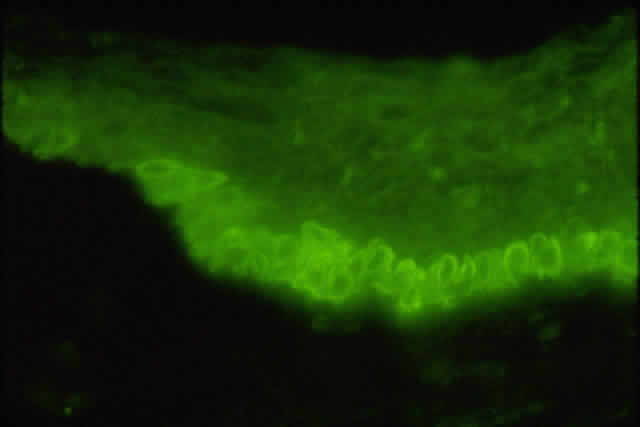
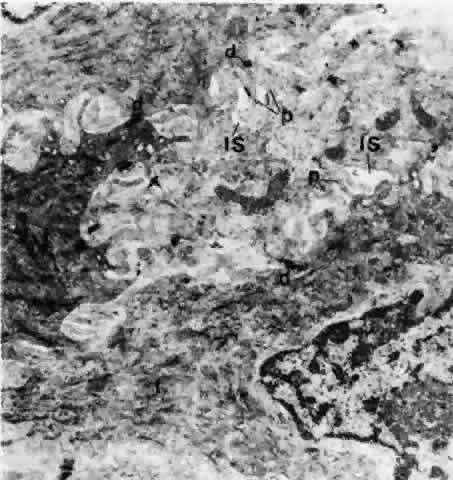
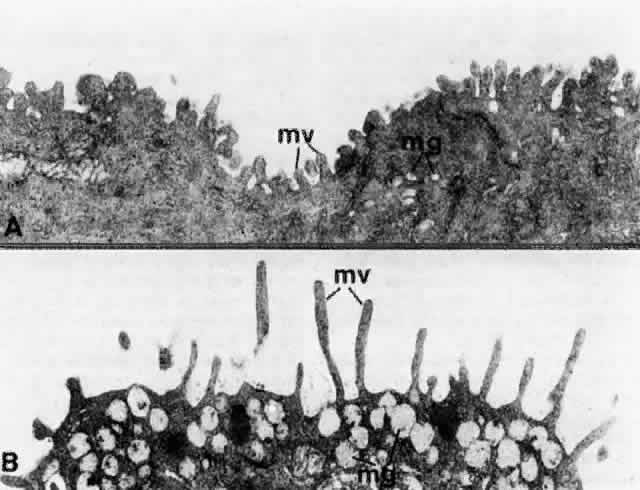
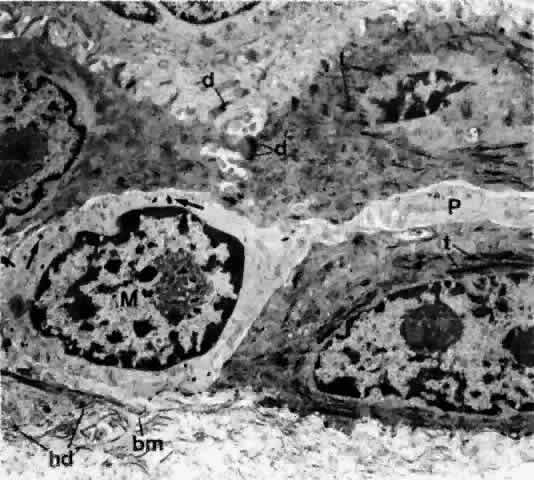
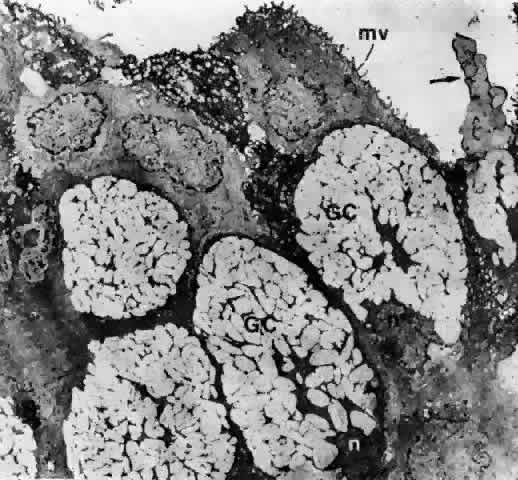
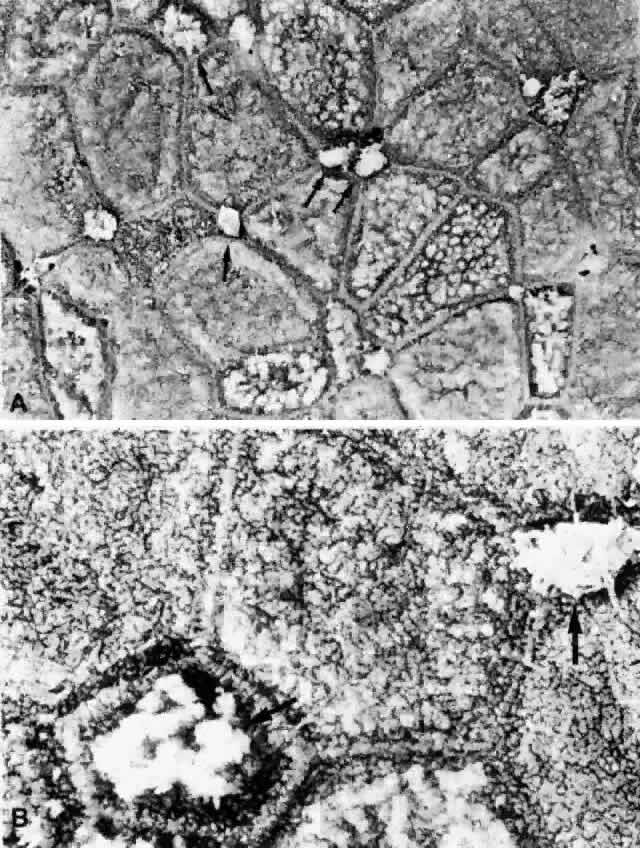
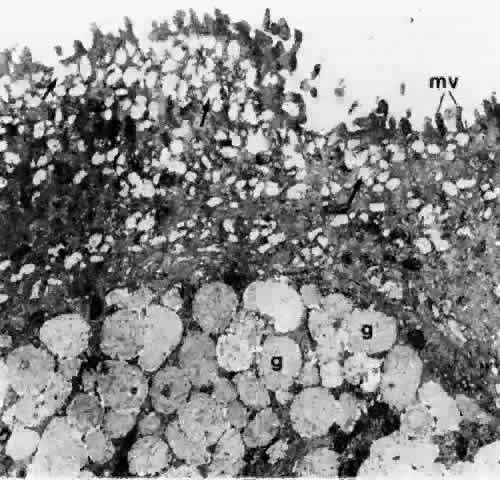
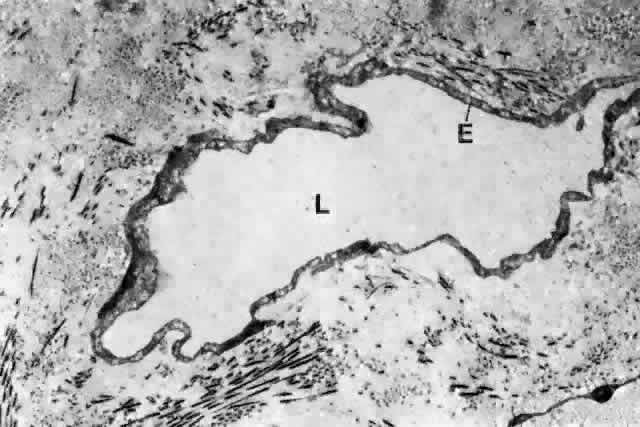
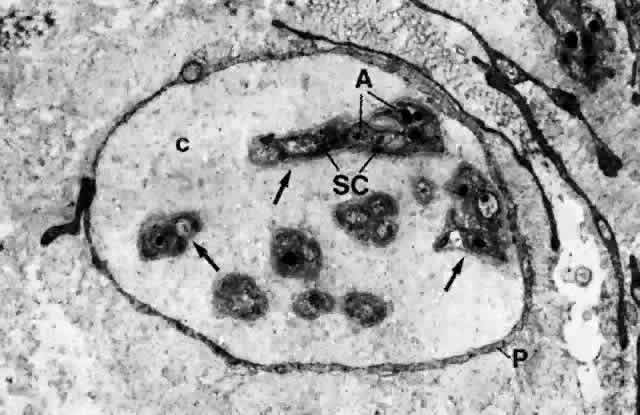
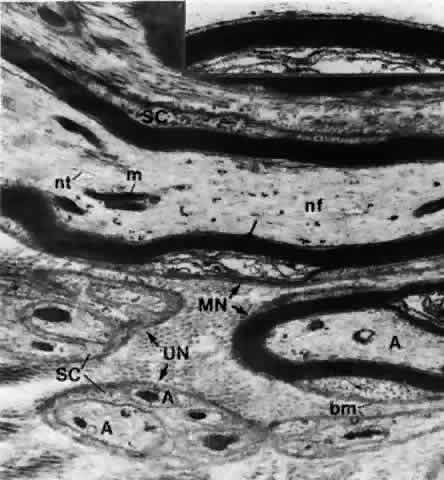
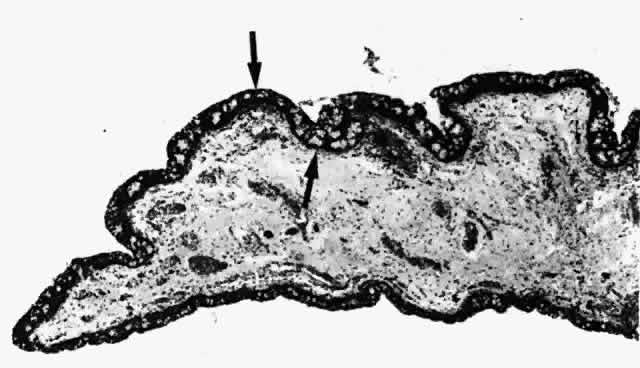
1. Holly RJ: Formation and stability of the tear film. Int Ophthalmol 13:73, 1973 2. Pfister RR: The normal surface of conjunctiva epithelium: A scanning electron microscopic
study. Invest Ophthalmol 14:267, 1975 3. Allansmith MR, O'Connor GR: Immunoglobulins: Structure, function and relation to the eye. Surv Ophthalmol 14:367, 1970 4. Gundersen T, Pearlson HR: Conjunctival flaps for corneal disease: Their usefulness and complications. Trans Am Ophthalmol Soc 67:78, 1969 5. Thoft RA: Conjunctival transplantation. Arch Ophthalmol 95:1425, 1977 6. Nagpal KC, Asdourian GK, Goldbaum MH: The conjunctival sickling sign, hemoglobin S, and irreversibly sickled
erythrocytes. Arch Ophthalmol 95:808, 1977 7. Duke-Elder S: Diseases of the Outer Eye, Vol 8, p 1061. St Louis, CV Mosby, 1965 8. Parakkal PF, Alexander NJ: Keratinization, pp 44–45. New York, Academic
Press, 1972 9. Ralph RA: Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol 14:299, 1975 10. Warwick R: Eugene Wolff's Anatomy of the Eye and Orbit, Seventh Edition. Philadelphia, WB
Saunders, 1976 11. Kurpakus MA, Maniaci MT, Esco M: Expression of keratins K12, K4 and K14 during development of ocular surface
epithelium. Curr Eye Res 13:805, 1994 12. Spinak M: Cytological changes of the conjunctiva in immunoglobulin-producing dyscrasias. Ophthalmology 88:1207, 1981 13. Trocme SD, Raizman MB, Bartley GB: Medical therapy for ocular allergy. Mayo Clin Proc 67:557, 1992 14. Duke-Elder S: System of Ophthalmology, Vol 2, p 543. St Louis, CV Mosby, 1961 15. Jordan DR, Anderson RB, Mamalis N: Accessory lacrimal glands. Ophthalmic Surg 21:146, 1990 16. Rivas L, Oroza MA, Perez-Esteban A, Murube-delCastillo J: Topographical distribution of ocular surface cells by the use of impression
cytology. Acta Ophthalmol 69:371, 1991 17. Oduntan AO: The inferior conjunctiva of the monkey. Acta Anat 143:178, 1992 18. Steuhl KP: Ultrastructure of the conjunctival epithelium. Dev Ophthalmol 19:1, 1989 19. Weingeist TA: Fine structure and function of ocular tissues: The conjunctiva. Int Ophthalmol Clin 13(3):85, 1973 20. Nicolaissen B Jr, Eidal K, Haaskjold E et al: Outgrowth of cells from human conjunctival explants onto cornea in vitro. Acta Ophthalmol 69:723, 1991 21. Sandvig KU, Haaskjold E, Bjerknes R et al: Cell kinetics of conjunctival and corneal epithelium during regeneration
of different-sized corneal epithelial defects. Acta Ophthalmol 72:43, 1994 22. Tseng SC, Tsai RJ: Limbal transplantation for ocular surface reconstruction—a review. Fortschr Ophthalmol 88:236, 1991 23. Zieske JD: Perpetuation of stem cells in the eye. Eye 8:163, 1994 24. Zieske JD, Bukusoglu G, Yankauckas MA: Characterization of a potential marker of corneal epithelial stem cells. Invest Ophthalmol Vis Sci 33:143, 1992 25. Schermer A, Galvin S, Sun T-T: Differentiation-related expression of a major 64K corneal keratin in vivo
and in culture suggests limbal location of corneal epithelial stem
cells. J Cell Biol 103:49, 1986 26. Cotsarelis G, Cheng S-Z, Dong G et al: Evidence of slow-cycling limbal epithelial basal cells that can be preferentially
stimulated to proliferate: Implications on epithelial stem
cells. Cell 57:201, 1989 27. Lindberg K, Brown ME, Chaves HV et al: In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci 34:2672, 1993 28. Sandvig KU, Haaskjold E: The proliferative response during regeneration of a ringshaped defect in
the corneal epithelium. Acta Ophthalmol 71:39, 1993 29. Sorensen T, Jensen FT: Conjunctival transport of technetium-99m pertechnetate. Acta Ophthalmol 57:691, 1979 30. Ahmed I, Patton TF: Importance of the noncorneal absorption route in topical ophthalmic drug
delivery. Invest Ophthalmol Vis Sci 26:584, 1985 31. Lantz E, Andersson A: Release of fibrinolytic activators from the cornea and conjunctiva. Graefes Arch Clin Exp Ophthalmol 219:263, 1982 32. Liu SH, Tagawa Y, Prendergast RA et al: Secretory component of IgA: A marker for differentiation of ocular epithelium. Invest Ophthalmol Vis Sci 20:100, 1981 33. Inatomi T, Spurr-Michaud S, Tisdale AS et al: Mucin genes MUC4 and MUC5 are
expressed by human conjunctival epithelia. Invest Ophthalmol Vis
Sci (in press) 34. Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK: Human corneal and conjunctival epithelia express MUCI mucin. Invest Ophthalmol Vis Sci 36:1818, 1995 35. Watanabe H, Fabricant M, Tisdale AS et al: Human corneal and conjunctival epithelia produce a mucin-like glycoprotein
for the apical surface. Invest Ophthalmol Vis Sci 36:337, 1995 36. Tsai RJ, Ho YS, Chen JK: The effects of fibroblasts on the growth and differentiation of human bulbar
conjunctival epithelial cells in an in vitro conjunctival equivalent. Invest Ophthalmol Vis Sci 35:2865, 1994 37. Wei Z-G, Cotsarelis G, Sun T-T, Lavker RM: Labelretaining cells are preferentially located in forniceal epithelium: Implications
on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci 36:236, 1995 38. Farquhar MG, Palade GE: The Golgi apparatus (complex) (1954-1981): From artifact to center stage. J Cell Biol 91(suppl 3, Pt 2):77S, 1981 39. Janssen PT, van Bijsterveld OP: Origin and biosynthesis of human tear fluid proteins. Invest Ophthalmol Vis Sci 24:623, 1983 40. Dua HS, Gomes JA, Jindal VK et al: Mucosa specific lymphocytes in the human conjunctiva, corneoscleral limbus
and lacrimal gland. Curr Eye Res 13:87, 1994 41. Latkovic S: Ultrastructure of M cells in the conjunctival epithelium of the guinea
pig. Curr Eye Res 8:751, 1989 42. Chin GN, Chi EY, Bunt AH: Ultrastructural and histochemical studies of conjunctival concretions. Arch Ophthalmol 98:720, 1980 43. Abdel-Khalek LM, Williamson J, Lee WR: Morphological changes in the human conjunctival epithelium: I. In the normal
elderly population. Br J Ophthalmol 62:792, 1978 44. Wanko T, Lloyd BJJ, Matthews J: The fine structure of human conjunctiva in the perilimbal zone. Invest Ophthalmol 3:285, 1964 45. Paridaens AD, McCartney AC, Curling OM et al: Impression cytology of conjunctival melanosis and melanoma. Br J Ophthalmol 76:198, 1992 46. Kessing SV: Epithelial cysts in the conjunctiva. Acta Ophthalmol 47:642, 1969 47. Srinivasan BD, Jakobiec FA, Iwamoto T, Devoe AG: Epibulbar mucogenic subconjunctival cysts. Arch Ophthalmol 96:857, 1978 48. Kessing SV: Mucous gland system of the conjunctiva: A quantitative normal
anatomical study. Acta Ophthalmol (Copenh) (suppl 95), 1968 49. Steida L: Ueber den Bauder Augenlidbindehaut des Menschen. Arch Mikr Anat 3:357, 1867 50. Apple DJ, Rabb MF: Ocular Pathology: Clinical Applications and Self-Assessment, Fourth
Edition, pp 454–473. St Louis, CV Mosby, 1991 51. Dilly PN: Structure and function of the tear film. Adv Exp Med Biol 350:239, 1994 52. Kessler TL, Mercer HJ, Zieske JD et al: Stimulation of goblet cell mucous secretion by activation of nerves in
rat conjunctiva. Curr Eye Res 14:985, 1995 53. Dartt DA, McCarthy DM, Mercer HJ et al: Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res 14:993, 1995 54. Kessler TL, Dartt DA: Neural stimulation of conjunctival goblet cell mucous secretion in rats. Adv Exp Med Biol 350:393, 1994 55. Huang AJ, Tseng SC, Kenyon KR: Morphogenesis of rat conjunctival goblet cells. Invest Ophthalmol Vis Sci 29:969, 1988 56. Qi LP: Determination of the conjunctival goblet cell density in 43 normal subjects. Chung Hua Yen Ko Tsa Chih 25(3):161, 1989 57. Knop E, Brewitt H: Conjunctival cytology in asymptomatic wearers of soft contact lenses. Graefes Arch Clin Exp Ophthalmol 230:340, 1992 58. Ehlers N, Kessing SV, Norn MS: Quantitative amounts of conjunctival mucous secretion and tears. Acta Ophthalmol 50:210, 1972 59. Jones DT, Monroy D, Ji Z et al: Sjogren's syndrome: Cytokine and Epstein-Barr viral gene expression
within the conjunctival epithelium. Invest Ophthalmol Vis Sci 35:3493, 1994 60. Carney LG, Hill RM: Human tear pH: Diurnal variations. Arch Ophthalmol 94:821, 1976 61. Iwata T, Ohkawa K, Uyama M: The fine structural localization of peroxidase activity in goblet cells
of the conjunctival epithelium of rats. Invest Ophthalmol 15:40, 1976 62. Srinivasan BD, Worgul BV, Iwamoto T, Merriam GRJ: The conjunctival epithelium: Part 2. Histochemical and ultrastructural
studies on human and rat conjunctiva. Ophthalmic Res 9:65, 1977 63. Greiner JV, Weidman TA, Korb DR, Allansmith MR: Histochemical analysis of secretory vesicles in nongoblet conjunctival
epithelial cells. Acta Ophthalmol 63:89, 1985 64. Rivas L, Oroza MA, Perez-Esteban A, Murube-delCastillo J: Morphological changes in ocular surface in dry eyes and other disorders
by impression cytology. Graefes Arch Clin Exp Ophthalmol 230:329, 1992 65. Driot JY, Bonne C: Beneficial effects of a retinoic acid analog, CBS-211 A, on an experimental
model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci 33:190, 1992 66. Tseng SC: Topical tretinoin treatment for dry-eye disorders. Int Ophthalmol Clin 27:47, 1987 67. Nelson JD, Wright JC: Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol 102:1049, 1984 68. Marner K: ‘Snake-like’ appearance of nuclear chromatin in conjunctival
epithelial cells from patients with keratoconjunctivitis sicca. Acta Ophthalmol 58:849, 1980 69. Kinoshita S, Kiorpes TC, Friend J, Thoft RA: Goblet cell density in ocular surface disease: A better indicator than
tear mucin. Arch Ophthalmol 101:1284, 1983 70. Friend J, Kiorpes T, Thoft RA: Conjunctival goblet cell frequency after alkali injury is not accurately
reflected by aqueous tear mucin content. Invest Ophthalmol Vis Sci 24:612, 1983 71. Versura P, Maltarello MC, Bonvicini F et al: Detection of mucus glycoconjugates in human conjunctiva by using the lectin
colloidal gold technique in TEM: I. A quantitative study in normal
subjects. Acta Ophthalmol 64:445, 1986 72. Srinivasan BD, Jakobiec FA, Iwamoto T, DeVoe AG: Giant papillary conjunctivitis with ocular prostheses. Arch Ophthalmol 97:892, 1979 73. Greiner JV, Kenyon KR, Henriquez AS et al: Mucus secretory vesicles in conjunctival epithelial cells of wearers of
contact lenses. Arch Ophthalmol 98:1843, 1980 74. Allansmith MR, Kajiyama G, Abelson MB et al: Plasma cell contents of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol 82:819, 1976 75. Allansmith MR, Greiner JV, Baird RS: Number of inflammatory cells in the normal conjunctiva. Am J Ophthalmol 86:250, 1978 76. Baum JL: Ocular infections. N Engl J Med 299:28, 1978 77. Yanoff M, Fine BS: Ocular Pathology. New York, Harper & Row, 1975 78. Fraunfelder FT, Garner A, Barras TC: Subconjunctival and episcleral lipid deposits. Br J Ophthalmol 60:532, 1976 79. Spencer WH: Ophthalmic Pathology: An Atlas and Textbook, Third Edition. Philadelphia, WB
Saunders, 1985 80. Robbins SL, Cotran RS, Kumar V: Pathologic Basis of Disease, Third Edition. Philadelphia, WB
Saunders, 1984 81. Ostler HB: Diseases of the External Eye and Adnexa: A Text and Atlas. Baltimore, Williams & Wilkins, 1993 82. Yanoff M, Fine BS: Ocular Pathology: A Text and Atlas, Second Edition. Philadelphia, JB
Lippincott, 1982 83. Trocmé SD, Kephart GM, Allansmith MR et al: Conjunctival deposition of eosinophil granule major basic protein in vernal
keratoconjunctivitis and contact lens-associated giant papillary
conjunctivitis. Am J Ophthalmol 108:57, 1989 84. Trocmé SD, Aldave AJ: The eye and the eosinophil. Surv Ophthalmol 39:241, 1994 85. Liesgang T: Disorders of the cornea, conjunctiva and lens. In Bartley GB, Liesgang
TJ (eds): Essentials of Ophthalmology. Philadelphia, JB Lippincott, 1992 86. Shields MB: Textbook of Glaucoma, Third Edition. Baltimore, Williams & Wilkins, 1992 87. Skuta GL, Parrish RK II: Wound healing in glaucoma filtering surgery. Surv Ophthalmol 32:149, 1987 88. Kook MS, Lee DA: Improving the success of glaucoma surgery by controlling wound healing. Ophthalmol Clin North Am 8:393, 1995 89. Mann I: Study of epithelial regeneration in living eye. Br J Ophthalmol 28:26, 1944 90. Dua HS, Gomes JA, Singh A: Corneal epithelial wound healing. Br J Ophthalmol 78:401, 1994 91. Haaskjold E, Sandvig KU, Bjerknes R, Kravik K: The early cell kinetic response during healing of corneal epithelial wounds. Ophthalmic Surg 23:680, 1992 92. Chung EH, DeGregorio PG, Wasson M, Zieske JD: Epithelial regeneration after limbus-to-limbus debridement: Expression
of alpha-enolase in stem and transient amplifying cells. Invest Ophthalmol Vis Sci 36:1336, 1995 93. Kruse FE, Chen JJ, Tsai RJ, Tseng SC: Conjunctival transdifferentiation is due to the incomplete removal of limbal
basal epithelium. Invest Ophthalmol Vis Sci 31:1903, 1990 94. Buck RC: Measurement of centripetal migration of normal corneal epithelial cells
in the mouse. Invest Ophthalmol Vis Sci 26:1296, 1985 95. Buck RC: Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci 18:767, 1979 96. Crosson CE, Klyce SD, Beuerman RW: Epithelial wound closure in the rabbit cornea: A biphasic process. Invest Ophthalmol Vis Sci 27:464, 1986 97. Soong HK, Cintron C: Disparate effects of calmodulin inhibitors on corneal epithelial migration
in rabbit and rat. Ophthalmic Res 17:27, 1985 98. Chen JJ, Tseng SC: Abnormal corneal epithelial wound healing in partial-thickness removal
of limbal epithelium. Invest Ophthalmol Vis Sci 32:2219, 1991 99. Danjo S, Friend J, Thoft RA: Conjunctival epithelium in healing of corneal epithelial wounds. Invest Ophthalmol Vis Sci 28:1445, 1987 100. Srinivasan BD, Worgul BV, Iwamoto T, Eakins KE: The reepithelialization of rabbit cornea following partial and complete
corneal denudation. Exp Eye Res 25:343, 1977 101. Murata T, Ishibashi T, Inomata H: Localizations of epidermal growth factor receptor and proliferating cell
nuclear antigen during corneal wound healing. Graefes Arch Clin Exp Ophthalmol 231:104, 1993 102. Zieske JD, Wasson M: Regional variation in distribution of EGF receptor in developing and adult
corneal epithelium. J Cell Sci 106:145, 1993 103. Addicks EM, Quigley HA, Green WR, Robin AL: Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol 101:795, 1983 104. Katz GJ, Higginbothan EJ, Lichter PR et al: Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology 102:1263, 1995 105. Ascher KW: Aqueous veins and their significance for pathogenesis of glaucoma. Arch Ophthalmol 42:66, 1949 106. Ascher KW: Aqueous veins: Preliminary note. Am J Ophthalmol 25:31, 1942 107. Jampol LM, Nagpal KC: Hemorrhagic lymphangiectasia of the conjunctiva. Am J Ophthalmol 85:419, 1978 108. Breinin GM: Scleredema adultorum: Ocular manifestations. Arch Ophthalmol 50:155, 1953 |