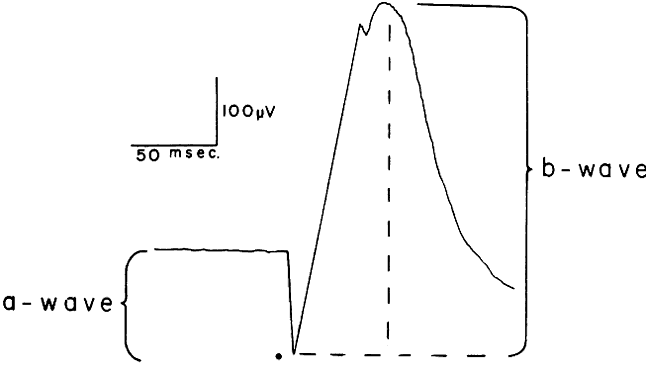
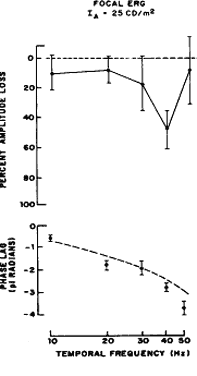
| The ERG is helpful in diagnosing a number of disorders. It can be used To aid in the diagnosis of a generalized degeneration of the retina or
to avoid the mistaken diagnosis of a generalized retinal degeneration
To assess family members where other individuals in the family have a known
hereditary retinal degeneration
To aid in the diagnosis of patients presenting with decreased vision and
nystagmus from birth
To assess retinal retinal function in the presence of vascular occlusions
To assess retinal function with opaque media
To aid in a diagnosis when subjective complaints outweigh objective findings
GENERALIZED DEGENERATION OF THE RETINA Among the multitude of generalized degenerations of the retina, retinitis
pigmentosa is the best known. While this term has been used generically
to describe any generalized retinal degeneration, attention to a
family history and evaluation of other family members, assessment of complaints
that may indicate systemic disease, long-standing uveitis, or
drug use, and careful evaluation of the ERG will help to clarify diagnosis
of disorders in this group and place them into better-defined entities (Table 1). It will also help to avoid a mistaken diagnosis of a generalized retinal
degeneration. TABLE 1. Generalized Retinal Degenerations
Retinitis pigmentosa
Other heredoretinal degenerations Retinitis pigmentosa sine pigmento
Retinitis punctata albescens
Inverse retinitis pigmentosa (cone-rod dystrophy)
Leber's congenital amaurosis
Choroideremia
Gyrate atrophy of retina and choroid
Favre's disease
Wagner's disease
Associated with systemic abnormalities
Pseudoretinitis pigmentosa (see Table 2)
Any patient with a generalized heredoretinal degeneration, of which retinitis
pigmentosa may be considered the prototype, has an abnormal ERG. In
most cases the ERG is extinguished or markedly reduced in amplitude, and
in most instances it has prolonged implicit times.6 In a few cases, usually early in the course of the disease, the ERG is
only slightly affected in terms of amplitude (usually reduction of the
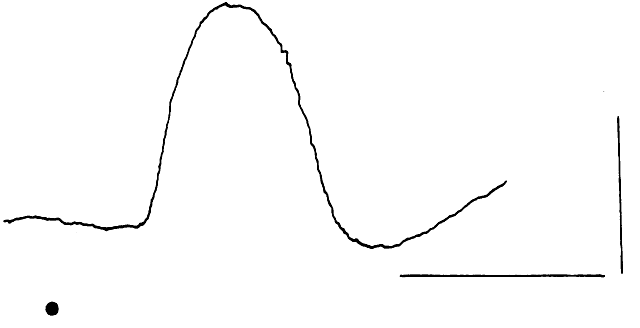
b-wave), but the prolonged photopic implicit time directs the examiner
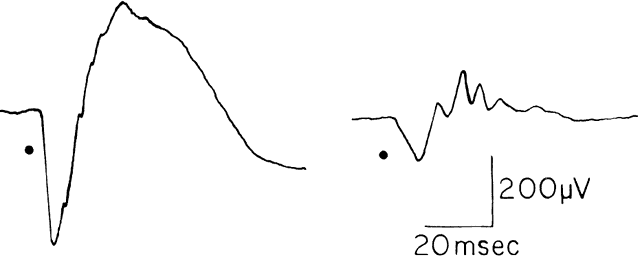
to the appropriate diagnosis7 (Fig. 7). 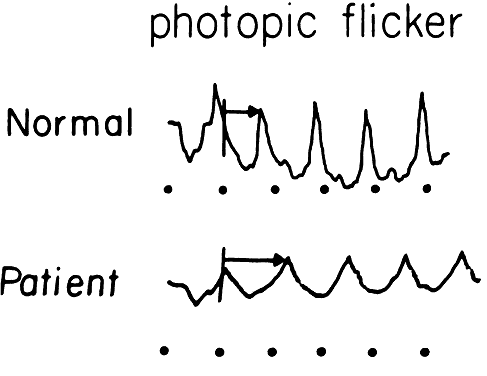 Fig. 7. ERG recordings from a 14-year-old boy with documented autosomal dominant
retinitis pigmentosa. His ERG shows a reduced amplitude and prolonged
photopic implicit time as compared with the normal. Fig. 7. ERG recordings from a 14-year-old boy with documented autosomal dominant
retinitis pigmentosa. His ERG shows a reduced amplitude and prolonged
photopic implicit time as compared with the normal.
|
CASE 1 (FIG. 8). A 49-year-old female had poor night vision from childhood and recent difficulty
going down stairs. There was no family history of any similar
disorder. Vision 20/25 OD, 20/20 OS. Both fundi showed slightly pale
discs, attenuated arterioles, a motheaten appearance of the retina, and
peripheral bone spicules. Visual fields were 10 degrees on a Goldmann
perimeter (III-4E). The ERG was extinguished. 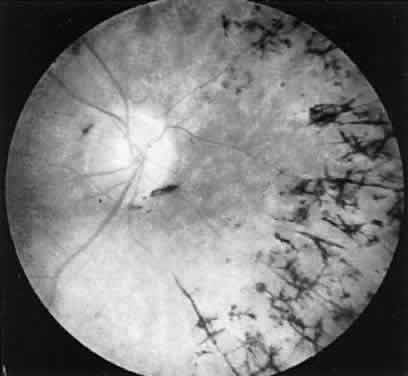 Fig. 8. Case I. See text for details. Fig. 8. Case I. See text for details.
|
This is a classic case of retinitis pigmentosa with the ERG providing confirmatory
evidence to the typical history, clinical picture, and visual
fields. CASE 2 (FIG. 9). A 5-year-old boy was seen for evaluation because of a family history of
choroideremia. His maternal grandfather had this disorder, but the patient
and all other family members had no complaints. Vision 20/20 in
each eye. Both fundi showed mild pigment granularity, but the discs and
arterioles were normal. The ERG was markedly reduced in amplitude under
all testing conditions. 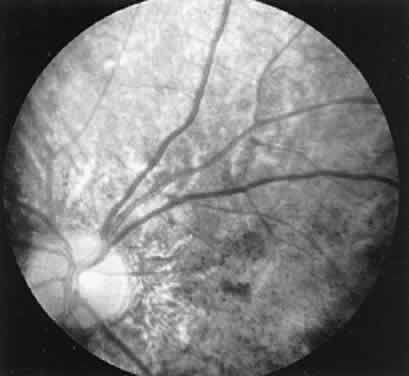 Fig. 9. Case 2. See text for details. Fig. 9. Case 2. See text for details.
|
This boy has electrophysiologic evidence of a generalized tapetoretinal
degeneration. More definitive fundus changes will appear in the future
and symptoms consistent with a widespread retinal degeneration will
ensue. Evaluation of his mother showed funduscopic evidence of a choroideremia
carrier state. All of her psychophysical and electrophysiologic
tests were normal. CASE 3 (FIG. 10). A 57-year-old female was referred because of increasing complaints of
difficulty with her night vision and her side vision. She had a long history
of low-grade uveitis and a progressive decrease in central vision. Visual
acuity 20/100 OD, 20/80 OS. The vitreous showed multiple small
cells. Both retinas showed narrowed arterioles and strands of pigment
in the far periphery. Multiple areas of atrophy of the RPE were seen
throughout. An ERG was extinguished. 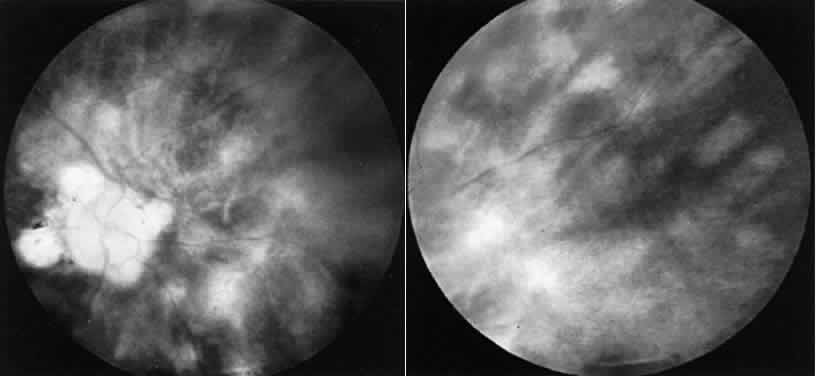 Fig. 10. Case 3. Left. Posterior pole. Right. Peripheral retina. See text for details. Fig. 10. Case 3. Left. Posterior pole. Right. Peripheral retina. See text for details.
|
This patient had birdshot choroiditis, an inflammatory disorder of the
choroid with severe secondary photoreceptor degeneration. The ERG gives
evidence of widespread degeneration, but the history and clinical findings
preclude the diagnosis of a generalized heredoretinal degeneration. This
disorder of birdshot choroiditis may produce a “pseudo-retinitis
pigmentosa” picture8 (Table 2). TABLE 2. Pseudoretinitis Pigmentosa
Infectious diseases Viral encephalitides (e.g., rubella); any of the childhood exanthematous disorders
Disseminated chorioretinitis (e.g., syphilis; birdshot)
Exudative disorders Harada's disease (after resolution of exudative detachments)
Toxemia of pregnancy (after resolution of exudative detachments)
Drug-induced retinal degenerations Phenothiazines
4-amino quinolines
Deferoxamine
Retinoids
Exogenous causes Ophthalmic artery occlusion
Trauma
Metallosis
Cancer associated retinopathy (CAR syndrome)
Miscellaneous Uniocular retinitis pigmentosa
Sector retinitis pigmentosa
Pericentral retinitis pigmentosa
Paravenous chorioretinal dystrophy
Fundus flavimaculatus
Senile reticular degeneration
CASE 4 (FIG. 11). A schizophrenic 29-year-old female was seen for routine eye exam with
vision of 20/20 in each eye and no ocular abnormalities noted. She was
seen for the second time 6 months later with complaints of a rapid decrease
in central vision. Vision was 20/100 OD and OS. Both fundi showed
heavy clumping of pigment throughout the macular area and a scattering
of pigment granules throughout the rest of the retina. The ERG was
extinguished. 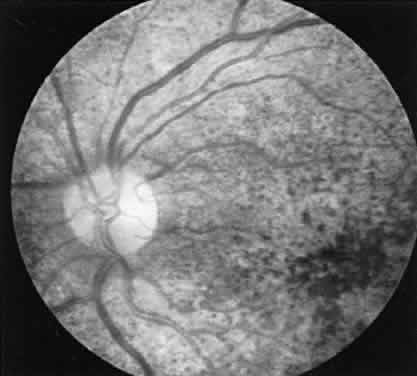 Fig. 11. Case 4. See text for details. Fig. 11. Case 4. See text for details.
|
The ERG gives evidence of a widespread retinal degeneration. The clinical
course and rapid change in the retinal picture is not found in retinitis
pigmentosa. Further history revealed this patient to have been on
high doses of Mellaril for the 5 months preceding her second evaluation. This
known retinotoxic drug was indicted as the cause of the bilateral
retinal degeneration.9 CASE 5 (FIG. 12). A 2-year-old deaf boy was referred with a diagnosis of Usher's syndrome (retinitis
pigmentosa and deafness). His vision seemed good in
both eyes and the parents were unsure as to his ability to see in darkness. Both
fundi showed a generalized granularity throughout. The ERG
was normal.  Fig. 12. Case 5. See text for details. Fig. 12. Case 5. See text for details.
|
The normal ERG precludes a diagnosis of retinitis pigmentosa. While the
mother denied rubella during pregnancy, the constellation of findings
makes this the most likely diagnosis. KNOWN HEREDITARY DEGENERATION Aside from a careful family history, evaluation of other relatives of an
individual with a known hereditary retinal problem may be necessary. For
example, the autosomal dominant form of retinitis pigmentosa is usually
the least severe of the genetic variants and young people with
the problem may have no symptoms and minimal fundus changes.10 Therefore, the ERG becomes the primary objective test to diagnose such
affected individuals. The importance of this also is noted in Case 2, where
a young patient with choroideremia had no symptoms but the ERG
provided the diagnosis. The physician who deals with a hereditary problem becomes the first line
in genetic counseling. Making the correct diagnosis is the initial step, but
it is likewise important to ascertain, as far as possible, the
hereditary mode of the disease. In the case of retinitis pigmentosa, where
all three modes of inheritance are seen, the importance becomes
obvious with regard to future generations. If an individual is seen without
a positive family history, autosomal recessive is statistically
the most likely mode of inheritance.11 But if one further considers an isolated case of a male presenting with
such a problem, the possible diagnosis of X-linked recessive retinitis
pigmentosa cannot be ruled out. In such a case it is important to examine
any female who might be the carrier of the X-linked gene. In a
large percentage of such cases the female carrier shows fundus changes
in the absence of any subjective complaints.12 These may consist of an unusual scintillating reflex in the macular area
or a clumping of pigment in the periphery (Fig. 13). However, these changes are not always seen. In such cases electrophysiologic
studies provide the answer, for it has been found that certain
electrophysiologic abnormalities also are seen in the majority of female
carriers, even those with no fundus abnormalities. These consist
of a prolonged photopic b-wave implicit time and/or a reduction in the
amplitude of the scotopic b-wave in a fully dark-adapted eye.13 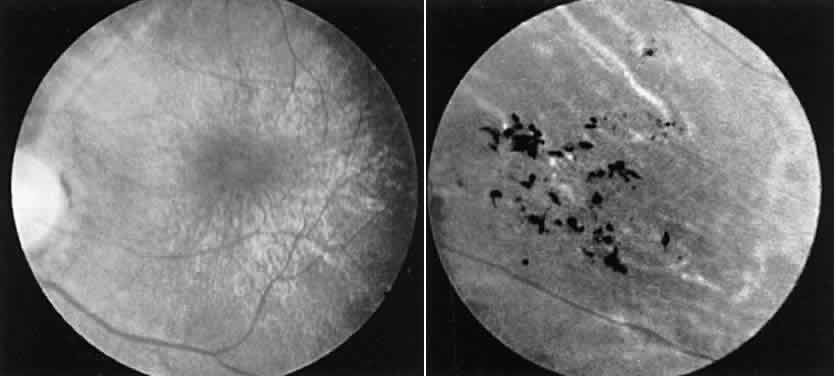 Fig. 13. Female carrier of X-linked retinitis pigmentosa. Fundus photographs of
a 48-year-old female with vision of 20/20 OD and OS. Left. Macular area shows an unusual scintillating reflex around the entire parafoveal
region. Right. Retinal periphery showing an isolated area of retinal pigment epithelial
loss with associated clumps of pigment. Fig. 13. Female carrier of X-linked retinitis pigmentosa. Fundus photographs of
a 48-year-old female with vision of 20/20 OD and OS. Left. Macular area shows an unusual scintillating reflex around the entire parafoveal
region. Right. Retinal periphery showing an isolated area of retinal pigment epithelial
loss with associated clumps of pigment.
|
Other disorders may be diagnosed in an asymptomatic individual on the basis
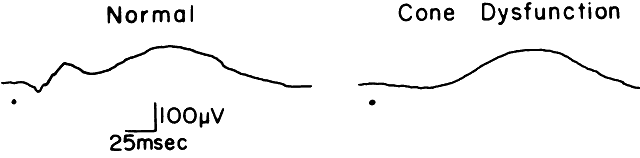
of ERG changes. CASE 6. A 12-year-old female was seen because of a family history of late onset
progressive cone dystrophy. She had no symptoms, vision was 20/30 in
each eye, the fundus exam was normal, and color vision was normal. An
ERG showed a markedly reduced photopic flicker response and a normal
rod response (Fig. 14). 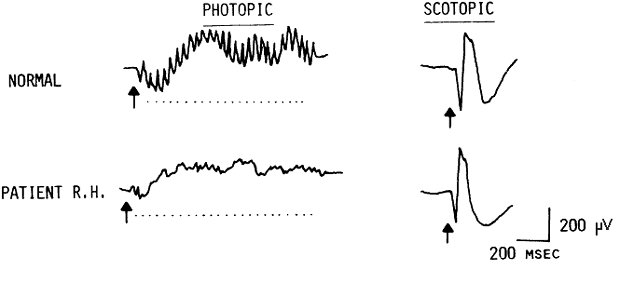 Fig. 14. Case 6. See text for details. Fig. 14. Case 6. See text for details.
|
The ERG provides the diagnosis of a cone dystrophy in this as yet asymptomatic
patient. This disorder appears to initially affect peripheral
cones with gradual progression centrally. As long as the central cones
are intact, vision will remain normal and color vision will likewise
be good. The ultimate visual outcome is an acuity of 20/200. DECREASED VISION AND NYSTAGMUS SINCE BIRTH Patients with this problem are invariably infants, for whom diagnostic
tests are limited to clinical evaluation and objective testing. A large
number of disorders result in these findings and all have in common
a bilateral decrease in central vision (Table 3). TABLE 3. Nystagmus and Decreased Vision from Birth
Disorders of the media (e.g., corneal opacities and cataracts)
Optic nerve diseases Coloboma
Hypoplasia
Atrophy Developmental
Hereditary
Retinal diseases Ophthalmoscopically visible Macular disease Colobomas Hereditary
Infectious Toxoplasmosis
Inclusion cell disease
Hypoplasia Albinism
Aniridia
Idiopathic
Rare bilateral associations Retinopathy of prematurity (ROP)
Persistent hyperplastic primary vitreous (PHPV)
Retinal dysplasia
Ophthalmoscopically variable Leber's congenital amaurosis
Rod monochromatism (achromatopsia)
CSNB with nystagmus and decreased vision
Congenital nystagmus
Many such diagnoses may be made by clinical examination, but three disorders
may have normal retinal evaluations and can be diagnosed only by
the ERG. CASE 7. An 11-month-old boy was evaluated under ketamine anesthesia because of
poor vision and nystagmus from birth. The eye exam was normal. ERG revealed
an absent photopic flicker response and a normal scotopic response. The ERG indicates an absence of cone function and normal rod function. This
congenital hereditary absence or near absence of cones, known as
rod monochromatism or achromatopsia, is inherited as an autosomal recessive
and is nonprogressive.14 The additional symptom of aversion to light and in older persons, poor
color vision, may help in making the diagnosis. CASE 8. A 6-month-old female was examined under ketamine anesthesia because of
poor vision and nystagmus from birth. The parents noted irregular wandering
eye movements and felt the vision to be extremely impaired. Examination
showed mild granularity of the retina and an extinguished ERG. The diagnosis of Leber's congenital amaurosis, a congenital form of
retinitis pigmentosa that is inherited as an autosomal recessive, can
be made on the basis of the ERG. The retina may look nearly normal in
infancy but invariably shows progressive changes during life.15 Vision is usually profoundly impaired but in rare instances may range
from 20/60 to 20/200. Associated somatic abnormalities may include cerebellar
dysfunction, deafness, and mental retardation.16 CASE 9. A 6-year-old boy was evaluated because of nystagmus and poor vision from
birth. The parents stated that the vision had seemed stable and the
child was able to read. Recently they were aware their child had difficulty
at night. Vision was 20/60 OD and OS with a -7.00 spherical equivalent. Fundus
evaluation showed myopic discs but was otherwise normal. The
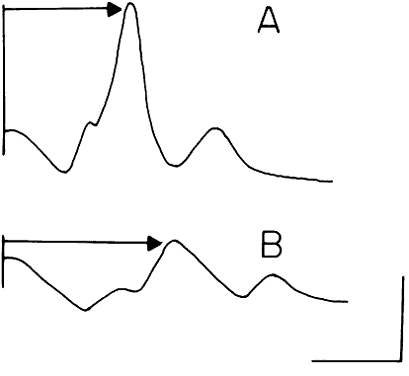
ERG showed a deep normal a-wave but an absent b-wave under scotopic
recording conditions (Fig. 15). 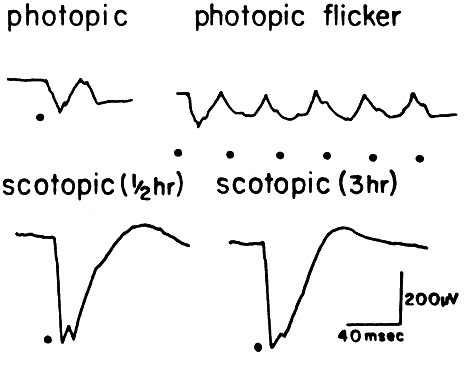 Fig. 15. Case 9. See text for details. Two scotopic recordings were made, one at 30 minutes
dark adaptation and the second after 3 hours of dark adaptation. There
is no change in the waveform in spite of the prolonged adaptation. Fig. 15. Case 9. See text for details. Two scotopic recordings were made, one at 30 minutes
dark adaptation and the second after 3 hours of dark adaptation. There
is no change in the waveform in spite of the prolonged adaptation.
|
The ERG indicates normal photoreceptors as evidenced by the normal a-wave
but an abnormality in the bipolar cell region as evidenced by the absent
b-wave. In this instance the combination of clinical and ERG findings
points to a diagnosis of congenital stationary nightblindness (CSNB), a
stationary disorder and in this variety usually inherited as an
X-linked recessive. This is one of several forms of CSNB (Table 4) and the typical ERG findings in such cases prevents confusion with more
serious disorders, particularly when nightblindness is a presenting
symptom. TABLE 4. Types of Congenital Stationary Nightblindness (CSNB)
CSNB with normal fundi Type I. Shubert-Bornschein Deep a-wave, absent b-wave
Type II. Nougaret or Riggs Reduced a- and b-waves
Type III. X-linked Associated with myopia, nystagmus, and decreased vision
CSNB with abnormal fundi Oguchi's disease Normal a-wave, absent b-wave. Abnormal retinal color disappears with prolonged
adaptation.
Fundus albipunctatus
ERG becomes normal only after prolonged adaptation. Abnormally slow regeneration of visual pigments.
VASCULAR OCCLUSIONS ERG is useful to assess retinal function in the presence of vascular occlusions. Central
retinal vein (CRV) occlusions can be differentiated
into ischemic and nonischemic varieties with the former having a much
more severe prognosis because of the poor visual outcome and the possibility
of developing neovascular glaucoma.17 If the areas of nonperfusion are great enough the ERG b-wave will be affected
since the capillary plexus that is ischemic supplies the midretinal
layers. The ERG thus provides a reliable ancillary test to differentiate
these two varieties of CRV occlusion, particularly if the hemorrhage
is sufficiently widespread so that the capillaries are not visible
on fluorescein angiography. A central retinal artery (CRA) occlusion affects the b-wave if the ischemia
is widespread enough. However, the clinical picture is usually sufficient
to make the diagnosis without electrophysiologic tests. In cases
of ophthalmic artery occlusion, however, where the clinical picture
in the acute stage is similar to central retinal artery occlusion, the
absent ERG can be the most helpful objective test to differentiate
between these disorders.18 CASE 10 (FIG. 16). A 23-year-old female awoke after cesarean section and stated she had no
vision in her right eye. Evaluation showed a normal left eye and a right
eye with no light perception, a dilated pupil that did not react
to light, and an edematous retina with a cherry red spot. The ERG was
extinguished. 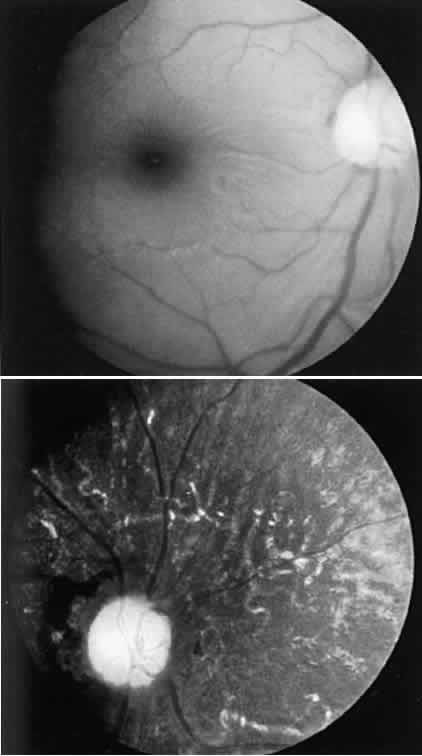 Fig. 16. Case 10. See text for details. Left. Fundus appearance 4 hours after cesarean section. Right. Fundus appearance 6 months later. Fig. 16. Case 10. See text for details. Left. Fundus appearance 4 hours after cesarean section. Right. Fundus appearance 6 months later.
|
This patient's ERG indicates a complete loss of all photoreceptor
function. Although the fundus picture resembles CRA occlusion, the ERG
findings point to ophthalmic artery occlusion, presumably due to pressure
on the eye from the face mask used for anesthesia. OPAQUE MEDIA Since the standard clinical ERG is a mass response reflecting overall viability
of the retina, it is used to assess overall retinal function
when the retina cannot be seen, either because of a cataract or due to
corneal or vitreous opacities. In the former case the cataract acts as
a diffuser of light and in some cases a “supernormal” ERG
is seen. A normal ERG in no way indicates whether central vision is
normal since macular degeneration or optic atrophy do not affect the ERG
amplitude. Corneal opacities likewise tend to diffuse light so a normal
ERG again gives information, but just regarding overall retinal function. If
the cornea is thin or if there is a reason for not recording
with a standard contact lens electrode a gold foil electrode that hooks
over the lid may be used. Vitreous opacities hinder the amount of light stimulating the retina so
a “bright flash” ERG may be necessary.19 This very intense stimulus is usually sufficient to get enough light to
the retina to generate a response and to assess overall retinal function. WHEN SUBJECTIVE COMPLAINTS OUTWEIGH OBJECTIVE FINDINGS As was noted previously, in children who cannot be tested subjectively
the ERG may be one of the major objective tests to evaluate visual function. In
some adults, either because there are no clinical findings to
account for symptoms or because of the patient's inability to communicate, the
ERG may be important for diagnosis. CASE 11. A 40-year-old female complained of progressive loss of side vision. She
had no prior eye problems. Vision was 20/30 OD and OS. Retinal exam
was normal. Visual fields were 5 degrees to a 3/1000 W and did not change
with distance or target size. The ERG was normal. The ERG shows that there are no widespread abnormalities to account for
the constricted fields. Since the ERG only measures outer retinal function, a
visual evoked response (VER) was also performed which was likewise
normal. The diagnosis was nonorganic visual loss. A change in the
patient's job to a less-demanding position and more within her
capabilities led to clearing of all symptoms. CASE 12. A 36-year-old female was seen because of poor night vision all of her
life. Her vision was 20/20 in each eye and visual fields and retinal evaluations
were normal. The ERG showed a deep negative a-wave and an absent
b-wave. These changes, along with the history, are indicative of another form of
CSNB (see Table 4).20 This group of disorders may show normal fundi with good vision. Other
forms (Oguchi's disease; fundus albipunctatus) may show fundus changes
and ERG abnormalities specific to the particular disorder. In Case 12 as
well as Case 9 the abnormality in the bipolar cell region is
probably a result of an abnormality in neural transmission.21 CASE 13. A 36-year-old male was seen because of difficulty seeing print over the
past several years. He also experienced severe glare problems and poor
color vision. Vision was 20/70 OD and 20/100 OS. Fundus exam was normal
with a good foveal reflex. Color testing on the Farnsworth Panel
D-15 was normal. Visual fields showed bilateral central scotoma. An ERG
showed a markedly reduced cone response with a normal rod response. The symptoms and the ERG findings are in keeping with the disorder of late
onset progressive cone dystrophy.22 This disorder is inherited as an autosomal dominant or is seen sporadically. The
disorder seems to start peripherally and gradually moves centrally
until vision ultimately falls to a level of 20/200. As noted in
Case 6, asymptomatic relatives of affected individuals should be checked
because in the early stages, although the intact central cones keep
visual acuity and color vision normal, the widespread loss of peripheral
cones will severely affect the ERG. CASE 14 (FIG. 17). A 6-year-old boy was noted at school to have a visual acuity of 20/60 in
each eye. The parents stated that he had no visual complaints. Both
macular areas showed a glistening reflex, which on stereoscopic examination
was found to represent cystic spaces in the foveal-parafoveal area. An
ERG showed a deep negative a-wave but no b-wave (Fig. 18). 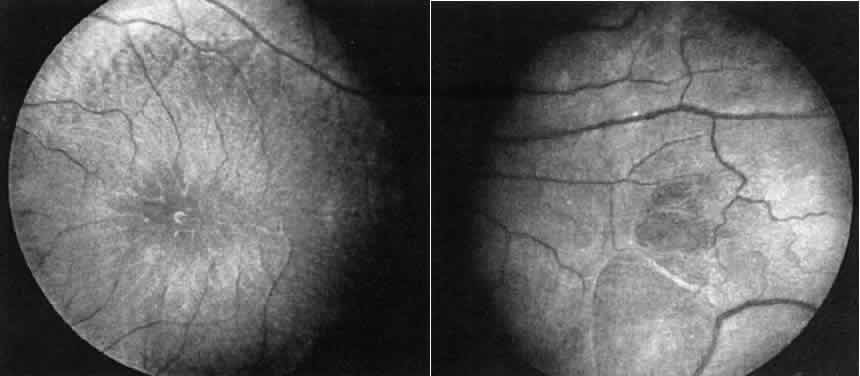 Fig. 17. Case 14. See text for details. Left. Macular area. Right. Peripheral retina of another patient with X-linked retinoschisis showing
the diaphanous “veils” or inner layer retinoschisis. Fig. 17. Case 14. See text for details. Left. Macular area. Right. Peripheral retina of another patient with X-linked retinoschisis showing
the diaphanous “veils” or inner layer retinoschisis.
|
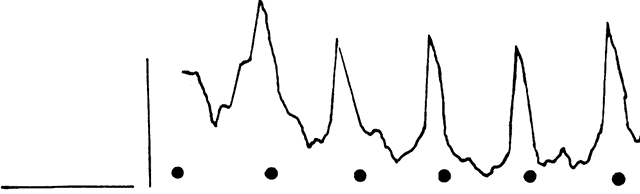
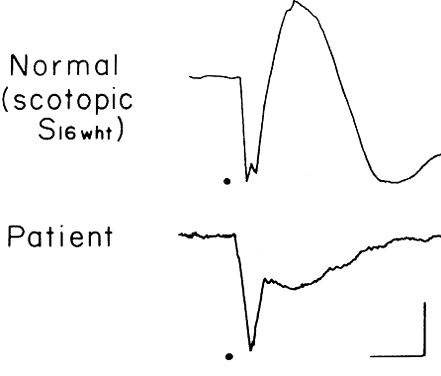 Fig. 18. Case 14. ERG in patient with X-linked retinoschisis. Fig. 18. Case 14. ERG in patient with X-linked retinoschisis.
|
These findings are typical of X-linked juvenile retinoschisis. The abnormality
of the ERG probably reflects widespread midretinal changes that
in some cases result in the peripheral inner layer retinoschisis seen
in 50% of such cases.23 In cases without the peripheral schisis vision usually remains in the 20/60 to 20/80 range. As
the patient gets older the central areas of schisis
may flatten leaving a nondescript central retinal pigment epithelium (RPE) change. In
such cases the typical ERG gives the appropriate
diagnosis. |