spitznas M, Breipohl W: Scanning electron microscopy of the zonule of Zinn: 1. Human eyes. Graefes Arch Clin Exp Ophthalmol 192:117–129, 1974 49. Reich ME, Roll P, Hofmann H, Scher A: Rasterelektronen mikroskopische Untersuchungen
des voreren Augenab-schnittes mit besonderer Berucksichtigung
des Verlaufs der Zonula Zinnii. Graefes Arch Clin Exp Ophthalmol 1975; 195:271–283 19 50. Davanger M: The suspensory apparatus of the lens: The surface of the ciliary body: A
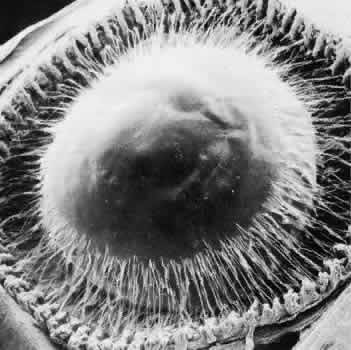
scanning electron microscopic study. Acta Ophthalmol 53:19–33, 1975 51. Farnsworth PN, Mauriello JA, Burke-Gadomski P et al: Surface ultrastructure of the human lens capsule and zonular attachments. Invest Ophthalmol Vis Sci 15:36–40, 1976 52. Streeten BW: The zonular insertion: A scanning electron microscopic study. Invest Ophthalmol Vis Sci 16:364–375, 1977 53. Farnsworth PNB, Burke P: Three-dimensional architecture of the suspensory apparatus of the lens
of the rhesus monkey. Exp Eye Res 25:563–576, 1977 54. Streeten BW, Pulaski JP: Posterior zonules and lens extraction. Arch Ophthalmol 96:132–138, 1978 55. Rohen JW: Scanning electron microscopic studies of the zonular apparatus in human
and monkey eyes. Invest Ophthalmol Vis Sci 18:133–144, 1979 56. Ober M, Rohen JW: Regional differences in the fine structure of the ciliary epithelium related
to accommodation. Invest Ophthalmol Vis Sci 18:655–664, 1979 57. Salzmann M: The Anatomy and Histology of the Human Eyeball in the Normal
State, EVL Brown (trans). Chicago, University of Chicago Press, 1912:155–162 58. Gamier RV: Uber den normalen und pathologischen Zustand der Zonula Zinnii. Arch Augenheilkd 24:32, 1892 59. Graf Spee H: Ueber den Bau der Zonulafasern und ihre Anordinung im menschlichen Aug. Anat Anz 21(suppl):236, 1892 60. Eisner G: Clinical examination of the vitreous. Trans Ophthalmol Soc UK 95:360–363, 1975 61. Streeten BW: A method for studying the zonule in gross specimens. Presented
at the Spring Meeting of the Association for Research in Vision and
Ophthalmology, Sarasota, FL, 1974, p 10 62. Foos RY: Zonular traction tufts of the peripheral retina in cadaver eyes. Arch Ophthalmol 82:620–632, 1969 63. Campbell DG: Pigmentary dispersion and glaucma: A new theory. Arch Ophthalmol 97:1667–1972, 1979 64. Duke-Elder S, Wybar KC: The Anatomy of the Visual System. London, Henry
Kimpton, 1961:324–338 65. Berger E: Beitrage zur Anatomie der Zonula Zinnii. Graefes Arch Clin Exp Ophthalmol 28:28, 1882 66. Kielty CM, Whittaker SP, Shuttleworth CA: Fibrillin: Evidence that chondroitin sulphate proteoglycans are components
of microfibrils and associate with newly synthesised monomers. FEBS Letters 386:169–173, 1996 67. Chan FL, Choi HL, Underhill CB: Hyaluronan and chondroitin sulfate proteoglycans are colocalized to the
ciliary zonule of the rat eye: a histochmeical and immunocytochemical
study. Histochem Cell Biol 107:289–301, 1997 68. Goldfischer S, Coltoff-Schiller B, Goldfischer M: Microfibrils, elastic anchoring components of the extracellular matrix, are
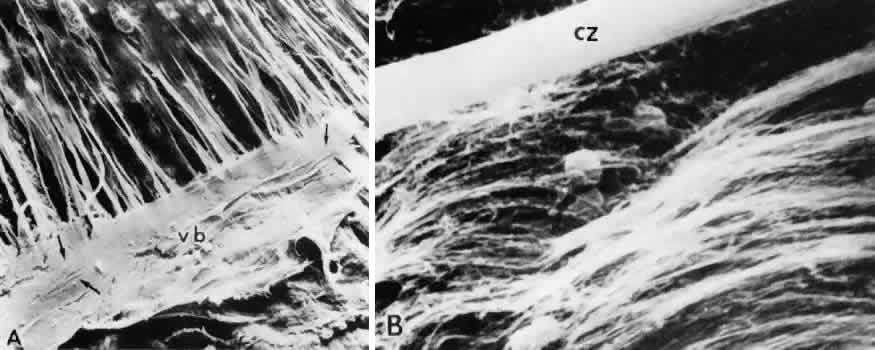
associated with fibronectin in the zonule of Zinn and aorta. Tissue Cell 17:441–450, 1985 69. Inoue S, Leblond CP, Rico P, Grant D: Association of fibronectin with the microfibrils of connective tissue. Am J Anat 186:43–54, 1989 70. Li Z-Y, Streeten BW, Wallace RN: Vitronectin localizes to pseudoexfoliative fibers in ocular and conjunctival
sites by immunoelectron microscopy. Invest Ophthalmol Vis Sci 32(suppl):777, 1991 71. Dark AJ, Streeten BW, Jones DB: Accumulation of fibrillar protein in the aging human lens capsule. Arch Ophthalmol 82:815–821, 1969 72. Seland JH: Ultrastructural changes in the normal human lens capsule from birth to
old age. Acta Ophthalmol 52:688–706, 1974 73. Kielty CM, Wooley DE, Whittaker SP et al: Catabolism of intact fibrillin microfibrils by neutrophil elastase, chymotrypsin
and trypsin. FEBS Letters 351:85–89, 1994 74. Qi Y, Streeten BW, Wallace RN: The pattern of zonular and matrix attachment proteins on the normal human
lens capsule. Invest Ophthalmol Vis Sci 35(suppl):2211, 1994 75. Wheatley HM, Traboulsi EI, Flowers BE et al: Immunohistochemical localization of fibrillin in human ocular tissues. Relevance
to th Marfan syndrome. Arch Ophthalmol 113:103–109, 1995 76. Mir S, Wheatley HM, Maumenee Hussels IE et al: A comparative histologic study of the fibrillin microfibrillar system in
the lens capsule of normal subjects with Marfan syndrome. Invest Ophthalmol Vis Sci 39:84–93, 1998 77. Brown NAP, Bron AJ, Sparrow JM: An estimate of the size and shape of the human lens fiber in vivo. Br J Ophthalmol 71:916–922, 1987 78. Guzek JP, Holm M, Cotter J: Risk factors for intraoperative complications in 1000 extracapsular cataract
cases. Ophthalmology 94:461–466, 1987 79. Wilson DJ, Jaeger MJ, Green WR: Effects of extracapsular cataract extraction on the lens zonules. Ophthalmology 94:467–470, 1987 80. Skuta GL: Zonular dialysis during extracapsular cataract extraction in pseudoexfoliation
syndrome. Arch Ophthalmol 105:632–634, 1987 81. Bock P: The distribution of disulfide groups in Descemet's membrane, lens
capsule, and zonular fibers. Acta Histochem 63:127–136, 1978 82. Buschmann W, Linnert D, Hofmann W, Gross A: The tensile strength of human zonule and its alteration with age. Graefes Arch Clin Exp Ophthalmol 206:183–190, 1978 83. Hanssen E, Franc S, Garrone R: Fibrillin-rich microfibrils: structural modifications during ageing in
normal human zonule. Submicrosc Cytol Pathol 30:365–369, 1998 84. Godfrey M, Nejezchleb PA, Schaeffer GB et al: Elastin and fibrillin on RNA and protein levels in the ontogeny of normal
human aorta. Connect Tissue Res 29:61–69, 1993 85. Farnsworth PN, Shyne SE: Anterior zonular shifts with age. Exp Eye Res 28:291–297, 1979 86. Stark WJ, Streeten BW: The anterior capsulotomy of extracapsular cataract extraction. Ophthalmic Surg 15:911–917, 1984 87. Duke-Elder A, Abrams D: Ophthalmic optics and refraction. In: Duke-Elder
S, ed. System of Ophthalmology, vol 5, pp 180–183. St. Louis, CV
Mosby, 1970 88. Neider MW, Crawford K, Kaufman PL et al: In vivo videography of the rhesus monkey accommodative apparatus: Age-related
loss ofciliary muscle response to central stimulation. Arch Ophthalmol 108:69–74, 1990 89. Brown N: The change in shape and internal form of the lens of the eye on accommodation. Exp Eye Res 15:441–459, 1973 90. Coleman DJ: Unified model for accommodative mechanism. Am J Ophthalmol 69:1063–1079, 1970 91. Koretz JF, Handelman GH: Model of the accommodative mechanism in the human eye. Vision Res 22:917–927,1982 92. Ludwig K, Wegscheider E, Hoops JP et al: In vitro imaging of the human zonular apparatus with high-resolution ultrasound
biomcroscopy. Graefes Arch Exp Ophthalmol 237:361–371, 1999 93. Schachar RA, Cudmore DP, Torti R et al: A physical model demonstrating Schachar's hypothesis of accommodation. Ann Ophthalmol 26:4–9, 1994 94. Schachar RA: Zonular function: A new hypothesis with clinical implications. Ann Ophthalmol 26:36–38, 1994 95. McKanna JA, Casagrande VA: Reduced lens development in lid-suture myopia. Exp Eye Res 26:715–723, 1978 |