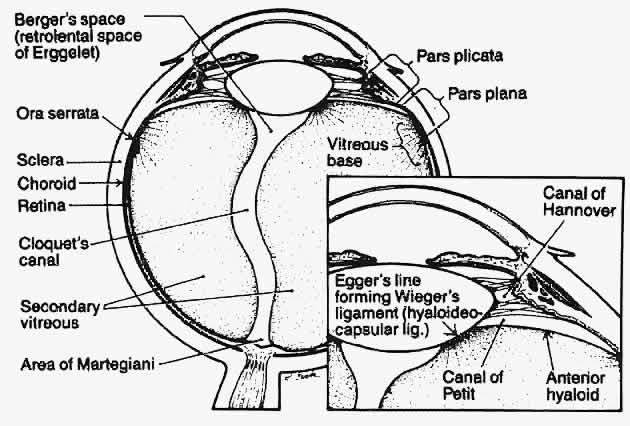
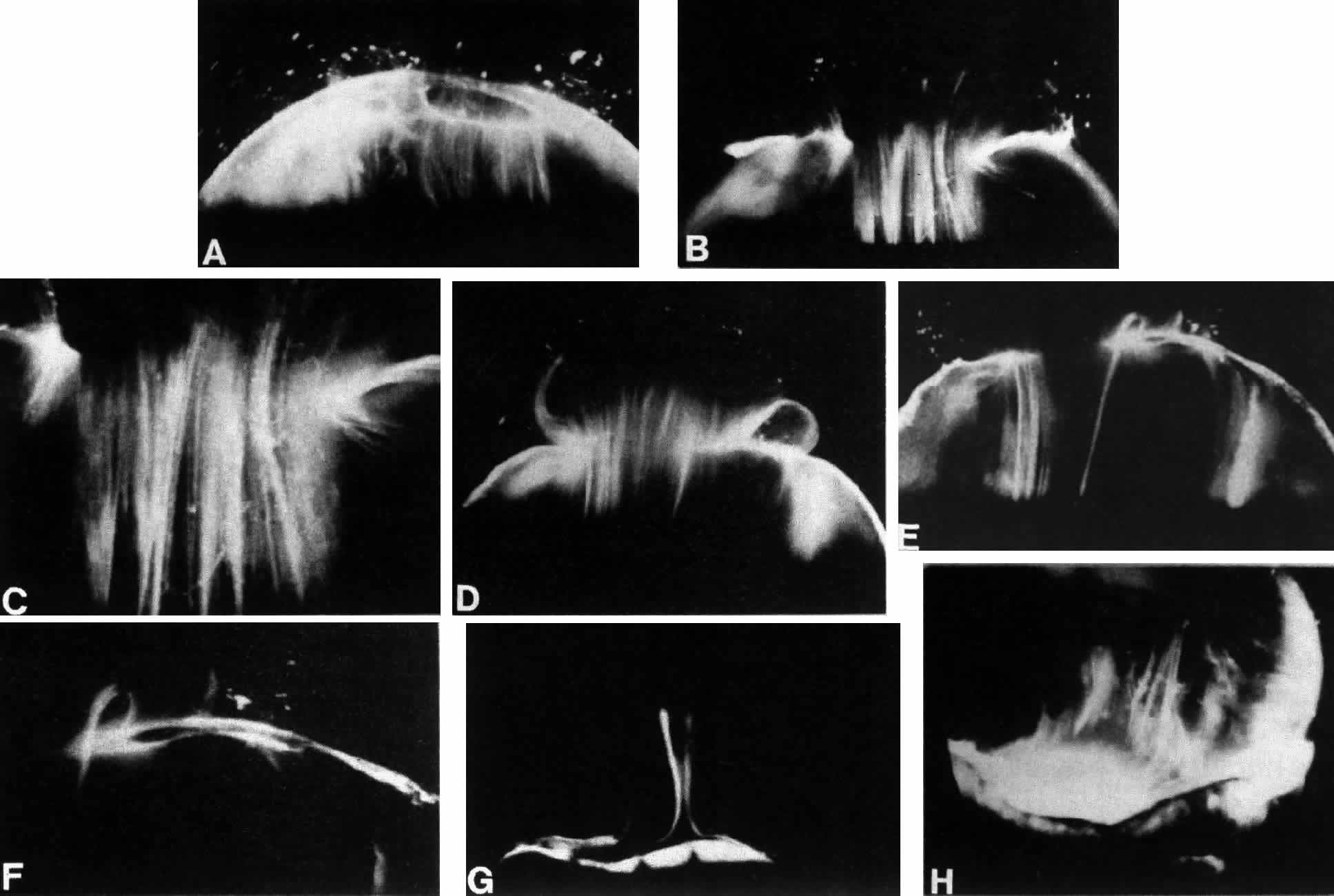
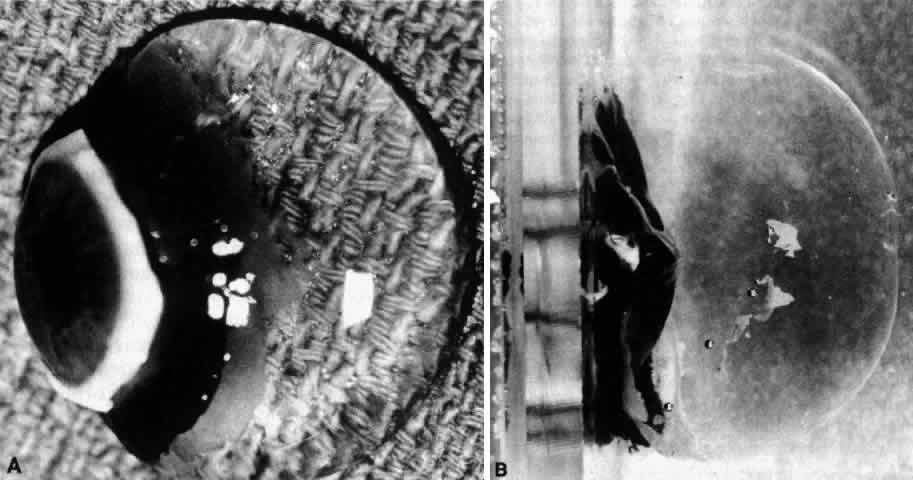
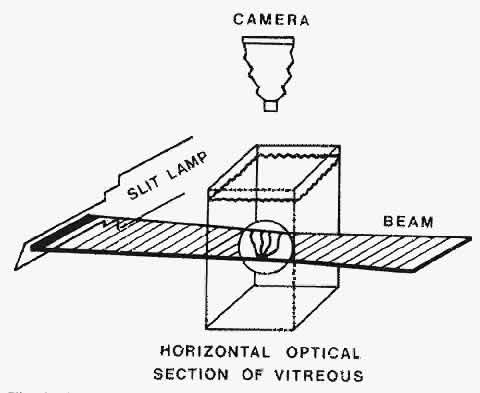
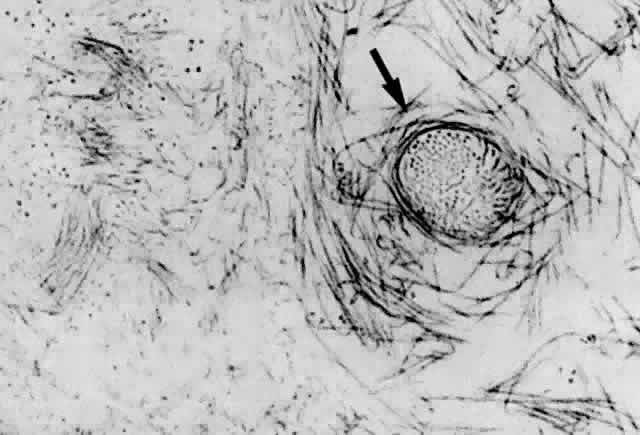
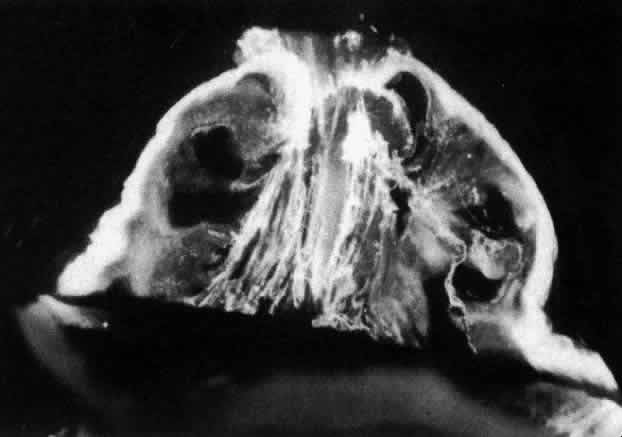
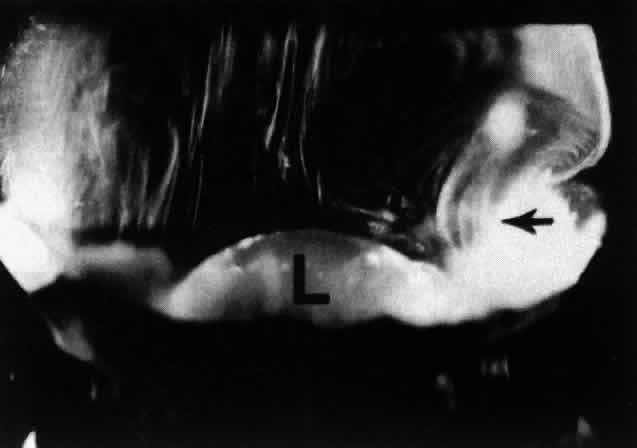
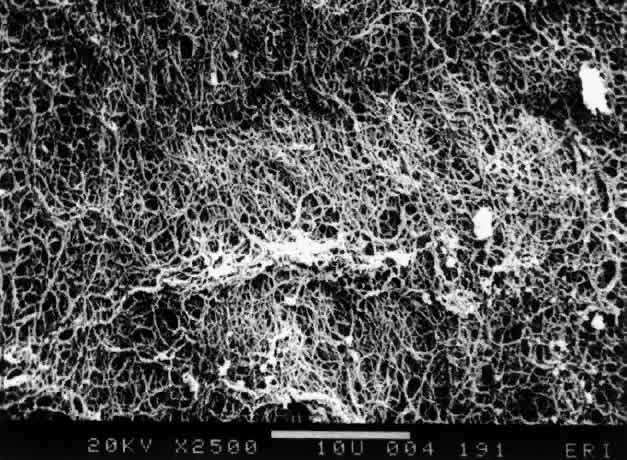
1. Duke-Elder WS: The nature of the vitreous body. Br J Ophthalmol 14(suppl):6, 1930 2. Demours: Observations anatomiques sur la structure cellulaire du corps
vitre. In: Memoires de Paris, 1741 3. Zinn: Descriptio anatomica oculi humani. Göttingen 4. Hannover A: Endeckung des baues des Glaskorpers. Muller Arch 00:467, 1845 5. Bowman W: Observations on the structure of the vitreous humour. Dublin Q J Med Sci 6:102, 1848 6. Retzius R: Om membrana limitans retinae interna. Nord Arch 3(2):1, 1871 7. Szent-Gyorgi A: Untersuchungen ueber die Struktur des Glaskoerpers des Menschen. Arch Mikroscop Anat 89:324, 1917 8. Baurmann M: Untersuchungen ueber die Struktur des Glaskoerpers bei Saeugetieren. Graefes Arch Ophthalmol 110:352, 1922 9. Stroemberg: Zur Frage nach dem Bau des Glaskoerpers. Acta Soc Med Suecanae, 57, 1931 10. Redslob E: Le Corps Vitre, pp 174–178. Paris, Masson, 1932 11. Gullstrand A: Die Nernspaltlampe in der ophthalmologischen Praxis. Klin Monatsbl Augenheilkd 50:483, 1912 12. Koeppe L: Clinical observations with the slit lamp. Arch Ophthalmol 90:232, 1917 13. Baurmann M: Ueber die Beziehungen der ultramikroscopischen Glaskorperstruktur zu den
Spaltlampenbefunden. Graefes Arch Ophthalmol 117:304, 1926 14. Goedbloed J: Studien am Glaskorper: I. Die Struktur des Glaskorpers. Graefes
Arch Ophthalmol p 323, 1934 15. Friedenwald JF, Stiehler RD: Structure of the vitreous. Arch Ophthalmol 14:789, 1935 16. Eisner G: Biomicroscopy of the Peripheral Fundus. New York, Springer-Verlag, 1973 17. Worst JGF: Cisternal systems of the fully developed vitreous body in the young adult. Trans Ophthalmol Soc UK 97:550, 1977 18. Kishi S, Shimizu K: Posterior precortical vitreous pocket. Arch Ophthalmol 108:979, 1990 19. Mann I: The vitreous and suspensory ligament of the lens. In Mann I (ed): The
Development of the Human Eye. New York, Grune & Stratton, 1964 20. Sebag J: The Treacher Collins Prize Essay: Lasers in Ophthalmic Diagnosis. Trans Ophthalmol Soc UK 105:607, 1986 21. Packer AJ, Newsome DA: Practical guidelines for posterior segment biomicroscopy. In
Newsome DA (ed): Retinal Dystrophies and Degenerations, pp 1–4. New
York, Raven Press, 1988 22. Jalkh AE, Trempe CL: Clinical methods of vitreous examination. In Schepens
CL, Neetens A (eds): The Vitreous and Vitreoretinal Interface, pp 73–83. New
York, Springer-Verlag, 1987 23. Kiryu J, Ogura Y, Shahidi M et al: Enhanced visualization of vitreoretinal interface by laser biomicroscopy. Ophthalmology 100:1040, 1993 24. Charles S: Vitreous Microsurgery, pp 7–8. Baltimore, Williams & Wilkins, 1981 25. Boruchoff SA: Corneo-vitreal contact. Trans Ophthalmol Soc UK 95:417, 1975 26. Lacqua H, Machemer R: Clinico-pathologic correlation in massive preretinal proliferation. Am J Ophthalmol 80:913, 1975 27. Scott JD: Treatment of massive vitreous retraction. Trans Ophthalmol Soc UK 95:429, 1975 28. Awan KJ, Thurmayan M: Changes in the contralateral eye in uncomplicated persistent hyperplastic
primary vitreous. Am J Ophthalmol 99:122, 1985 29. El Bayadi G: A new method of slit-lamp micro-ophthalmoscopy. Br J Ophthalmol 37:625, 1953 30. Takahashi M et al: Biomicroscopic evaluation and photography of posterior vitreous detachment. Arch Ophthalmol 98:665, 1980 31. Schepens CL: Retinal Detachment and Allied Diseases. Philadelphia, WB Saunders, 1983 32. Buzney SM, Weiter JJ, Furukawa H et al: Examination of the vitreous: Comparison of biomicroscopy using the Goldmann
and El Bayadi-Kajiura lenses. Ophthalmology 92:1745, 1985 33. Goldmann H: Slit-lamp examination of the vitreous and fundus. Br J Ophthalmol 33:242, 1949 34. Jaffe NS: Complications of acute posterior vitreous detachment. Arch Ophthalmol 79:568, 1968 35. Mainster MA, Grossman JL, Erickson PJ, Heacock GL: Retinal laser lenses: Magnification, spot size, and field of view. Br J Ophthalmol 74:177, 1990 36. Fischer YL, Slatter JS, Friedman RA, Yannuzzi LA: Kinetic ultrasound evaluation of the posterior vitreoretinal interface. Ophthalmology 98:1135, 1991 37. Arzabe CW, Akiba J, Jalkh AE et al: Comparative study of vitreoretinal relationships using biomicroscopy and
ultrasound. Graefes Arch Clin Exp Ophthalmol 220:66, 1991 38. Morner CT: Untersuchung der Proteinsubstanzen in der lichtbrechenden Medien des Auges. Z Physiol Chem 18:223, 1894 39. Young RA: The ground substance of connective tissue. J Physiol 16:325, 1894 40. Pirie A, Schmidt G, Waters JW: Ox vitreous humor: I. The residual protein. Br J Ophthalmol 32:321, 1948 41. Bembridge BA, Crawford CNC, Pirie A: Phase-contrast microscopy of the animal vitreous body. Br J Ophthalmol 36:131, 1952 42. Matolstsky AG, Gross J, Grignolo A: A study of the fibrous components of the vitreous body with the electron
microscope. Proc Soc Exp Biol Med 76:857, 1951 43. Schwarz W: Electron microscopic observations on the human vitreous body. In
Smelser GK (ed): Structure of the Eye, pp 283–291. New York, Academic
Press, 1961 44. Young RG, Williams HH: Biochemistry of the eye--gelatinous protein of the vitreous body. Arch Ophthalmol 51:593, 1954 45. Swann DA: Chemistry and biology of vitreous body. Int Rev Exp Pathol 22:1, 1980 46. Bishop P, McLeod D, Ayad S: Extraction and characterization of the intact form of bovine vitreous type
IX collagen. Biochem Biophys Res Commun 185:392, 1992 47. Snowden JM, Swann DA: Vitreous structure: V. The morphology and thermal stability of vitreous
collagen fibers and comparison to articular cartilage (type II) collagen. Invest Ophthalmol Vis Sci 19:610, 1980 48. Hogan MJ: The vitreous: Its structure in relation to the ciliary body and retina. Invest Ophthalmol 2:418, 1963 49. François J, Victoria-Troncoso V, Albarran E: The histochemical structure of the vitreous fibers studied by phase contrast
microscopy. Am J Ophthalmol 69:763, 1970 50. Streeten BA: Disorders of the vitreous. In Garner A, Klintworth GK (eds): Pathobiology
of Ocular Disease--A Dynamic Approach, part B, chap 49, pp 1381–1419. New
York, Marcel Dekker, 1982 51. Balazs EA: Physiology of the vitreous body in retina surgery with special
emphasis on reoperations. In Schepens CL (ed): Proceedings of the 11th
Conference of the Retina Foundation, pp 29–48. St. Louis, CV
Mosby, 1960 52. Balazs EA: The vitreous. Int Ophthalmol Clin 15:53–63, 1973 53. Berman ER, Michaelson IC: The chemical composition of the human vitreous body as related to age and
myopia. Exp Eye Res 3:9, 1964 54. Weber H, Landuehr G, Kilp H, Neubauer H: Mechanical properties of the vitreous in pig and human donor eyes. Ophthalmic Res 14:335, 1982 55. Balazs EA, Laurent TC, Laurent UBG et al: Studies on the structure of the vitreous body: VIII. Comparative biochemistry. Arch Biochem Biophys 81:464, 1959 56. Bettelheim FA, Balazs EA: Light-scattering patterns of the vitreous humor. Biochem Biophys Acta 158:309, 1968 57. Birck DE, Zychard EI: Collagen fibrillogenesis in situ: Fibril segments are intermediates in matrix assembly. Proc Natl Acad Sci USA 86:4549, 1989 58. Meyer K, Palmer JW: The polysaccharide of the vitreous humor. J Biol Chem 107:629, 1934 59. Meyer K: Chemical structure of hyaluronic acid. Fed Proc 17:1075, 1958 60. Balazs EA: Molecular morphology of the vitreous body. In Smelser GK (ed): The
Structure of the Eye, pp 293–310. New York, Academic Press, 1961 61. Comper WD, Laurent TC: Physiological functions of connective tissue polysaccharides. Physiol Rev 58:255, 1978 62. Balazs EA, Denlinger JL: Aging changes in the vitreous. In: Aging and Human
Visual Function, pp 45–57. New York, Alan R Liss, 1982 63. Atkins EDT, Sheehan JK: Structure for hyaluronic acid. Nature (New Biol) 235:253, 1972 64. Atkins EDT, Phelps CF, Sheehan JK: The conformation of the mucopolysaccharides--hyaluronates. Biochem J 128:1255, 1972 65. Chakrabarti B, Park JW: Glycosaminoglycans--Structure and interaction. CRC Crit Rev Biochem 8:255, 1980 66. Christiansson J: Changes in mucopolysaccharides during alloxan diabetes in the rabbit. Acta Ophthalmol 36:141, 1958 67. Xiong H, Cheng HM: Change of vitreous toxicity in “sugar” cataracts. Invest Ophthalmol Vis Sci 29:149, 1988 68. Yamashita H, Hori S: A new model of neovascularization caused by tractional force. Invest Ophthalmol Vis Sci 29:177, 1988 69. Tasman WS: Diabetic vitreous hemorrhage and its relationship to hypoglycemia. Mod Probl Ophthalmol 20:413, 1979 70. Osterlin SE, Balazs EA: Macromolecular composition and fine structure of the vitreous in the owl
monkey. Exp Eye Res 7:534, 1968 71. Balazs EA: Structure of vitreous gel. In: Acta XVII Concilium Ophthalmologicum, vol 11, p 1019. 1954 72. Balazs EA: In Acta XVIII Concilium Ophthalmologicum, vol II, p 1296. Brussels, 1958 73. Balazs EA: Die Mikrostruktur und Chimie des Glaskorpers. In Jaeger W (ed): Bericht
uber die 68 Zusammen Kunst der Deutschen Ophthalmologischen
Besemschaft, Heidelberg, 1967, pp 536–572. Munich, JF Bergmann
Verlag, 1968 74. Balazs EA: Functional anatomy of the vitreous. In Duane TD, Jaeger EA (eds): Biomedical
Foundations of Ophthalmology, vol 1, chap 17. Philadelphia, JB
Lippincott, 1982 75. Ali S, Bettelheim FA: Distribution of freezable and non-freezable water in bovine vitreous. Curr Eye Res 3:1233, 1984 76. Schwarz W: Electron microscopic study on the gel of the central part of the corpus
vitreum in the ox. Cell Tissue Res 168:271, 1976 77. Streeten BA: The nature of the ocular zonule. Trans Am Ophthalmol Soc 80:823, 1982 78. Asakura A: Histochemistry of hyaluronic acid of the bovine vitreous body as studied
by electron microscopy. Acta Soc Ophthalmol Jpn 89:179, 1985 79. Sebag J: The Vitreous--Structure, Function, Pathobiology, pp 60–61. New
York, Springer-Verlag, 1989 80. Scott JE: Proteoglycan: Collagen interactions and corneal ultrastructure. Biochem Soc Trans 19:877, 1991 81. Scott JE: The chemical morphology of the vitreous. Eye 6:553, 1992 82. Sebag J: Ageing of the vitreous. Eye 1:254, 1987 83. Sebag J, Buckingham B, Reiser KA, Charles MA: Biochemical abnormalities in vitreous collagen from patients with proliferative
diabetic retinopathy. Arch Ophthalmol 110:1472, 1992 84. Bettelheim FA, Popdimitrova N: Hyaluronic acid--a syneretic glycosaminoglycans. Curr Eye Res 11:411, 1992 85. Hamming NA, Apple DJ, Geiser DK, Vygantas CM: Ultrastructure of hyaloid vasculature in primates. Invest Ophthalmol Vis Sci 16:408, 1977 86. Bloom GD, Balazs EA, Ozanics V: The fine structure of the hyaloid arteriole in bovine vitreous. Exp Eye Res 31:129, 1988 87. Balazs EA, Denlinger JL: The vitreous. In Davson H (ed): The Eye, vol 1A, pp 533–589. London, Academic Press, 1984 88. Sebag J: La structure fibreuse du corps vitre humain. Bull Mem Soc Fr Ophthalmol 96:395, 1985 89. Sebag J, Balazs EA: Human vitreous fibres and vitreoretinal disease. Trans Ophthalmol Soc UK 104:123, 1985 90. Sebag J, Balazs EA: Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci 30:1867, 1989 91. Sebag J: Age-related differences in the human vitreoretinal interface. Arch Ophthalmol 109:966, 1991 92. Sebag J: Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol 225:89, 1987 93. Sebag J: Letter to the editor. Arch Ophthalmol 109:1059, 1991 94. Jongebloed WL, Humalda D, Worst JFG: A SEM correlation of the anatomy of the vitreous body: Making visible the
invisible. Doc Ophthalmol 64:117, 1986 95. Jongebloed WL, Worst JFG: The cisternal anatomy of the vitreous body. Doc Ophthalmol 67:183, 1987 96. O'Malley P: The pattern of vitreous syneresis: A study of 800 autopsy eyes. In
Irvine AR, O'Malley P (eds): Advances in Vitreous Surgery, pp 17–33. Springfield, IL, Charles C Thomas, 1976 97. Oksala A: Ultrasonic findings in the vitreous body at various ages. Graefes Arch Klin Exp Ophthalmol 207:275, 1978 98. Reeser FH, Aaberg T: Vitreous humor. In Records PE (ed): Physiology of
the Human Eye and Visual System, pp 1–31. Hagerstown, MD, Harper & Row, 1979 99. Gartner J: Electron microscopic study on the fibrillar network and fibrocyte-collagen
interactions in the vitreous cortex at the ora serrata of human eyes
with special regard to the role of disintegrating cells. Exp Eye Res 42:21, 1986 100. Gloor BP, Daicker BC: Pathology of the vitreo-retinal border structures. Trans Ophthalmol Soc UK 95:387, 1975 101. Gartner J: The fine structure of the vitreous base of the human eye and the pathogenesis
of pars planitis. Am J Ophthalmol 71:1317, 1971 102. Teng CC, Chi HH: Vitreous changes and the mechanism of retinal detachment. Am J Ophthalmol 44:335, 1957 103. Schepens CL: Personal communication, 1983 104. Gartner J: New research on the aetiology and surgery of retinal detachment. Mod Probl Ophthalmol 15:112, 1975 105. Foos RY: Posterior vitreous detachment. Trans Am Acad Ophthalmol Otolaryngol 76:480, 1972 106. Chaine G, Sebag J, Coscas G: The induction of retinal detachment. Trans Ophthalmol Soc UK 103:480, 1983 107. Best F: Der Glaskoerper bei Augen bewegangen. Klin Monatsbl Augenheilkd 42:538, 1984 108. Leber T, Graefe A, Saemisde ET: Handbuch der Gesamten Augenheilkunde, vol 7. Berlin, Springer, 1916 109. Teng CC, Katzin HM: An anatomic study of the peripheral retina: III. Congenital retinal rosettes. Am J Ophthalmol 36:169, 1953 110. Kanski JJ: Complications of acute posterior vitreous detachment. Am J Ophthalmol 80:44, 1975 111. Schepens CL: Subclinical retinal detachments. Arch Ophthalmol 47:593, 1952 112. Rutnin U, Schepens CL: Fundus appearance in normal eyes: III. Peripheral degeneration. Am J Ophthalmol 64:1040, 1967 113. Byer NE: Lattice degeneration of the retina. Surv Ophthalmol 23:213, 1979 114. Foos RY: Vitreous base, retinal tufts and retinal tears: Pathogenic relationships. In
Pruett RC, Regan CDJ (eds): Retina Congress. New York, Appleton-Century-Crofts, 1974 115. Sigelman J: Vitreous base classification of retinal tears: Clinical application. Surv Ophthalmol 25:59, 1980 116. Straatsma BR, Allen RA: Lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol 66:600, 1962 117. Sato K, Tsunakawa N, Inaba K, Yanagisawa Y: Fluorescein angiography on retinal detachment and lattice degenerations: I. Equatorial
degeneration with idiopathic retinal detachment. Acta Soc Ophthalmol Jpn 75:635, 1971 118. Foos RY, Wheeler NC: Vitreoretinal juncture--synchisis senilis and posterior vitreous detachment. Ophthalmology 89:1502, 1982 119. Foos RY, Simons KB: Vitreous in lattice degeneration of retina. Ophthalmology 91:452, 1984 120. Byer NE: Discussion: Vitreous in lattice degeneration of retina. Ophthalmology 91:457, 1984 121. Tolentino FI, Schepens CL, Freeman HM: Vitreoretinal Disorders, p 1976. Philadelphia, WB
Saunders, 1967 122. Daicker B: Sind die symptome “Weiss mit Druck” und “Weiss ohne Druck” durch
die peripherie netzshautsklerose bedingt? Mod Probl Ophthalmol 15:82, 1975 123. Watzke RC: The ophthalmoscopic sign “white with pressure”: A clinicopathologic
correlation. Arch Ophthalmol 66:812, 1961 124. Green WR: Vitreoretinal juncture. In Ryan SJ (ed): Retinal Disease, pp 13–69. St. Louis, CV
Mosby, 1989 125. Byer NE: The Peripheral Retina in Profile: A Stereoscopic Atlas. Torrance, CA, Criterion
Press, 1982 126. Lindner B: Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol 87(suppl):1, 1966 127. Tasman WS: Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol 72:217, 1968 128. Novak MA, Welch RB: Complications of acute symptomatic posterior vitreous detachment. Am J Ophthalmol 97:308, 1984 129. Hyams SW, Neumann E: Peripheral retina in myopia with particular reference to retinal breaks. Br J Ophthalmol 53:300, 1969 130. Pickett-Seltner RL, Doughty MJ, Pasternak JJ, Sivak JG: Proteins of the vitreous humor during experimentally induced myopia. Invest Ophthalmol Vis Sci 33:3424, 1992 131. Hyams SW, Neumann E, Friedman Z: Myopia-aphakia: II. Vitreous and peripheral retina. Br J Ophthalmol 59:483, 1975 132. Hovland KR: Vitreous findings in fellow eyes of aphakic retinal detachment. Am J Ophthalmol 86:350, 1978 133. Bradford JD, Wilkinson CP, Fransen SR: Pseudophakic retinal detachments--the relationships between retinal tears
and the time following cataract surgery at which they occur. Retina 9:181, 1989 134. Byer NE: Clinical study of retinal breaks. Trans Am Acad Ophthalmol Otolaryngol 71:461, 1967 135. Combs JL, Welch RB: Retinal breaks without detachment: Natural history, management, and long-term
follow-up. Trans Am Ophthalmol Soc 80:64, 1982 136. Lindner K: Zur Klinik des Glaskorpers: III. Glaskorper und Netzhautabhebung. Graefes Arch Ophthalmol 137:157, 1937 137. Rosengren B, Osterlin S: Hydrodynamic events in the vitreous space accompanying eye movements: Significance
for the pathogenesis of retinal detachment. Ophthalmologica 173:513, 1976 138. Oksala A: Ultrasonic findings in the vitreous space in patients with detachment of
the retina. Graefes Arch Klin Exp Ophthalmol 202:197, 1977 139. Duke-Elder WS, MacFaul PA: Concussion changes in the vitreous. In Duke-Elder
WS (ed): System of Ophthalmology, vol 14, p 197. London, Henry Kimpton, 1972 140. Tolentino FI, Lee PF, Schepens CL: Biomicroscopic study of the vitreous cavity in diabetic retinopathy. Arch Ophthalmol 75:238, 1966 141. Scheiffarth OF, Kampik A, Gunther H et al: Proteins of the extracellular matrix in vitreoretinal membranes. Graefes Arch Clin Exp Ophthalmol 226:357, 1988 142. Bellhorn MB, Friedman AH, Wise GN, Henkind P: Ultrastructure and clinicopathologic correlation of idiopathic preretinal
macular fibrosis. Am J Ophthalmol 79:366, 1975 143. Clarkson JG, Green WR, Massof D: A histopathologic review of 168 cases of preretinal membranes. Am J Ophthalmol 84:1, 1977 144. Wallow IHL, Tso MOM: Proliferation of the RPE over malignant choroidal tumors. Am J Ophthalmol 73:914, 1972 145. Mueller-Jensen J, Machemer R, Azuronia R: Auto-transplantation of retinal pigment epithelium in intravitreal diffusion
chamber. Am J Ophthalmol 80:530, 1975 146. Campochiaro PA, Jerdan JA, Glaser BM et al: Vitreous aspirates from patients with PVR stimulate RPE cell migration. Arch Ophthalmol 103:1403, 1985 147. Sebag J, Boulton M, Tang M et al: Surface markers and growth factors in human hyalocytes. Invest Ophthalmol Vis Sci 34:1419, 1993 148. Lutty GA, Merges C, Threlkeld AB et al: Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous
and choroid. Invest Ophthalmol Vis Sci 34:477, 1993 149. Fine BS, Tousimis AJ: The structure of the vitreous body and the suspensory
ligaments of the lens. Arch Ophthalmol 65:95, 119, 1961 150. Faulborn J, Bowald S: Combined macroscopic, light microscopic, scanning and transmission electron
microscopic investigation of the vitreous body: II. The anterior
vitreous cortex. Ophthalmic Res 14:117, 1982 151. Rhodes RH: An ultrastructural study of complex carbohydrates in the posterior chamber
and vitreous base of the mouse. Histochem J 17:291, 1985 152. Theopold H, Faulborn J: Scanning electron microscopic aspects of the vitreous body. Mod Probl Ophthalmol 20:92, 1979 153. Jaffe NS: Vitreous traction at the posterior pole of the fundus due to alterations
in the vitreous posterior. Trans Am Acad Ophthalmol Otolaryngol 71:642, 1967 154. Jaffe NS: Macular retinopathy after separation of vitreoretinal adherence. Arch Ophthalmol 78:585, 1967 155. Sebag J: Vitreoretinal interface and the role of vitreous in macular disease. In
Brancato R, Coscasa G, Lumbrosos B (eds): Proceedings of the
Retina Workshop, pp 3–6. Amsterdam, Kugler & Ghedini, 1987 156. Hageman G, Russel G: Enzymatic disinsertion of the primate vitreous body. Exp Eye Res 55:751, 1992 157. Verstraeten TC, Chapman C, Hartzer M et al: Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol 111:849, 1993 158. Schwalbe G: In Engelmann W (ed): Von Graefe--Saemisch's Handbuch der
Gesamnten Augenheilkunde, vol I, p 457. Leipzig, 1874 159. Schwalbe G: Lehrbuch der Anatomie des Auges, p 288. Erlangen, E Besold, 1887 160. Balazs EA, Toth LZ, Eckl EA, Mitchell AP: Studies on the structure of the vitreous body: XII. Cytological and histochemical
studies on the cortical tissue layer. Exp Eye Res 3:57, 1964 161. Gloor BP: Cellular proliferation on the vitreous surface after photocoagulation. Graefes Arch Clin Exp Ophthalmol 178:99, 1969 162. Bloom GD, Balazs EA: An electron microscope study of hyalocytes. Exp Eye Res 4:249, 1965 163. Hogan MJ, Alvarado JA, Weddel JE: Histology of the Human Eye: An Atlas
and Textbook, p 607. Philadelphia, WB Saunders, 1971 164. Saga T, Tagawa Y, Takeuchi T et al: Electron microscopic study of cells in vitreous of guinea pig. Jpn J Ophthalmol 28:239, 1984 165. Balazs EA: Studies on structure of vitreous body: Absorption of ultraviolet light. Am J Ophthalmol 38:21, 1954 166. Jacobson B, Osterlin S, Balazs EA: A soluble hyaluronic acid synthesizing system from calf vitreous. Proc Fed Am Soc Exp Biol 25:588, 1966 167. Osterlin SE: The synthesis of hyaluronic acid in the vitreous. III. In vivo metabolism
in the owl monkey. Exp Eye Res 7:524, 1968 168. Osterlin SE: The synthesis of hyaluronic acid in the vitreous: IV. Regeneration in the
owl monkey. Exp Eye Res 8:27, 1969 169. Berman ER, Gambos GM: Studies on the incorporation of U-14 C-glucose into vitreous polymers in vitro and in vivo. Invest Ophthalmol 18:521, 1969 170. Balazs EA, Sundblad L, Toth LZJ: In vitro formation of hyaluronic acid by cells in the vitreous body and
by lamb tissue. Abstr Fed Proc 17:184, 1958 171. Bleckmann H: Glycosaminoglycan metabolism of cultured fibroblasts from bovine vitreous. Graefes Arch Clin Exp Ophthalmol 222:90, 1984 172. François J, Victoria-Troncoso V, Maudgal PC: Immunology of the vitreous body. Mod Probl Ophthalmol 16:196, 1976 173. Rhodes RH, Mandelbaum SH, Minckler DS, Cleary PE: Tritiated fucose incorporation in the vitreous body, lens and zonules of
the pigmented rabbit. Exp Eye Res 34:921, 1982 174. Jacobson B: Degradation of glycosaminoglycans by extracts of calf vitreous hyalocytes. Exp Eye Res 39:373, 1984 175. Haddad A, Almeida JC, Laicine EM et al: The origin of the intrinsic glycoproteins of the rabbit vitreous body: An
immunohistochemical and autoradiographic study. Exp Eye Res 50:555, 1990 176. Haddad A, Laicine EM: Studies on the origin of the glycoproteins of the rabbit vitreous body
using a protein synthesis inhibitor and radioactive fucose and amino acids. Germ J Ophthalmol 2:127, 1993 177. Newsome DA, Linsemayer TF, Trelstad RJ: Vitreous body collagen: Evidence for a dual origin from the neural retina
and hyalocytes. J Cell Biol 71:59, 1976 178. Ayad S, Weiss JB: A new look at vitreous humour collagen. Biochem J 218:835, 1984 179. Hoffmann K, Baurwieg H, Riese K: Uber Gehalt und Vertailang niederund hoch molekularer Substanzen in Glaskorper: II. Hoch
molekulare Substanzen (LDH, MDH, GOT). Graefes Arch Clin Exp Ophthalmol 191:231, 1974 180. Teng CC: An electron microscopic study of cells in the vitreous of the rabbit eye: I. The
macrophage. Eye Ear Nose Throat Month 48:91, 1969 181. Freeman MI, Jacobson B, Toth LZ, Balazs EA: Lysosomal enzymes associated with hyalocyte granules: I. Intercellular
distribution patterns of enzymes. Exp Eye Res 7:113, 1968 182. Grabner G, Baltz G, Forster O: Macrophage-like properties of human hyalocytes. Invest Ophthalmol Vis Sci 19:333, 1980 183. Forrester JV, Balazs EA: Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology 40:435, 1980 184. Sebag J, Balazs EA, Eakins KE, Kulkarni P: The effect of Na-hyaluronate on prostaglandin synthesis and phagocytosis
by mononuclear phagocytes. Invest Ophthalmol Vis Sci 20:33, 1981 185. Balazs EA, Toth LZ, Ozanics V: Cytological studies on the developing vitreous as related to the hyaloid
vessel system. Graefes Arch Clin Exp Ophthalmol 213:71, 1980 186. Kefalides NA: The biology and chemistry of basement membranes. In Kefalidese
NA (ed): Proceedings of the First International Symposium on the
Biology and Chemistry of Basement Membranes, pp 215–228. New York, Academic
Press, 1978 187. Goodnight R, Nagy AR, Ryan SJ: Differential distribution of laminin in rabbit and monkey retinas. Invest Ophthalmol Vis Sci 29:203, 1988 188. Yamada E: Some structural features of the fovea centralis in the human retina. Arch Ophthalmol 82:151, 1969 189. Anderson DR: Ultrastructure of the optic nerve head. Arch Ophthalmol 83:63, 1970 190. Uga A: Some structural features of the retinal Muellerian cells in the juxtaoptic
nerve region. Exp Eye Res 19:105, 1974 191. Heergaard S, Jensen OA, Prause JU: Structure of the vitread face of the monkey optic disc (Macacca mulatta): SEM on frozen resin-cracked optic nerve heads supplemented by TEM and
immunohistochemistry. Graefes Arch Clin Exp Ophthalmol 226:377, 1988 192. Sebag J, Balazs EA: Pathogenesis of C.M.E.: Anatomic consideration of vitreoretinal adhesions. Surv Ophthalmol 28(suppl):493, 1984 193. Zimmerman LE, Straatsma BR: Anatomic relationships of the retina to the
vitreous body and to the pigment epithelium. In Schepens CL (ed): Importance
of the Vitreous Body in Retina Surgery with Special Emphasis on
Regeneration. St. Louis, CV Mosby, 1960 194. Kuwabara T, Cogan DG: Studies of retinal vascular patterns: I. Normal architecture. Arch Ophthalmol 64:904, 1960 195. Pedler C: The inner limiting membrane of the retina. Br J Ophthalmol 45:423, 1961 196. Wolter JR: Pores in the internal limiting membrane of the human retina. Acta Ophthalmol 42:971, 1964 197. Mutlu F, Leopold IH: Structure of the human retinal vascular system. Arch Ophthalmol 71:93, 1964 198. Gartner J: Electron microscopic observations on the ciliozonular border of the human
eye with particular reference to the aging changes. Z Anat Entwickl Gesch 131:263, 1970 199. Gersh I, Catchpole HR: The organization of ground substance and basement membrane and its significance
in tissue injury, disease and growth. Am J Anat 85:457, 1949 200. Caulfield JB: Medical progress in the application of the electron microscope to renal
disease. N Engl J Med 270:183, 1964 201. Michaelson IC: The mode of development of the vascular system of the retina with some
observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 68:137, 1948 202. Sebag J, McMeel JW: Diabetic Retinopathy: Pathogenesis and role of retina-derived growth factor
in angiogenesis. Surv Ophthalmol 30:377, 1986 203. Glaser BM: Extracellular modulating factors and the control of intraocular neovascularization. Arch Ophthalmol 106:603, 1988 204. Jalkh A, Takahashi M, Topilow HW et al: Prognostic value of vitreous findings in diabetic retinopathy. Arch Ophthalmol 100:432, 1982 205. Foos RY, Krieger AE, Forsythe AB et al: Posterior vitreous detachment in diabetic subjects. Ophthalmology 87:122, 1980 206. Tagawa H, McMeel JW, Furukawa H et al: Role of the vitreous in diabetic retinopathy: I. Vitreous changes in diabetic
retinopathy and in physiologic aging. Ophthalmology 93:596, 1986 207. Sebag J: Abnormalities of human vitreous structure in diabetes. Graefes Arch Clin Exp Ophthalmol 231:257, 1993 208. Sebag J: Letter to the editor. Ophthalmology 100:1599, 1993 209. Kishi S, Shimizu K: Clinical manifestations of posterior precortical vitreous pocket in proliferative
diabetic retinopathy. Ophthalmology 100:225, 1993 210. Chu TG, Green RL, Cano MR et al: Schisis of the posterior vitreous cortex: An ultrasonographic finding in
diabetic retinopathy. Invest Ophthalmol Vis Sci 32:1028, 1992 211. Raviola G: The fine structure of the ciliary zonule and ciliary epithelium. Invest Ophthalmol 10:851, 1971 212. Barraquer J: Totale Linsenextraktion nach Aulosung der Zonula durch Chymotrypsin enzymatische
zonulyse. Klin Monatsbl Augenheilkd 133:609, 1958 213. Takei Y, Smelsen GK: Electron microscopic studies on zonular fibers: II. Changes of the zonular
fibers after the treatment with collagenase, alpha-chymotrypsin and
hyaluronidase. Graefes Arch Klin Exp Ophthalmol 194:153, 1975 214. Swann DA, Sotman SS: The chemical composition of bovine vitreous humour collagen fibres. Biochem J 185:545, 1980 215. Streeten BA, Licari PA, Marucci AA et al: Immunohistochemical comparison of ocular zonules and the microfibrils of
elastic tissue. Invest Ophthalmol Vis Sci 21:130, 1981 216. Kaczurowski MI: The surface of the vitreous. Am J Ophthalmol 63:419, 1967 217. Hilton GF, Grizzard WS: Pneumatic retinopexy: A two-step outpatient operation without conjunctival
incision. Ophthalmology 93:626, 1986 218. Tornambe PE: Pneumatic retinopexy. Surv Ophthalmol 32:270, 1988 219. Sebag J, Tang M: Pneumatic retinopexy using only air. Retina 13:8, 1993 220. Busacca A, Goldmann H, Schiff-Wertheimer S: Biomicroscopie du Corps Vitre
et du Fond d'Oeil. Paris, Masson, 1957 221. Rieger M: Uber die Bedeutung der Aderhausveranderungen fur die Entstehung der Glaskorperabhebung. Graefes Arch Klin Ophthalmol 136:119, 1936 222. Smith TJ, Murata Y, Horwitz AL et al: Regulation of glycosaminoglycan synthesis by thyroid hormone in vitro. J Clin Invest 70:1066, 1982 223. Smith TJ: Dexamethasone regulation of glycosaminoglycan synthesis in cultured human
skin fibroblasts: Similar effects of glucocorticoid and thyroid hormones. J Clin Invest 74:2157, 1984 224. Likar LH, Likar IN, Robinson RW: Levels of acid mucopolysaccharides of the bovine aorta at different stages
of the sexual cycle. J Atheroscl Res 5:388, 1965 225. Sirek OV, Sirek A, Fikar K: The effect of sex hormones on glycosaminoglycan content of canine aorta
and coronary arteries. Atherosclerosis 17:227, 1977 226. Larsen JS: The hyaluronic acid in the rabbit vitreous: Variations following hormonal
treatment. Arch Ophthalmol 60:815, 1971 227. Larsson L, Osterlin S: Posterior vitreous detachment: A combined clinical and physiochemical study. Graefes Arch Clin Exp Ophthalmol 223:92, 1985 228. Spencer WH: Vitreous. In Spencer WH (ed): Ophthalmic Pathology--An Atlas
and Textbook, vol 2, pp 548–588. Philadelphia, WB Saunders, 1985 229. Kuhn W, Hargitay B, Katcholsky M, Eisenberg H: Reversible dilation and contraction by changing the state of ionization
of high polymer acid networks. Nature 165:514, 1956 230. Akiba J, Ueono N, Chakrabarti B: Molecular mechanisms of posterior vitreous detachment. Graefes Arch Clin Exp Ophthalmol 231:408, 1993 231. Foos RY, Gloor BP: Vitreoretinal juncture: Healing of experimental wounds. Graefes Arch Klin Exp Ophthalmol 196:213, 1975 232. Jost BF, Hutton WL, Fuller DG et al: Vitrectomy in eyes at risk for macular hole formation. Ophthalmology 97:843, 1990 233. Berman ER, Voaden M: The vitreous body. In Graymore CN (ed): Biochemistry
of the Eye, pp 373–471. New York, Academic Press, 1970 234. Osterlin SE: Changes in the vitreous with age. Trans Ophthalmol Soc UK 95:372, 1975 235. Wolf E, Gardiner JS: Studies on the scatter of light in the dioptric media of the eye as a basis
of visual glare. Arch Ophthalmol 74:338, 1965 236. Deiter P, Wolf E, Geer S: Glare and the scatter of light in the vitreous: Effect in postoperative
retinal detachment patients. Arch Ophthalmol 87:12, 1972 237. Cibis GE, Watzke RC, Chua J: Retinal hemorrhages: Posterior vitreous detachment. Am J Ophthalmol 79:358, 1975 238. Foos RY, Roth AM: Surface structure of the optic nerve head: II. Vitreopapillary attachments
and posterior vitreous detachment. Am J Ophthalmol 76:622, 1973 239. Murakami K et al: Vitreous floaters. Ophthalmology 90:1271, 1983 240. Moore RF: Subjective “lightning streak.” Br J Ophthalmol 19:545, 1935 241. Wise GN: Relationship of idiopathic preretinal macular fibrosis to posterior vitreous
detachment. Am J Ophthalmol 79:358, 1975 242. Voerhoeff FH: Are Moore's lightning streaks of serious portent. Am J Ophthalmol 41:837, 1956 243. Schachat AP, Sommer A: Macular hemorrhages associated with posterior vitreous detachment. Am J Ophthalmol 102:647, 1986 244. Spencer LM, Foos RY: Paravascular vitreoretinal attachments: Role in retinal tears. Arch Ophthalmol 84:557, 1970 245. Foos RY: Vitreoretinal juncture, epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci 16:416, 1977 246. Kishi S, Demaria C, Shimizu K: Vitreous cortex remmants at the fovea after spontaneous vitreous detachment. Int Ophthalmol Clin 9:253, 1986 247. Gartner J: Photoelastic and ultrasonic studies on the structure and senile changes
of the intervertebral disc and of the vitreous body. Mod Probl Ophthalmol 8:136, 1969 248. Smiddy WE, Michels RG, Glaser BM et al: Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol 197:624, 1988 249. Sebag J, de Bustros S, Wendell R: Disorders of the vitreomacular interface. In
Margo CE, Hamed LM, Mames RN (eds): Diagnosis Problems in Clinical
Ophthalmology, pp 556-563. Philadelphia, WB Saunders, 1994 250. Michels RG: A clinical and histopathologic study of epiretinal membranes affecting
the macula and removed by vitreous surgery. Trans Am Ophthalmol Soc 80:580, 1982 251. Smiddy WE, Maguire AM, Green WR et al: Idiopathic epiretinal membranes: Ultrastructural characteristics and clinicopathologic
correlation. Ophthalmology 96:811, 1989 252. Kelly NE, Wendell RT: Vitreous surgery for idiopathic macular holes. Arch Ophthalmol 109:654, 1991 253. Glaser BM, Michels RG, Kuppermann BD et al: Transforming growth factor ß2 for the treatment of full-thickness macular holes. Ophthalmology 99:1162, 1992 254. Liggett P, Alfaro DV, Horio B et al: Autologous serum as a tissue adhesive in the treatment of idiopathic macular
holes. Ophthalmology 100 (suppl):73, 1993 |