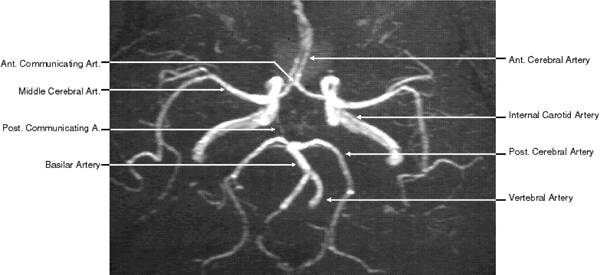
| To diagnose pathologic changes when shown by a particular imaging technique, it
is important to recognize the appearance of normal structures
as they are imaged by these techniques. Not all normal anatomical structures
can be clearly imaged, and their absence on a particular scan
does not necessarily imply the presence of any abnormalities. In particular, small
nerves and vessels, certain muscular structures, particular
regions of the brain, and bony suture lines are frequently beyond the
resolution of current imaging modalities or are missed during the scanning
process owing to slice thickness or interstice skip areas. However, the
vast majority of significant structures related to the eye, orbit, cranial
nerves, and visual pathways can be seen by both MRI and
CT when standard techniques are used.1,43–47 In the future, continued improvements in imaging equipment and techniques
will undoubtedly improve our ability to visualize clearly smaller
and less easily defined structures that currently are beyond the limits
of our technology. The following sections describing the appearance
of normal anatomical structures when imaged by CT and MRI are not meant
to be comprehensive, nor are the images included with the text all inclusive. Rather, what
follows is a detailed overview of the important
structures of interest to the ophthalmologist in understanding the visual
system. The interested reader is referred to the references cited
in the text for additional information and other sources of CT and MRI
images.1,43–47 The globe is shown in Figure 12. The orbit and periorbital structures are shown in Figures 13 through 16, and the optic canal is shown in Figures 17 through 26. The cavernous sinus and optic chiasm are shown in Figures 27 and 28, and the posterior visual pathway and cranial nerves are shown in Figures 29 through 33. 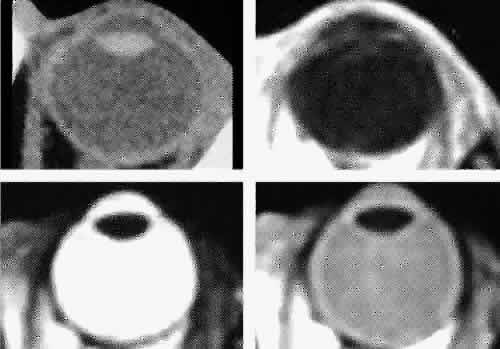 Fig. 12. Axial cuts through the eye. Computed tomography (upper left), T1-weighted
magnetic resonance imaging (upper right), T2-weighted magnetic resonance
imaging (lower left), proton-density magnetic resonance imaging (lower
right). Fig. 12. Axial cuts through the eye. Computed tomography (upper left), T1-weighted
magnetic resonance imaging (upper right), T2-weighted magnetic resonance
imaging (lower left), proton-density magnetic resonance imaging (lower
right).
|
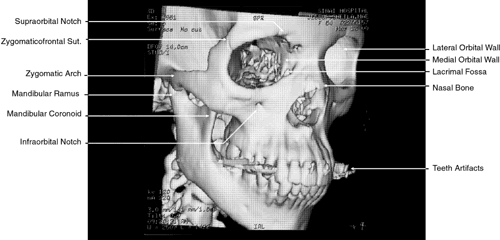
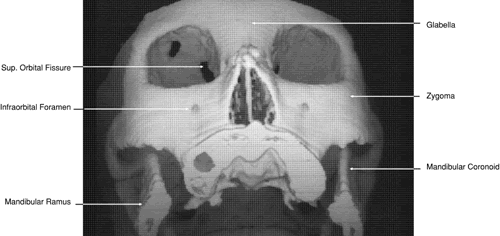 Fig. 13. Three-dimensional reconstruction of orbit and infraorbital structures (Water's
view). Fig. 13. Three-dimensional reconstruction of orbit and infraorbital structures (Water's
view).
|
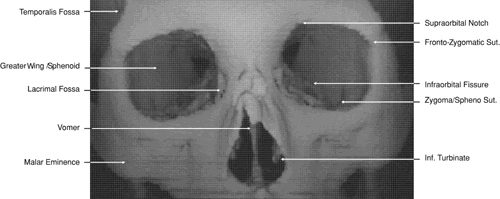 Fig. 14. Three-dimensional reconstruction of orbit (anterior view). Fig. 14. Three-dimensional reconstruction of orbit (anterior view).
|
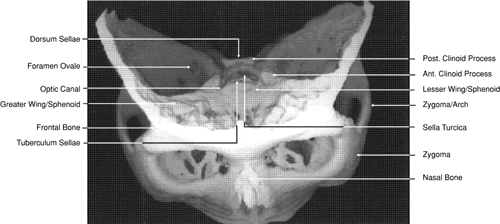 Fig. 15. Three-dimensional reconstruction of orbit and cranial cavity (superior
view). Fig. 15. Three-dimensional reconstruction of orbit and cranial cavity (superior
view).
|
 Fig. 16. Three-dimensional bone reconstruction of cranial cavity structures (view
from posterior cranial fossa). Fig. 16. Three-dimensional bone reconstruction of cranial cavity structures (view
from posterior cranial fossa).
|
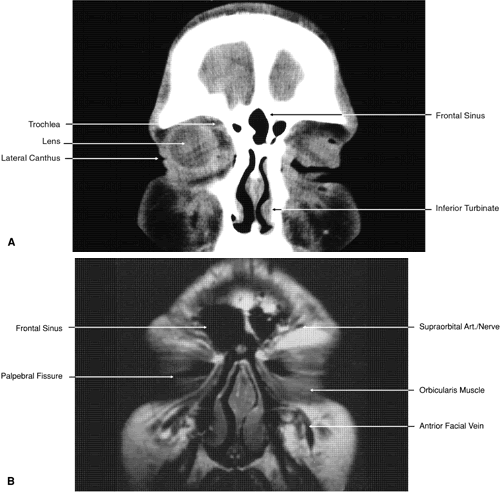 Fig. 17. Coronal images through anterior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 17. Coronal images through anterior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
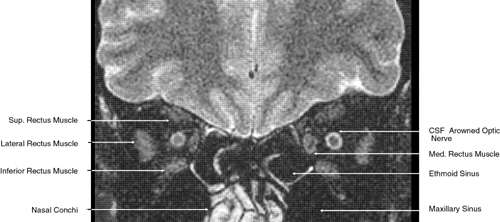
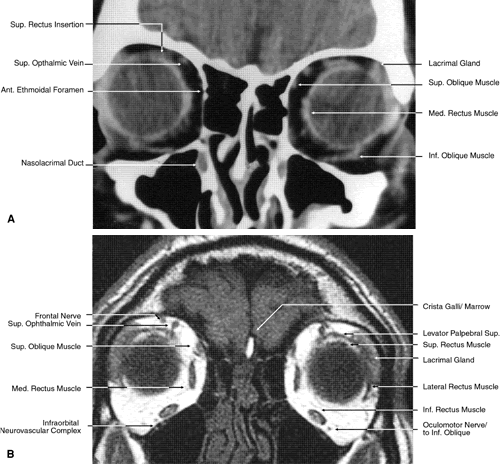 Fig. 18. Coronal images through midglobe. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 18. Coronal images through midglobe. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
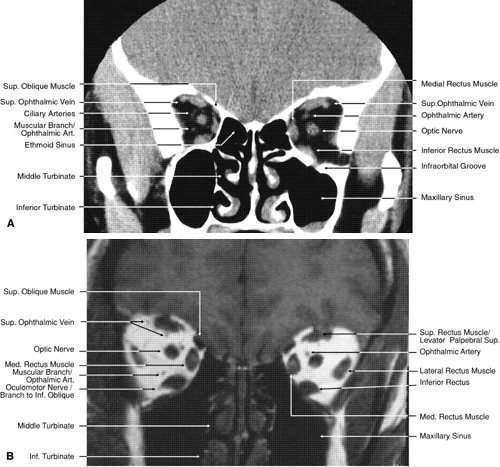 Fig. 19. Coronal images through midorbit posterior to the globe. A. Computed tomography scan.B. T1-weighted magnetic resonance imaging. Fig. 19. Coronal images through midorbit posterior to the globe. A. Computed tomography scan.B. T1-weighted magnetic resonance imaging.
|
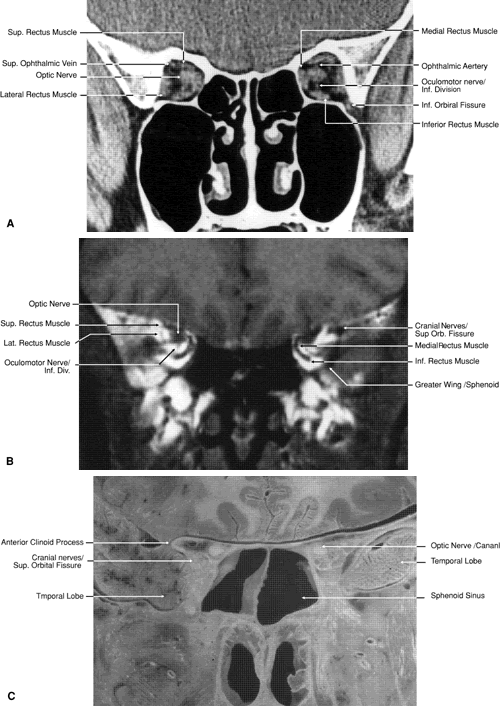 Fig. 20. Coronal images through orbital apex. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. C. Anatomic section of a cadaver head at the level of the orbital apex. Fig. 20. Coronal images through orbital apex. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. C. Anatomic section of a cadaver head at the level of the orbital apex.
|
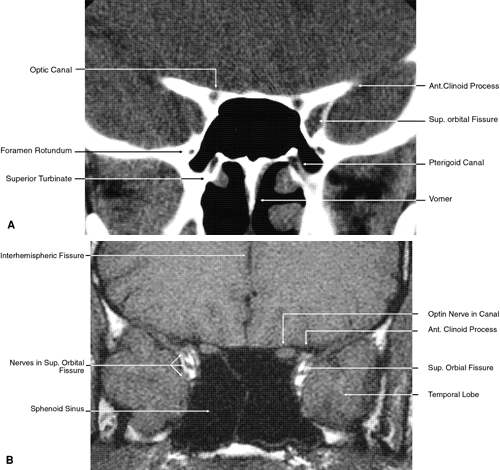 Fig. 21. Coronal images through optic canal. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 21. Coronal images through optic canal. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
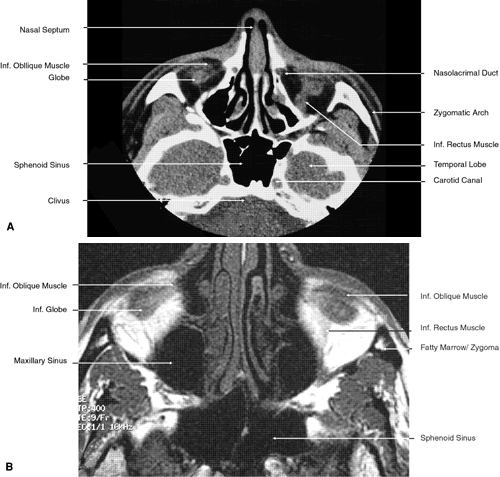 Fig. 22. Axial images at the level of inferior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 22. Axial images at the level of inferior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
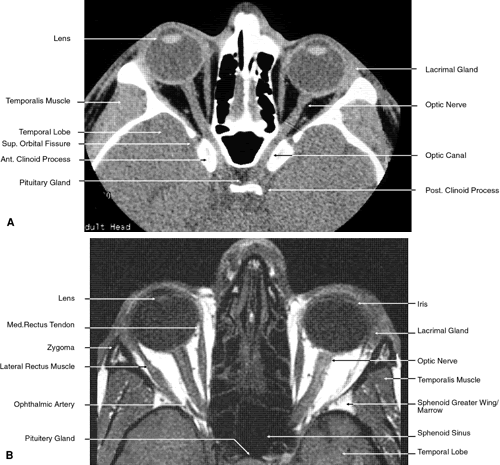 Fig. 23. Axial images at the level of midorbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 23. Axial images at the level of midorbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
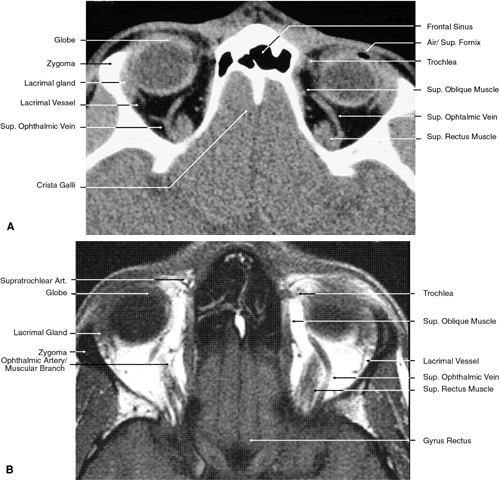 Fig. 24. Axial images at the level of superior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 24. Axial images at the level of superior orbit. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
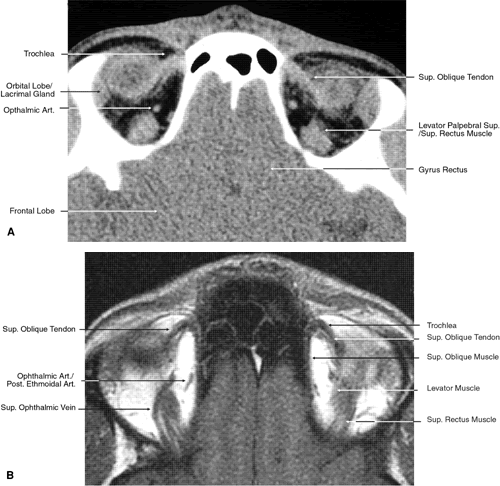 Fig. 25. Axial images at the level of tendon of the superior oblique. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging. Fig. 25. Axial images at the level of tendon of the superior oblique. A. Computed tomography scan. B. T1-weighted magnetic resonance imaging.
|
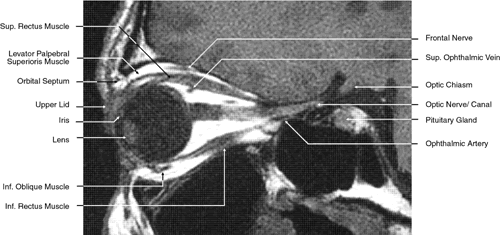 Fig. 26. T1-weighted magnetic resonance imaging; sagittal image through optic nerve. Fig. 26. T1-weighted magnetic resonance imaging; sagittal image through optic nerve.
|
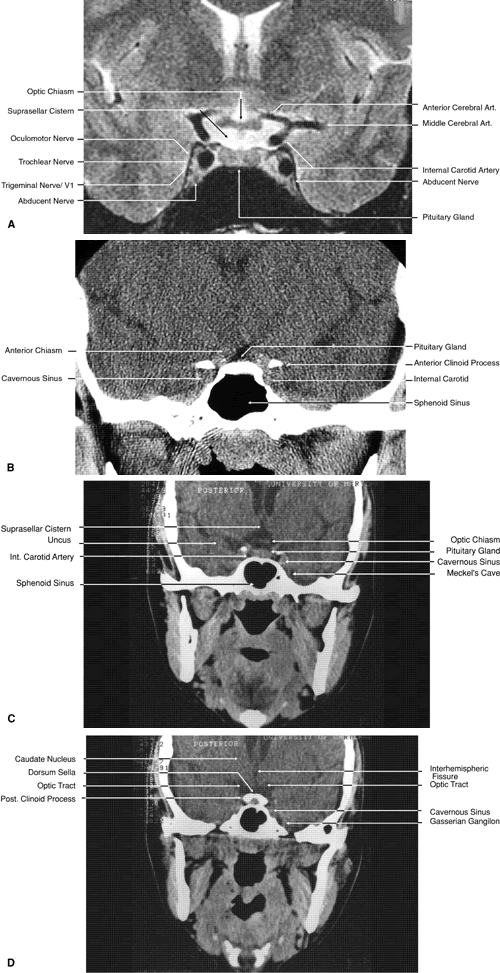 Fig. 27. Coronal images through cavernous sinus and optic chiasm. A. T1-weighted magnetic resonance imaging through anterior chiasm. B. Computed tomography image through anterior chiasm. C. Computed tomography image through posterior chiasm. D. Computed tomography image through optic tract. Fig. 27. Coronal images through cavernous sinus and optic chiasm. A. T1-weighted magnetic resonance imaging through anterior chiasm. B. Computed tomography image through anterior chiasm. C. Computed tomography image through posterior chiasm. D. Computed tomography image through optic tract.
|
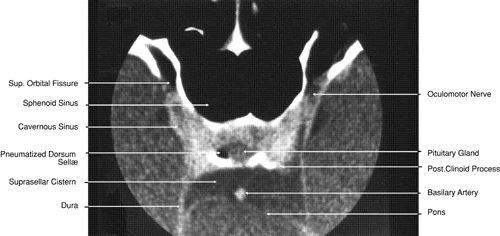 Fig. 28. Axial computed tomography image with contrast medium through cavernous
sinus and pituitary gland. Fig. 28. Axial computed tomography image with contrast medium through cavernous
sinus and pituitary gland.
|
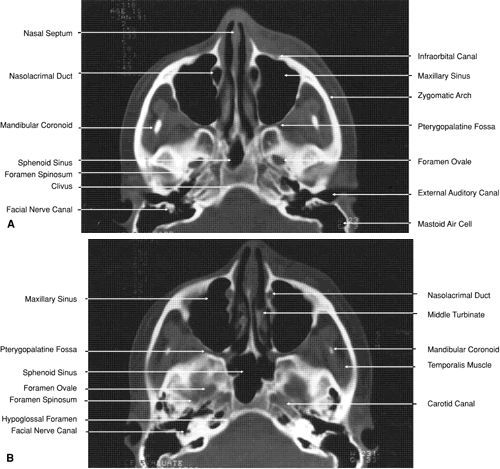 Fig. 29. A. Axial computed tomography soft tissue image at the level of the base of
skull. B. Axial computed tomography bone window image at the level of the base of
skull. Fig. 29. A. Axial computed tomography soft tissue image at the level of the base of
skull. B. Axial computed tomography bone window image at the level of the base of
skull.
|
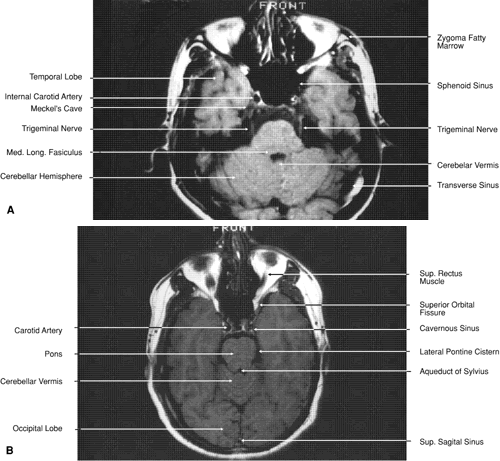 Fig. 30. A. Axial T1-weighted image at the level of floor of orbit and trigeminal
nerve. B. Axial T1-weighted image at the level of oculomotor nerve. Fig. 30. A. Axial T1-weighted image at the level of floor of orbit and trigeminal
nerve. B. Axial T1-weighted image at the level of oculomotor nerve.
|
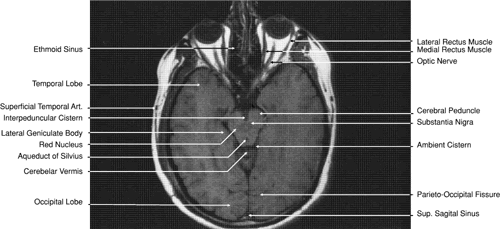 Fig. 31. Axial T1-weighted image through the cerebral peduncle at the level of oculomotor
nerve. Fig. 31. Axial T1-weighted image through the cerebral peduncle at the level of oculomotor
nerve.
|
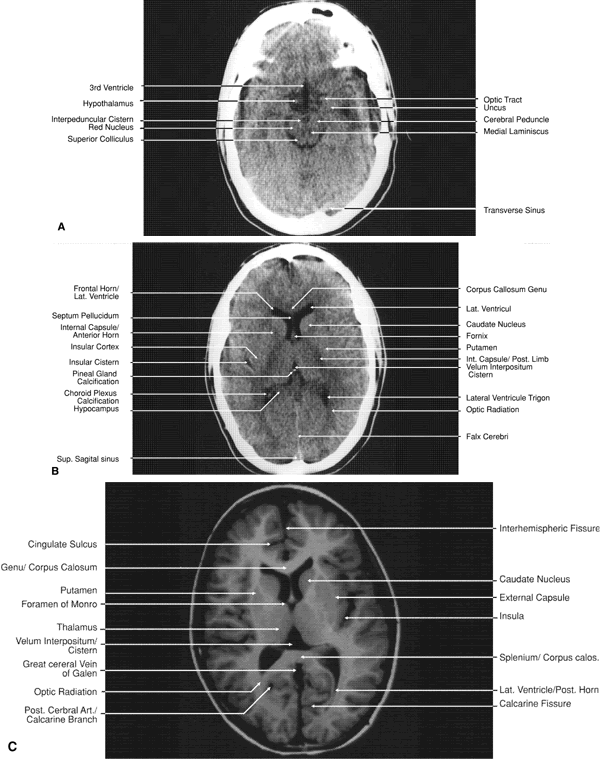 Fig. 32. A. Axial computed tomography soft tissue image at the level of suprasellar
cistern. B. Axial computed tomography soft tissue image at the level of thalamus. C. Axial T1-weighted image at the level of thalamus. Fig. 32. A. Axial computed tomography soft tissue image at the level of suprasellar
cistern. B. Axial computed tomography soft tissue image at the level of thalamus. C. Axial T1-weighted image at the level of thalamus.
|
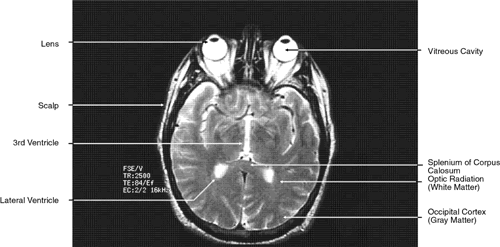
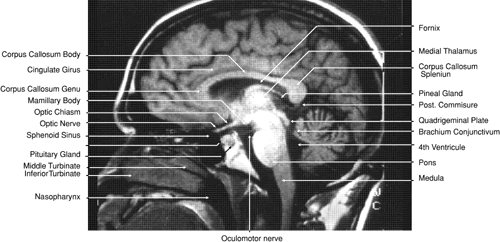 Fig. 33. Sagittal T1-weighted image of the brain through the interhemispheric fissure. Fig. 33. Sagittal T1-weighted image of the brain through the interhemispheric fissure.
|
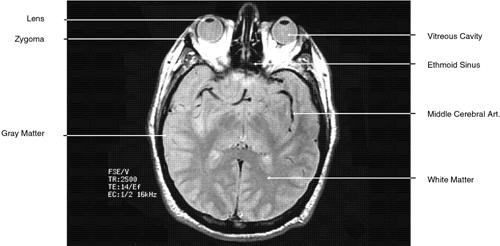
GLOBE Both MRI and CT are limited in their ability to image clearly the normal
intraocular structures (see Fig. 12). For the most part this is due to the small size and ultrastructural
composition of these structures. The cornea and sclera cannot be differentiated
from each other but are quite distinct on both MRI and CT owing
to their contrast with both the vitreous and aqueous internally and
the orbital fat and, if present, air trapped behind the eyelids externally. The
only other readily visible intraocular structure is the lens. On
CT this appears uniformly dense and similar in appearance to the
sclera. However, on T1-weighted MRI, the external lens capsule can be
clearly differentiated from the internal lens structure owing to the
presence of a significant number of hydrogen proteins within the central
portion of the lens. In addition, the normal choroid, ciliary body, and
iris can occasionally be visualized on MRI but not on CT. The normal
retina cannot be seen by either technique; neither can the conjunctive, Tenon's
capsule, angle structures, or the vessels and nerves
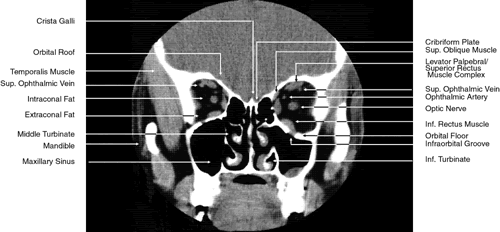
penetrating the globe. ORBIT AND PERIORBITAL STRUCTURES The bony orbital and periorbital anatomy is best visualized with CT, whereas
the soft tissue anatomy can be visualized with either CT or MRI. The
orbital cavities are roughly shaped like quadrilateral pyramids parallel
to each other medially and lying on one side with their apex facing
posteriorly. The widest portion of the orbit is approximately 1.5 cm
posterior to the orbital rim (see Fig. 2). On average the adult orbit is 40 to 45 mm deep, with the anterior orbit
measuring 40 mm wide and 35 mm high. The interorbital distance in
the normal adult is 25 mm. In contrast, the newborn orbit is more rounded, with
a width and height of 27 mm, and the orbit of a 7-year-old measures 28 mm
high and 33 mm wide.48 The orbital volume is approximately 30 mL, in comparison to the globe, whose
diameter of24 mm gives it a volume of 6.5 to 7.0 mL. The orbital roof is approximately triangular and is composed of the frontal
bone anteriorly and the lesser wing of the sphenoid posteriorly. The
roof is markedly concave, with the greatest degree of this concavity
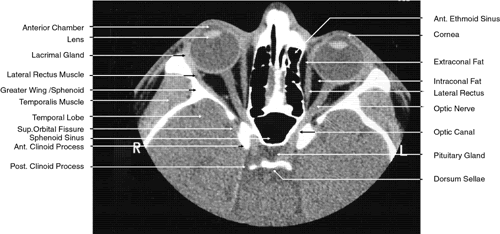
in the area of the equator of the globe (see Fig. 26). At the anterior and lateral portion of the orbital roof lies the lacrimal
gland in the lacrimal fossa (see Figs. 18 and 23). This gland consists of a large orbital portion and a smaller palpebral
portion. The orbital portion normally measures 20 × 12 × 5 mm, whereas
the palpebral portion is about one third of this size.49 The supraorbital notch is at the junction of the nasal third and the lateral
two thirds of the bony orbital margin (see Fig. 1). The trochlea of the superior oblique muscle is located 4 mm posterior
to the orbital margin in the medial and anterior portion of the orbital
roof (see Figs. 17, 24, and 25). Although usually a cartilaginous structure, it is occasionally partially
or wholly ossified. It measures 4 × 6 mm and is firmly attached
by connective tissue to the periosteum. The frontal bone portion
of the orbital roof is extremely thin and like the orbital floor is subject
to so-called blow-in fractures as well as to penetrating injury.50 The posterior portion of the roof is more substantial, measuring 3 mm
thick. Except for the anterior portion of the orbit, the intracranial
cavity lies directly superior to the orbital cavity (see Fig. 26). The levator muscle and the superior rectus muscle just inferior to it
are present along the midportion of the orbital roof for all but its
most anterior portion (see Fig. 26). The superior oblique muscle, after it changes direction at the trochlea, is
present inferior to the anterior portion of the roof and inserts
onto the globe inferior to the superior rectus muscle. Anteriorly and medially, the frontal sinuses are superior to the orbit
and lie between the two plates of the frontal bone. Occasionally, the
ethmoid air cells are also found invading the orbital roof. The frontal
sinus measures approximately 3 cm high,2.5 cm wide, and 2 cm deep. This
is quite variable, and it is not unusual for one sinus to be considerably
larger or smaller than the other or even completely absent. The
two sinus cavities are separated by a bony septum that is usually deviated
to one side. Medially the ethmoid air cells and nasal cavity lie
below the frontal sinuses and are separated from them by a thin wall
of bone. Superiorly and posteriorly the frontal sinuses are separated
from the intracranial cavity and the frontal lobes by the thin frontal
bone. Immediately beneath the central portion of the orbital roof lies the frontal
nerve, a branch of the ophthalmic division of the trigeminal nerve (see Fig. 18). Along with the trochlear nerve and lacrimal nerve, another branch of
the ophthalmic division of the trigeminal nerve, it enters the orbit
through the superior orbital fissure superior to the annular tendinous
insertions of the extraocular muscles. The lacrimal nerve enters the
orbit medial and superior to the superior orbital vein and travels laterally
below the orbital roof and superior to the lateral rectus muscle
to enter the lacrimal gland (see Fig. 24). The lacrimal artery arises from the ophthalmic artery lateral to the
optic nerve and travels with the distal two thirds of the lacrimal nerve. The
supraorbital and supratrochlear arteries branch from the ophthalmic
artery superior to the optic nerve, passing medially to the superior
rectus and levator muscles to accompany the supraorbital and supratrochlear
nerves, the two branches of the frontal nerve, as they pass
above the levator muscle. The supraorbital vein and artery accompany
the nerve along the anterior two thirds of its course before exiting
with it at the supraorbital notch. The other branch of the frontal nerve, the
supratrochlear nerve, travels medially after separating from the
frontal nerve at approximately the junction of the posterior one third
and anterior two thirds of the orbit. The trochlear nerve diverges
from the frontal nerve in the posterior orbit, passing medially below
the orbital roof and above the levator and superior rectus muscles to
enter the superior aspect of the posterior half of the superior oblique
muscle. The orbital floor is similar in shape to the triangular orbital roof and
is composed of the maxillary, zygomatic, and palatine bones. Medially, the
bony lacrimal canal containing the nasolacrimal duct lies just
posterior to the inferior orbital rim (see Fig. 22). At this point the canal is formed by the maxillary and lacrimal bones. Just
lateral to the bony canal is the origin of the inferior oblique
muscle. Laterally, the floor is separated from the lateral orbital wall
by the inferior orbital fissure, which begins lateral and inferior
to the optic foremen and near the inferior aspect of the superior orbital
fissure. It is approximately 20 mm long, ending 20 mm posterior to
the lateral portion of the inferior orbital margin (see Fig. 14). The boundaries of the fissure are the maxillary and palatine bones medially, the
greater wing of the sphenoid bone posteriorly, and the zygomatic
bone laterally and anteriorly. Inferior to the orbital floor over
most of its area is the maxillary sinus (see Figs. 5, 19, and 26). The bone of the floor is 0.5 to 1.0 mm thick, being thinnest at the
inferior orbital groove and canal. The fragility of this bone is the reason
it is commonly fractured during orbital trauma and the reason for
orbital extension of sinus tumors. The ethmoid air cells are occasionally
found within the orbital floor medially, and posteriorly there may
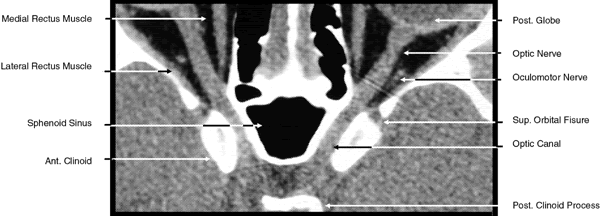
be a sinus within the orbital portion of the palatine bone.48 The medial rectus muscle runs along the middle aspect of the floor until
it inserts into the globe. It is in contact with the floor posteriorly, but
anteriorly it is superior to the inferior oblique muscle (see Fig. 19). The inferior orbital fissure is associated with several important soft
tissue and bony structures. The maxillary division of the trigeminal nerve
enters the fissure through the foremen rotundum and pterygopalatine
fossa, dividing into the infraorbital and zygomatic nerves (see Figs. 20 and 21). This latter nerve separates into the zygomaticofacial and zygomaticotemporal
nerves entering the zygomatic bone of the lateral orbital wall
through small canals before exiting onto the face. The pterygopalatine
fossa is bounded anteriorly by the pterygoid process of the sphenoid
bone, the greater wing of the sphenoid, the maxilla, and the palatine
bone (see Figs. 21 and 29). In addition to the maxillary division of the trigeminal nerve, it contains
the pterygopalatine ganglion and its sensory, parasympathetic, and
sympathetic branches and a portion of the maxillary artery. It communicates
with the orbit by way of the inferior orbital fissure, the nasal
cavity through the sphenopalatine foremen, and with the infratemporal
fossa by way of the pterygomaxillary fissure. In addition to the
foremen rotundum, sphenopalatine foremen, and pterygomaxillary fissure, the
pterygoid and pharyngeal canals also enter into the fossa (see Fig. 29). After leaving the pterygopalatine fossa, the infraorbital nerve travels
in the inferior orbital fissure a short distance before turning directly
anteriorly into the infraorbital groove and canal within the maxillary
bone. Finally it exits from the anterior surface of the bone 4 to 6 mm
inferior to the midportion of the inferior orbital rim (see Fig. 19). The infraorbital artery, a terminal branch of the internal maxillary
artery, accompanies the nerve along most of its course. Other branches
of the maxillary division of the trigeminal nerve include branches to
the pterygopalatine ganglion and the posterior, medial, and anterior
superior alveolar nerves, which supply sensation to the upper teeth and
gums. The full extent of the orbital roof and floor as well as the superior and
inferior rectus muscles and the levator muscle is best evaluated using
sagittal views of the orbit (see Figs. 17 through 20, 26). Reconstructed images are generally too crude to provide detailed imaging
of these structures. Coronal views of the orbit are excellent for
showing the vertical dimension of the lateral and medial orbital walls
and the horizontal dimension of the orbital roof and floor. This view
is also important for showing the cross-sectional areas of the globe
and orbital soft tissue structures, including the muscles, nerves, vessels, and
orbital fat. The lateral wall of the orbit is composed of the zygomatic bone anteriorly
and the greater wing of the sphenoid bone posteriorly (see Fig. 14). Although the zygomatic bone also forms a portion of the orbital floor
and is separated from the frontal bone and orbital roof by the frontozygomatic
suture, posteriorly the sphenoid bone is prominently demarcated
from the floor of the orbit by the inferior orbital fissure and from
the roof of the orbit by the superior orbital fissure. The lateral
wall is triangular and diverges at a 45-degree angle from the medial
wall. Posteriorly it is slightly convex; anteriorly it is slightly concave; centrally
it is flat. Posteriorly the sphenoid bone portion has
a small protuberance of spine that separates the thin superolateral portion
of the superior orbital fissure from the wider inferomedial portion. The
lateral rectus muscle inserts onto this projection, which is
formed in part by the groove containing the superior ophthalmic vein as
it passes through the superior orbital fissure. Anteriorly the zygomatic
bone has a small bony protuberance, the lateral orbital tubercle. This
is just inside the lateral orbital rim and approximately 11 mm below
the zygomaticofrontal suture. The levator aponeurosis, lateral canthal
ligament, and check ligaments from the lateral rectus muscle insert
onto this structure. A small lateral fat pad may also be present in
this area.51 Anteriorly, where it is subjected to the greatest stress, the lateral
orbital wall is quite thick. In contrast, posteriorly it is quite thin, only 1 mm
thick.48 The temporal fossa and temporalis muscle are lateral to the lateral orbital
wall for its anterior half, whereas the middle cranial fossa and
temporal lobe are lateral to it for its posterior half. The lateral rectus
muscle runs along the middle aspect of the wall until it inserts
into the globe (see Fig. 23). The superior orbital fissure separates the orbital roof from the lateral
orbital wall and the lesser and greater wings of the sphenoid bone (see Figs. 13 and 14). The common tendinous ring (annulus of Zinn) of the extraocular muscles
and the spine for the insertion of the lateral rectus muscle separates
the fissure into a thin superolateral portion and a wide inferomedial
portion (see Fig. 21). The fissure is approximately 22 mm long, and its superior end is 30 to 40 mm
from the frontozygomatic suture.48 Directly posterior to the fissure are the middle cranial fossa and temporal
lobe. Passing through the superior portion of the fissure above
the tendinous ring are the lacrimal (cranial nerve V), frontal (cranial
nerve V), and trochlear (cranial nerve IV) nerves, the superior ophthalmic
vein, and the recurrent lacrimal artery. Passing within the ring
are the superior division of the oculomotor nerve (cranial nerve III) and
the abducens nerve (cranial nerve VI) laterally, the nasociliary
nerve (cranial nerve V) and the inferior division of the oculomotor nerve
medially, and the sympathetic root of the ciliary ganglion and the
inferior ophthalmic vein, which on occasion may pass below the ring. The
tendinous insertions of the lateral and inferior rectus muscles form
the superior, lateral, and inferior portions of the ring in the area
of the superior orbital fissure. There is no muscle tendon medial to
the fissure. The medial border of the fissure is formed by a strut of
bone from the lesser wing of the sphenoid bone, which separates it from
the optic canal. The medial wall of the orbit is formed by four bones: the frontal process
of the maxillary bone, the lacrimal bone, the orbital portion of the
ethmoid bone, and a small portion of the lesser wing of the sphenoid
bone. Generally, this wall is parallel to the sagittal plane and is somewhat
quadrilateral, unlike the other walls of the orbit. Superiorly
and inferiorly this wall blends into the orbital roof and floor (see Fig. 14). Anteriorly the wall contains the lacrimal sac within the lacrimal fossa. This
is formed by portions of the lacrimal and maxillary bones. The
fossa is bounded anteriorly by the anterior lacrimal crest and posteriorly
by the posterior lacrimal crest. The anterior lacrimal crest blends
into the inferior orbital rim. Inferiorly the lacrimal fossa turns
into the bony nasolacrimal canal, which exits beneath the inferior
turbinate bone approximately 4 cm posterior to the opening of the nares (see Figs. 14 and 17). This structure is bounded by parts of the lacrimal, maxillary, and inferior
turbinate bones. The fossa measures approximately 14 mm vertically
and 5 mm anteroposteriorly, whereas the canal measures approximately 15 mm
vertically.48 Medial to the lacrimal fossa, the anterior portion of the ethmoid air
cells is present superiorly as well as the middle meatus of the nasal
cavity inferiorly. Medial to the bony nasolacrimal canal is the middle
meatus and inferior turbinate superiorly and the inferior meatus inferiorly. The ethmoid portion of the medial wall contains the thinnest bone of the
orbit (lamina papyracea). It is translucent to light and is only 0.2 to 0.4 thick (see Fig. 23).48 Because of its thinness, this area of the medial orbital wall is frequently
prone to fracture. Anteriorly the medial wall is adjacent to the
nasal cavity, middle turbinate, lateral wall of the nose, and the ethmoid
sinus cavity. At its far posterior aspect the wall is adjacent laterally
to the sphenoid sinus and medially to the optic canal (see Fig. 3). The superior oblique muscle runs along the superior aspect of the wall
for most of its length before changing direction at the trochlea. The
medial rectus muscle runs along the middle aspect of the wall until
it inserts into the globe. The cribriform plate of the ethmoid bone is
present medial to the superior aspect of the medial orbital wall at
the point where the frontal and ethmoid bones join (see Figs. 5 and 24). Superior to the midportion of the cribriform plate and midway between
the two medial orbital walls is a vertical portion of the ethmoid called
the crista galli. Inferior to the cribriform plate and directly below
the crista galli is the portion of the ethmoid bone forming the bony
vertical aspect of the nasal septum (vomer bone). The anterior and
posterior ethmoidal canals, which contain the posterior and anterior
ethmoidal arteries, both branches of the ophthalmic artery, are present
at the junction of the medial orbital wall and the orbital roof. The
anterior canal is about 25 mm posterior to the anterior lacrimal crest, whereas
the posterior canal is about 13 mm posterior to the anterior
canal and about 8 mm anterior to the orbital opening of the optic canal. The optic canal is contained entirely within the lesser wing of the sphenoid
bone, leading from the middle cranial fossa to the orbit (see Figs. 3 and 21). The anterior opening of the canal measures approximately 6 mm vertically
by 5 mm horizontally and is larger than the posterior opening, which
is wider horizontally than vertically.48 Anteriorly, the canals form a 36-degree angle with the sagittal plane
and are about 30 mm apart. Posteriorly, the canals form a 90-degree angle
if they are invisibly extended to the dorsum sellae. Intracranially, they
are about 20 to 25 mm apart. The canals vary in length from 5 to 12 mm. Medial
to the optic canal is the anterior portion of the sphenoid
sinus and the posterior portion of the ethmoid sinus (see Fig. 3). Optic nerve injury can occur during sinus surgery when the lateral walls
of these sinuses are inadvertently violated. Superior to the canal
is the gyrus rectus of the frontal lobe and the olfactory tracts (see Fig. 21). The canal transmits the optic nerve with its meningeal covering, sympathetic
nerve fibers, and the ophthalmic artery. The intraorbital portion
of the optic nerve is serpentine and 25 to 30 mm long. The intracanalicular
nerve is 6 to 10 mm long, and the intracranial portion is 10 to 15 mm
long. The ophthalmic artery is initially below and then lateral
to the nerve as it exits the canal (see Figs. 23 and 26). It then moves superiorly between the optic nerve and superior rectus
muscle to travel medially between the medial rectus and superior oblique
muscles before ending in its terminal branches, the supratrochlear
and dorsal nasal arteries. The medial rectus muscle inserts into the
annulus of Zinn medial to the optic nerve, whereas the superior rectus
muscle inserts into the annulus superior to the nerve. Because of the
close approximation of these two muscles to the nerve, movement of these
muscles can be painful in cases of retrobulbar neuritis. The levator
muscle inserts into the annulus superior to the superior rectus muscle, and
the suerior oblique muscle inserts into the annulus medial and
somewhat superior to the medial rectus muscle. As noted previously, the
lateral and inferior rectus muscles insert into the common tendinous
ring in the area of the superior orbital fissure, lateral to the optic
canal. Within the orbit the muscles are interconnected through intermuscular
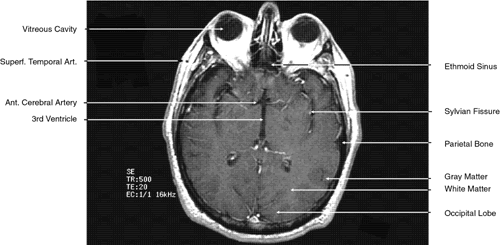
septa, which also extend to insert into the periorbita.52 INTRACRANIAL STRUCTURES After exiting the optic canals, the optic nerves enter an area known as
the cisterna basalis. This region is almost completely surrounded by
the intracranial portions of the sphenoid bone, being bounded anteriorly
by the tuberculum sellae, anterior cranial fossa, and anterior clinoid
processes; posteriorly by the clivus, dorsum sellae, and posterior
clinoid processes; inferiorly by the sella turcica; laterally by the
cavernous sinuses; and superiorly by the frontal lobe and third ventricle (see Figs. 2, 26, and 27). The tentorium cerebelli, a transaxial fold of aura separating the cerebellum
from the posterior portion of the cerebral hemispheres, extends
from the petrous portion of the sphenoid bone and anterior clinoid
processes to the straight sinus and falx cerebri separating the cerebral
hemispheres in the sagittal plane. The free edge of the tentorium is
superior to the cavernous sinus. The diaphragma sellae connects the clinoid processes, forming a roof over
the sella turcica, which contains the hypophysis (pituitary gland). The
hypophyseal stalk extends through an opening in the diaphragma sella, posterior
to the optic chiasm, and connects to the hypothalamus and
floor of the third ventricle. In this area the posterior portion of
the chiasm is separated from the ventricle by the thin plate of hypothalamus
with which it is in direct contact. Inferior to the hypophysis
are the sphenoid sinuses. Laterally are the cavernous sinuses, with the
intracavernous portions of the internal carotid artery in close approximation
to the gland. The intercavernous sinuses or circular sinus
connecting the two cavernous sinuses are both directly above and below. The optic nerves converge at an angle of approximately 90 degrees to join
at the optic chiasm. The optic tracts diverge from the chiasm posteriorly. The
chiasm measures approximately 12 mm transversely and 8 mm
anteroposteriorly.48 It lies at an oblique angle of 45 degrees, with its posterior portion
higher than its anterior portion (see Figs. 26 and 33).53 It lies directly above the diaphragma sella, being separated from it by 5 to 10 mm. Portions
of the interpeduncular and chiasmatic cisterns
are inferior and posterior to the chiasm. The former contains the circle
of Willis, which is superior to the pituitary fossa. In most cases (79%), the
posterior aspect of the chiasm is directly over the dorsum
sellae. However, in 12% of cases it is over the diaphragma sellae, in 5% it
is over the tuberculum sellae, and in 1% to 2% it is behind the
dorsum sellae.53 The close approximation to the optic nerves, chiasm, and optic tracts
of the meninges covering the bony structures, cisterns, and venous sinuses
surrounding the cisterna basalis is the reason meningiomas in this
area frequently result in visual symptomatology. The extracavernous
portion of the internal carotid artery is lateral to the chiasm along
with the anterior perforated substance of the frontal lobe. Anteriorly, the
chiasm is bounded by the anterior cerebral arteries and the anterior
communicating artery. Posteriorly, it is bounded by a portion of
the third ventricle and beyond that the tuber cinereum, a thin plate of
gray matter, connected superiorly to the mammillary bodies and separated
from the infundibulum by the infundibular cavity of the third ventricle. Inferoposteriorly
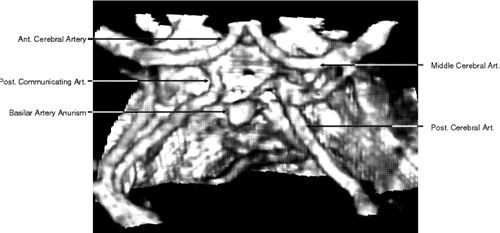
within the interpeduncular cistern are the basilar, posterior
cerebral, and posterior communicating arteries (see Fig. 33). The oculomotor nerve (cranial nerve III) is also posterior and inferior
as it passes from the midbrain under the posterior cerebral artery
and above the superior cerebellar artery before entering the cavernous
sinus. Also sitated posteriorly are the pons and cerebral peduncles, which
are separated from the dorsum sellae and clivus by the interpeduncular
cistern. Aneurysms involving the posterior cerebral and communicating
arteries frequently affect the third nerve and posterior aspect
of the chiasm, whereas aneurysms involving the anterior cerebral and
communicating arteries may affect the anterior portion of the chiasm. The
blood supply to the chiasm is from the anterior cerebral and internal
carotid arteries. The cavernous sinuses lie on either side of the pituitary fossa in the
middle cranial fossa, lateral and superior to the sphenoid sinus. These
endothelial-lined structures extend from the superior orbital fissure
to the apex of the petrous bone and are completely covered by dura (see Fig. 27). The sinuses receive venous blood from the superior and inferior ophthalmic
veins, cerebral veins, and the sphenoparietal sinus. They have
connections with the transverse sinus, pterygoid plexus, angular and facial
veins, and the contralateral sinus through the intercavernous sinuses. They
drain into the inferior petrosal sinus and internal jugular
vein. Several structures pass through each sinus. In the lateral wall
of the sinus, starting superiorly and going inferiorly, are the oculomotor
nerve (cranial nerve III), trochlear nerve (cranial nerve IV), the
ophthalmic division of the trigeminal nerve (cranial nerve V), and
the maxillary division of the trigeminal nerve (see Fig. 27). In the anterior portion of the sinus, the trochlear nerve is above the
oculomotor nerve. The carotid artery and abducens nerve (cranial nerve
VI) are suspended within the sinus by fibrous septations. The abducens
nerve is in the midportion of the sinus just medial to the ophthalmic
division of the trigeminal nerve. The internal carotid artery travels
upward through the sinus after it passes through the carotid canal
and foremen lacerum in the petrous portion of the temporal bone. Sympathetic
nerve fibers from the superior cervical ganglion travel with
it. Anteriorly, before entering the cavernous sinus, the artery is separated
from the trigeminal ganglion by a thin plate of bone and by a fibrous
membrane. Within the cavernous sinus, the artery first travelsposteriorly
toward the posterior clinoid process. It then turns anteriorly
in the medial aspect of the sinus and curves to travel upward toward
the anterior clinoid process, finally perforating the sinus. After
passing between the optic and oculomotor nerves, it turns toward the anterior
perforted substance and divides into its terminal branches, the
anterior and middle cerebral, posterior communicating, and anterior
choroidal arteries (see Fig. 20). The ophthalmic artery arises from the carotid as it leaves the cavernous
sinus on the medial side of the anterior clinoid process. It travels
through the optic canal inferior and lateral to the optic nerve before
entering the orbit, where it rotates to a position superior and medial
to the nerve. The oculomotor nerve nuclei arise in the midbrain and consist of a paired
group of motor cells in the form of a V, measuring approximately 10 mm
long.53 The nuclei are just ventral to the central gray matter of the mesencephalon
that surrounds the cerebral aqueduct. Superiorly the nuclei extend
almost as far as the floor of the third ventricle; inferiorly they
end just below the superior colliculus. The medial longitudinal fasciculus
is ventral and lateral to these nuclei. Inferiorly the nuclei blend
with the trochlear nerve nuclei. The oculomotor nerve fibers travel
ventrally after leaving the nuclei, passing through the brain stem. They
successively cross the medial longitudinal fasciculus, the red nucleus, the
substantia nigra, and the anterior border of the pons to exit
as paired nerve trunks in the interpeduncular fossa at the angle of
the pons and cerebral peduncle. The nerve then travels anteriorly and
laterally below the posterior cerebral and above the superior cerebellar
arteries to enter the cavernous sinus. As it emerges from the cavernous
sinus, it passes lateral to the carotid artery near the uncus of
the temporal lobe to enter the orbit through the superior orbital fissure. It
then divides into a superior branch, innervating the levator and
superior rectus muscles, and an inferior branch, innervating the medial
and inferior rectus and the inferior oblique muscles (see Fig. 20). Sympathetic fibers from the carotid plexus travel with the superior branch
of the third nerve to innervate Muller's muscle. After synapsing
in the pretectal nuclei near the superior colliculi, the parasympathetic
fibers for pupillary constriction travel in the superior peripheral
portion of the nerve as it leaves the brain stem. They then follow
the inferior nerve branch to the inferior oblique muscle, leaving it
to synapse in the ciliary ganglion. This structure, measuring about 2 × 1 mm, is
located in the posterolateral portion of the orbit between
the optic nerve and lateral rectus muscle and 1 cm anterior to
the optic foramen. It is adjacent to the nerve and near the ophthalmic
artery. Both sympathetic fibers to the pupil and ocular blood vessels
and sensory fibers to the cornea, iris, and ciliary body pass through
this ganglion. The paired nuclei for the trochlear nerve are located just below the oculomotor
nerve nuclei just ventral to the central gray substance in the
mesencephalon. Like the oculomotor nerve nuclei, they are ventral and
lateral to the cerebral aqueduct and dorsal and medial to the medial
longitudinal fasciculus. They are on the same level as the superior aspect
of the inferior colliculus. The nerve fibers arising from these
nuclei travel dorsally and laterally around the central gray substance
and cross dorsally to this structure before exiting the brain stem as
nerve roots near the inferior aspect of the inferior colliculi. This
is the only cranial nerve to exit from the dorsal surface of the brain
stem, and it has the longest intracranial course (75 mm) of any cranial
nerve.48 The nerve then courses anteriorly and ventrally around the superior portion
of the cerebral peduncles to the anterior aspect of the brain stem. It
proceeds forward between the posterior cerebral and superior cerebellar
arteries, medial and superior to the trigeminal nerve and below
the free edge of the tentorium cerebelli, to enter the cavernous sinus
below the oculomotor nerve. The motor and sensory nuclei of the trigeminal nerve are in the dorsal
part of the pons, lateral and ventral to the fourth ventricle. The sensory
nuclei are a superior continuation of the sensory column of the spinal
cord and are present throughout the entire brain stem. The motor
nuclei are ventral and medial to the superior cerebellar peduncles and
lateral to the nuclei of the abducens nerves and nuclei and fibers of
the facial nerve. Proprioceptive fibers arise from the main sensory (mesencephalic) nuclei
located in the superior aspect of the pons near
the superior cerebral peduncle and lateral to the cerebral aqueduct. Motor
fibers arise from the pontine area medial to the sensory nuclei
and closer to the fourth ventricle. The spinal nuclei are the location
for thermal and tactile sensation. The fibers from the mandibular division
go to the most superior portion of these nuclei, those from the
maxillary division to the middle portion, and those from the ophthalmic
division to the most inferior portion. The motor and sensory nerve fibers
travel through the middle cerebral peduncle to exit the pons near
the middle of its lateral aspect (see Fig. 30). The nerve roots then travel approximately 1 cm anteriorly and slightly
superiorly in the pontine cistern toward a slight depression in the
petrous portion of the temporal bone lateral to the oculomotor, trochlear, and
abducens nerves. The free edge of the tentorium and the cerebellum
are above the nerve fibers, and the facial and vestibulocochlear
nerves are lateral and inferior to them. The nerve fibers enter the trigeminal (gasserian) ganglion, which is located
in Meckel's cave, a aural pocket lateral to the cavernous sinus
and carotid artery in a depression formed at the apex of the petrous
portion of the temporal bone. Below the ganglion are the greater and
lesser petrosal nerves and the foremen lacerum containing the carotid
artery. Laterally is the foremen spinosum, which transmits the middle
meningeal artery. Superiorly are the uncus and temporal lobe. The motor
branch of the nerve travels below the ganglion to exit with the mandibular
division of the nerve through the foremen ovale. The ganglion
measures approximately 1 × 2 cm and is bean-shaped.48 The ophthalmic division of the nerve leaves the ganglion from its anterior
and superior aspect, passing through the lateral aspect of the cavernous
sinus within its own separate aural sheath. It passes into the
orbit through the superior orbital fissure with sympathetic fibers from
the carotid plexus. As described earlier, in the orbit it divides into
three branches, the frontal, lacrimal, and nasociliary nerves. The
ophthalmic division supplies sensation to the eye, conjunctive, cornea, lacrimal
gland, a portion of the nasal and sinus mucosa and skin of
the eyelids, forehead, and nose. The maxillary division of the nerve
leaves the ganglion positioned between the two other divisions. It travels
in the inferior aspect of the cavernous sinus close to the lateral
wall and below the ophthalmic division before exiting the cranial cavity
through the foremen rotundum, crossing the pterygopalatine fossa, and
entering the infraorbital fissure. It supplies sensation to portions
of the aura, midface, lower eyelid, side of the nose, upper lip, and
mucosa of the maxillary sinus, soft palate, upper gums, hard and soft
palate, and nasopharynx and to the upper teeth. The paired motor nuclei of the abducens nerves are located in the floor
of the fourth ventricle on either side of the midline. The fibers of
the facial nerves course around these nuclei, forming a small elevation
in the floor of the ventricle called the facial colliculus. The medial
longitudinal fasciculus is medial to the nuclei, and the vestibular
nuclei are lateral to the nuclei and the facial nerve fibers. The abducens
nerve fibers travel ventrally without decussating through the tegmentum
and pons. The nerve roots exit the lower portion of the pons on
either side of the midline. They course forward, upward, and somewhat
laterally in the middle cranial fossa toward the petrous process of
the temporal bone. The two nerve roots are approximately 1 cm apart at
their origin. The basilar artery is medial to them and the origin of
the facial nerve is lateral to them. The abducens nerves are crossed near
their origin by the anteroinferior cerebellar arteries. They travel
superiorly between the pons and basilar portion of the occipital bone
to reach the petrous process of the temporal bone about 2 cm below and
lateral to the posterior clinoid process and slightly medial or posterior
to the inferior petrosal sinus.48 Each nerve root then passes forward and laterally over the petrous portion
of the temporal bone in a small groove (Dorello's canal) to
which it is held by the petrosphenoidal (Gruber's) ligament. It then
passes through the aura to enter the cavernous sinus together with
the inferior petrosal sinus. In the sinus the nerve courses around the
lateral aspect of the internal carotid artery. It lies in the sinus
in a more medial position than the other nerves and within its own separate
sheath. The nerve enters the orbit through the annulus of Zinn below
the oculomotor nerve. The nuclei of the facial nerve are located deep in the reticular formation
of the brain stem ventral and lateral to the abducens nuclei. The
fibers from the nuclei travel dorsally toward the fourth ventricle and
circle laterally around the abducens nuclei. They then turn ventrally
again to exit the pons between the olive and the inferior cerebellar
peduncle. At this point the nerve is medial to the auditory nerve and
lateral to the abducens nerve. The nervus intermedius, a portion of the
facial nerve carrying sensory fibers for taste as well as parasympathetic
fibers, lies on the lateral side of the facial nerve. The nervus
intermedius and facial and auditory nerves travel together anteriorly
and laterally into the internal auditory meatus. The facial nerve and
nervus intermedius separate from the auditory nerve and enter the facial
canal in the petrous portion of the temporal bone. Initially the nerve
continues its lateral course between the cochlea and semicircular
canals. Near the tympanic cavity it turns posteriorly, running in the
medial wall of the cavity. At this point the frontal nerve merges into
the geniculate ganglion. From this structure the greater petrosal, vidian, and
lesser petrosal nerves and the afferent division of the nervus
intermedius arise. The nerve then dips into the mastoid air cells
and exits from the stylomastoid foramen. At this point the posterior auricular, digastric, and
stylohyoid branches of the nerve arise. It then
enters the substance of the parotid gland, where its various facial
branches, the temporal, zygomatic, buccal, mandibular, and cervical divisions, arise. The medial longitudinal fasciculus is close to the midline just ventral
to the aqueduct and fourth ventricle. It extends from the pons to the
medulla and into the spinal cord. This tract coordinates the nuclei of
the oculomotor, trochlear, and abducens nerves to allow conjugate movement
of the eyes. In addition, it integrates these movements with impulses
from the vestibular apparatus as well as with motor movements of
the neck. It is particularly important in relaying impulses between
the medial rectus muscle and the contralateral pontine gaze center. The optic tracts extend posteriorly and laterally from the chiasm, passing
around the basilar artery, mammillary bodies, and cerebral peduncle
and between the anterior perforated substance and tuber cinereum. Each
tract is attached to the lateral surface of the peduncle just lateral
to the hypothalamic nuclei and third ventricle as well as the internal
capsule.48 The anterior choroidal and posterior cerebral arteries run parallel and
across the tracts. The tracts ascend slightly as they move laterally
around the peduncle crossing the pyramidal tracts. Posteriorly the globus
pallidus is above and the hippocampus is below the tracts, which
come very close to the inferior horn of the lateral ventricle. The tracts
and the afferent visual fibers then pass into the lateral geniculate
bodies (see Fig. 31). These triangular areas of the thalamus are located inferior and lateral
to the major portions of the thalamus. Medial to the lateral geniculate
body is the medial geniculate body and portions of the internal
capsule. The lateral geniculate body is adjacent to the hippocampal convolution
inferiorly, the pulvinar superiorly, and the inferior horn of
the lateral ventricle posteriorly. As the optic tracts enter the lateral
geniculate body, they are just lateral and adjacent to the major
motor tracts, including the pyramidal tracts. The optic tract enters the
lateral geniculate anteriorly and ventrally to synapse with the neuronal
components in its various layers. The blood supply to this structure
is from the anterior choroidal artery anteriorly and the posterior
cerebral artery posteriorly. The optic radiations exit from its dorsal
surface. The superior colliculi, situated on the dorsal aspect of the
midbrain, are connected to the lateral geniculate bodies by the superior
brachium. This structure transmits afferent pupillary fibers to
the pretectal region and afferent fibers from the optic tract to the superior
colliculi for reflex control of the ocular muscles. After leaving the lateral geniculate bodies, the optic radiations, also
called the geniculocalcarine radiations, turn anteriorly and then laterally
at the optic peduncle to bypass the lateral ventricle (see Fig. 32). The posterior and medial aspect of this tract, representing fibers from
the upper portions of the retina and macula, proceeds directly through
the parietal lobe to the occipital cortex, terminating superior to
the calcarine fissure. The anterior and lateral aspect of this tract, representing
fibers from the lower portions of the retina and macula, turns
anteriorly and traverses the temporal horn of the lateral ventricle
before proceeding to the occipital cortex terminating inferior to
the calcarine fissure. These fibers pass through the temporal lobe. Their
most anterior position is called Meyer's loop. Injury to this
area results in a superior homonymous quadrantic hemianopia. Just posterior
and lateral to the internal capsule as the optic radiations proceed
posteriorly, they cross the temporal isthmus. This is an area approximately 1.5 cm
in diameter where visual, somesthetic, motor, speech, and
intracerebral association pathways are in close proximity.53 Lesions in this area, often resulting from anterior choroidal artery occlusion, are
associated with hemianopia, aphasia, numbness in the opposite
extremities, and motor weakness of the leg. The blood supply to
the optic radiations is from the anterior choroidal artery anteriorly, the
middle cerebral artery in its middle portion, and the posterior cerebral
artery posteriorly. |