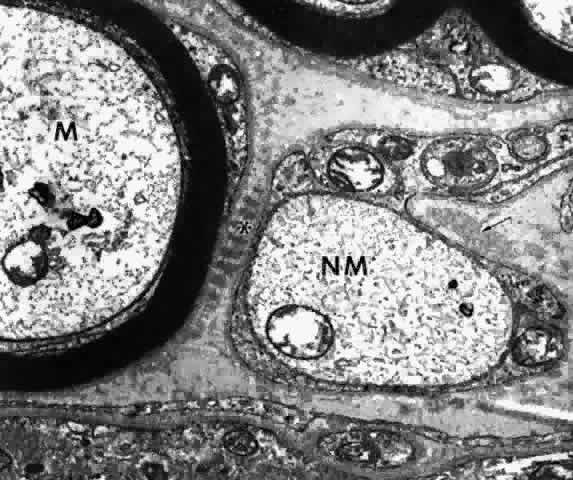
NONPIGMENTED CILIARY EPITHELIUM The ciliary epithelium consists of two layers: an inner nonpigmented (NPE) and
an outer pigmented layer (PE). These two layers derive from infolding
of the single cell layer of the optic vesicle against itself, to
form the optic cup. The potential space left between the two ciliary
layers rarely opens, owing to the frequency of junctional complexes
uniting the cells. A peculiar result of the infolding affects nomenclature
in this region, since the apices of the epithelial cells now face
each other across the potential space (Fig. 11). The bases of the cells face outward, toward the ciliary body stroma
for the PE, and toward the posterior chamber for the NPE. Basement membrane
covers the bases of both cell layers as is characteristic of epithelial
cells. 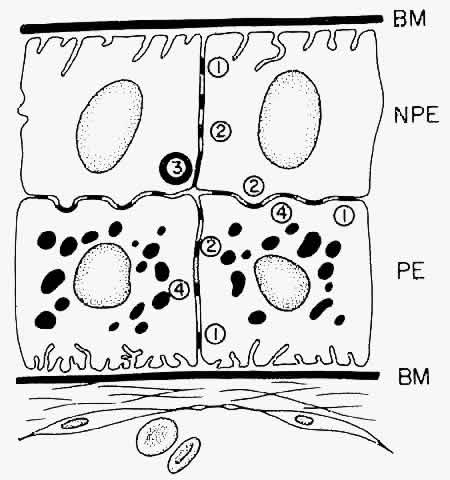 Fig. 11. Diagram showing the relationship of the two layers of ciliary epithelium. Basement
membrane (BM) covers their bases and their apices face each
other across a potential space. The cells are united by several types
of junctional complexes: 1, desmosomes; 2, gap junctions; and 4, puncta
adherentes. Onlythe apices of the nonpigmented epithelial cells (NPE) are
joined by “tight” junctions (3), composed of a zonula
occludens usually combined with a zonular adherens complex. PE, pigmented
epithelium. Fig. 11. Diagram showing the relationship of the two layers of ciliary epithelium. Basement
membrane (BM) covers their bases and their apices face each
other across a potential space. The cells are united by several types
of junctional complexes: 1, desmosomes; 2, gap junctions; and 4, puncta
adherentes. Onlythe apices of the nonpigmented epithelial cells (NPE) are
joined by “tight” junctions (3), composed of a zonula
occludens usually combined with a zonular adherens complex. PE, pigmented
epithelium.
|
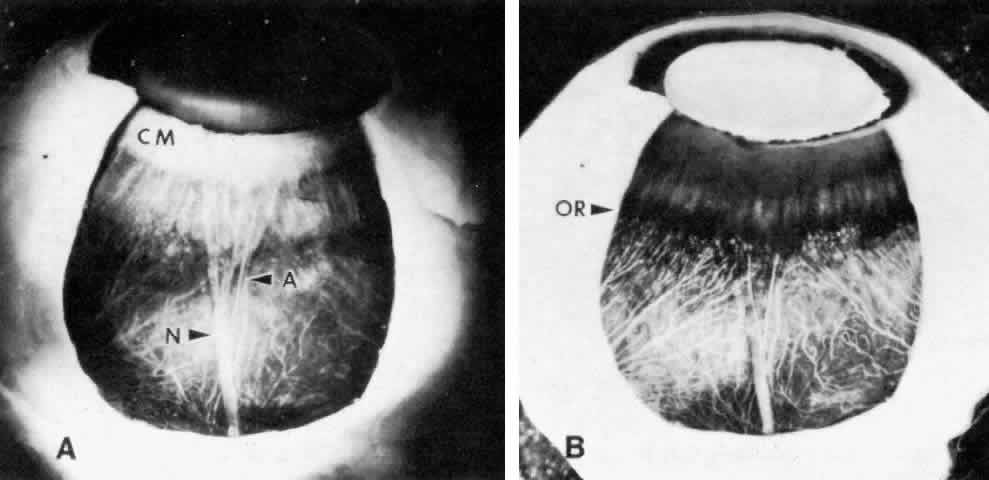
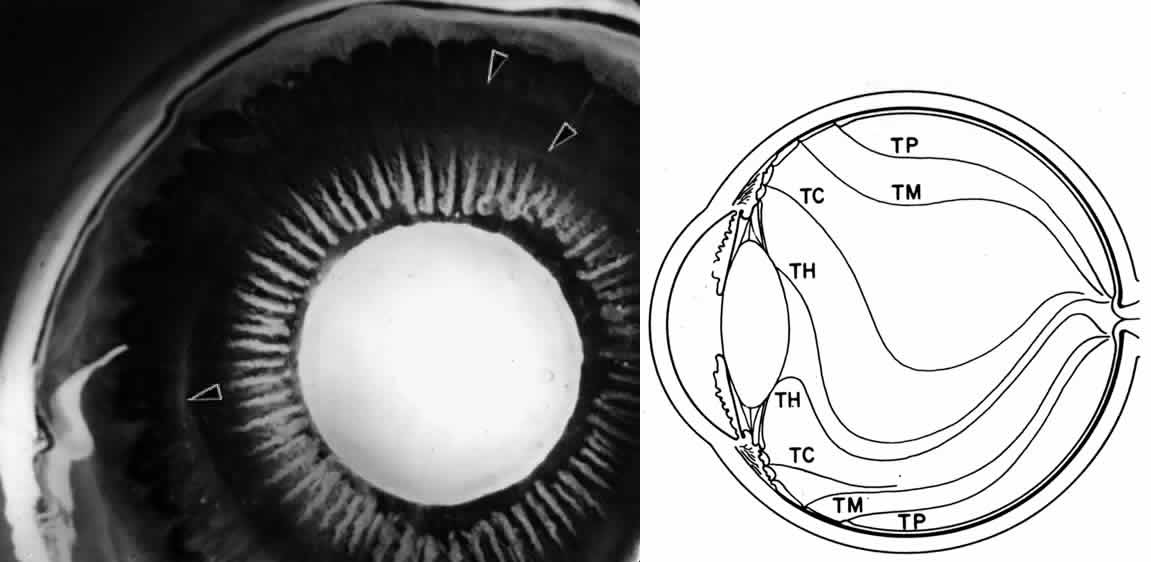
The NPE of the ciliary body stretches in a continuous layer from the root
of the iris to the ora serrata. As the transition from pigmented iris
epithelium occurs, melanin granules in the inner layer suddenly decrease
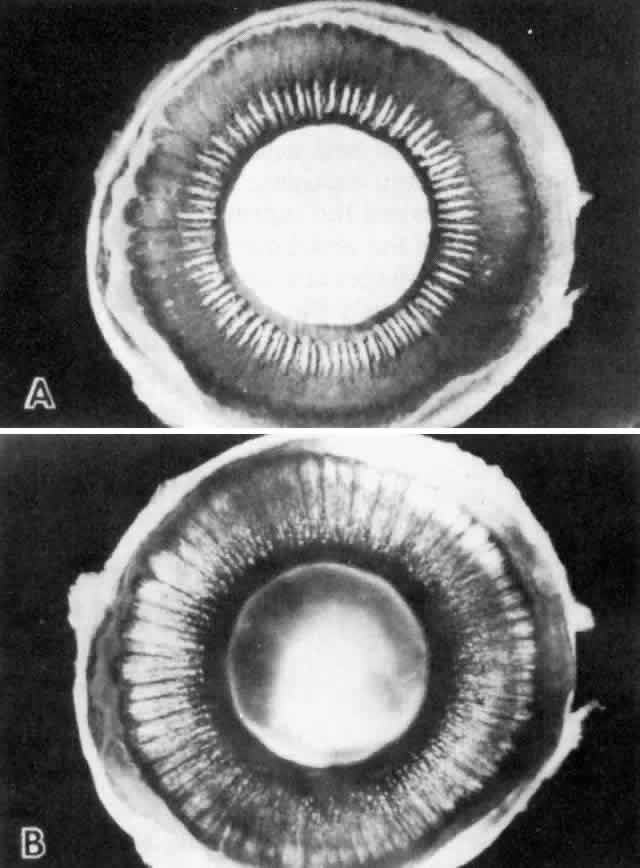
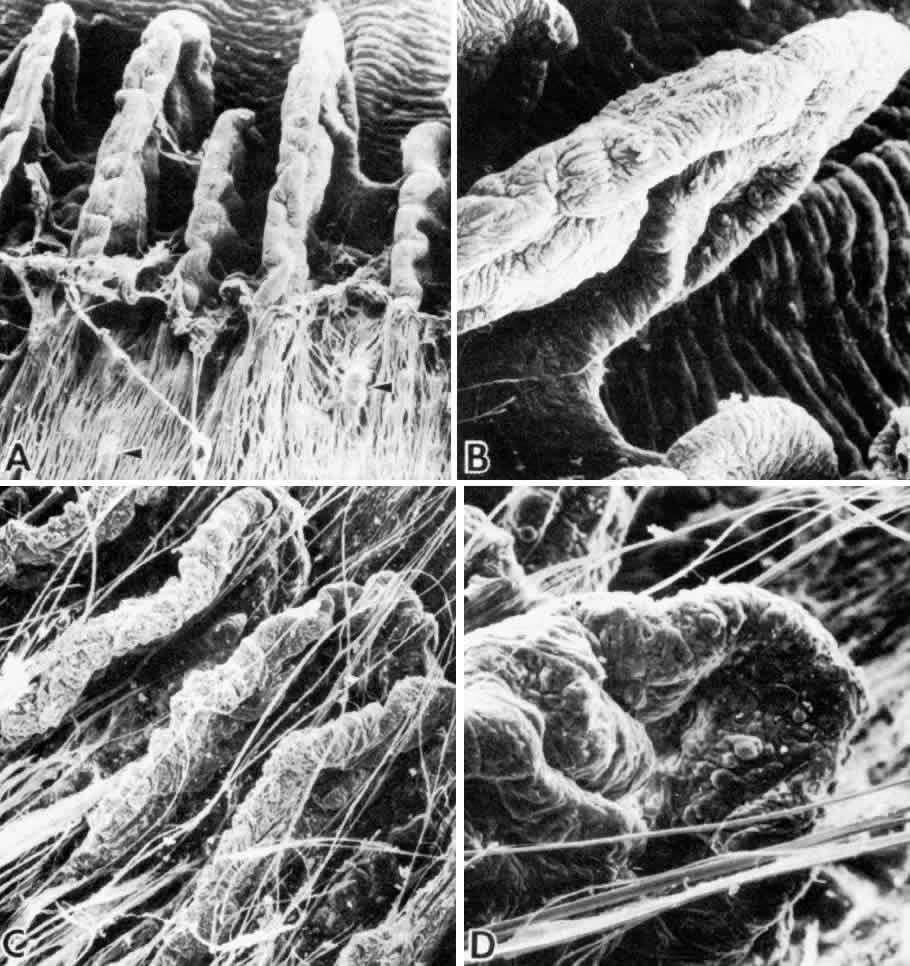
in number, and the cells become slightly smaller (Fig. 12). In the pars plicata the NPE cells are cuboidal, 12 to 15 μm in width, with
central nuclei (Figs. 13A and 13B). The knobbiness that develops during aging is due to small nodular proliferations
of NPE cells, especially on the minor plicae (Fig. 13C). In the young eye the cells of the pars plana are also cuboidal, but
with growth they become thinner and more columnar, sometimes reaching
up to 30 μm in height and 5 to 10 μm in width (Fig. 13D). In the posterior half of the pars plana, some NPE cells tilt forward
as though responding to anterior zonular traction, while others may be
inclined posteriorly, suggesting complex vectors of force in this region. The
nuclei are vertically oval and lie near the apex of the cells. The
epithelium here becomes very irregular with aging, showing hyperplastic
toothlike cell processes intertwining and extending up into the
vitreous and among the zonular fibers. At the ora serrata the ciliary
NPE joins the retina abruptly, highlighting the difference in thickness
of these two layers (Fig. 14).  Fig. 12. Frontal view of a ciliary process at its junction with iris, showing conversion
to thicker, double-layered iris pigment epithelium (arrows). (Toluidine
blue, X 200) Fig. 12. Frontal view of a ciliary process at its junction with iris, showing conversion
to thicker, double-layered iris pigment epithelium (arrows). (Toluidine
blue, X 200)
|
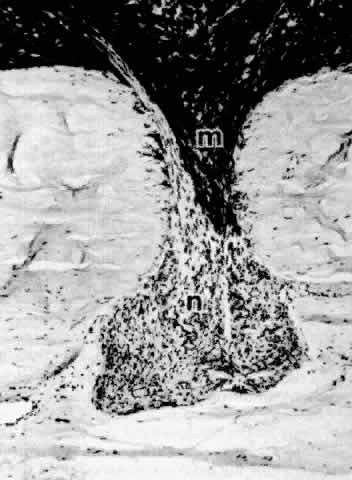
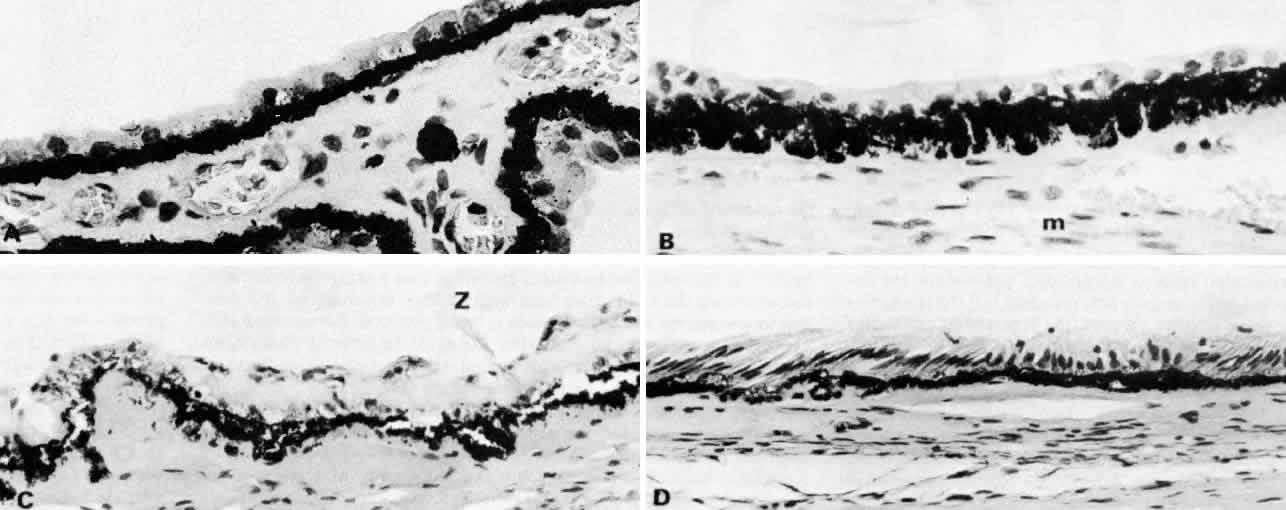 Fig. 13. Ciliary nonpigmented epithelium. A. Anterior pars plicata, age 3. (hematoxylin-eosin, X 800) B. Posterior pars plicata with areas of thickened, pigmented
epithelium, age 3. m, ciliary muscle (hematoxylin-eosin, X 800) C. Nodular
proliferation of the nonpigmented epithelium appears
as small cellular caps over the surface at age 70. Z, zonule. (hematoxylin-eosin, X 400) D. Distorted epithelium of pars plana shows evidence
of traction in both anterior and posterior directions (adult). (hematoxylin-eosin, X 400) Fig. 13. Ciliary nonpigmented epithelium. A. Anterior pars plicata, age 3. (hematoxylin-eosin, X 800) B. Posterior pars plicata with areas of thickened, pigmented
epithelium, age 3. m, ciliary muscle (hematoxylin-eosin, X 800) C. Nodular
proliferation of the nonpigmented epithelium appears
as small cellular caps over the surface at age 70. Z, zonule. (hematoxylin-eosin, X 400) D. Distorted epithelium of pars plana shows evidence
of traction in both anterior and posterior directions (adult). (hematoxylin-eosin, X 400)
|
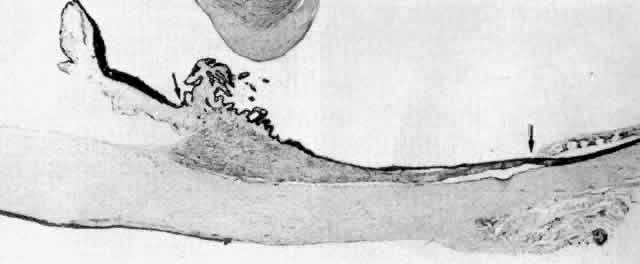
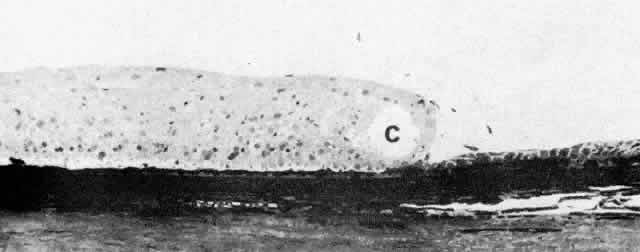 Fig. 14. Ora serrata in young adult, showing abrupt junction of ciliary nonpigmented
epithelium and sensory retina. A few hyalocytes are present in the
adjacent vitreous, and a degenerative cyst (C) is present in the peripheral
retina. (Toluidine blue, X 200) Fig. 14. Ora serrata in young adult, showing abrupt junction of ciliary nonpigmented
epithelium and sensory retina. A few hyalocytes are present in the
adjacent vitreous, and a degenerative cyst (C) is present in the peripheral
retina. (Toluidine blue, X 200)
|
While the ultrastructure of the PE is similar in most areas of the ciliary
body, that of the NPE shows noticeable regional differences that appear
to be of functional importance. These differences have been studied
more extensively in the monkey than in the human but are similar in
both species.2,16–26 The pars plicata, and particularly its anterior portion, appears to be
the predominant site of aqueous formation and has many special characteristics
of this secretory function. The NPE here has marked cytoplasmic
infolding at its base and redundant interdigitations at its basolateral
margins, greatly increasing the area of the cell surface facing
the posterior chamber (Fig. 15, Inset A). These cell membranes and, to a lesser degree, those of the
pigmented epithelium contain the enzyme complex Na+ /K+ -ATPase, evidence of anenergy-dependent active transport system.27 The presence of the enzyme carbonic anhydrase in the NPE cells of the
pars plicata of all species studied is further evidence of fluid-pumping
activity.28 In the moderately electron-lucent cytoplasm of the anterior NPE particularly, there
are large numbers of mitochondria near the base of the cell
and rough endoplasmic reticulum (RER) in single cisternae or parallel
stacks near the nucleus, where Golgi complexes are also common22 (Fig. 16). The mitochondria are of importance in providing energy for transport, and
the RER for processing of new protein. Clusters of free ribosomes
and occasional cilia are found in all areas. With aging, unusual whorled
formations of RER are described near the cell base in the pars plicata, along
with lipid droplets and lysosomal residual bodies.17,22 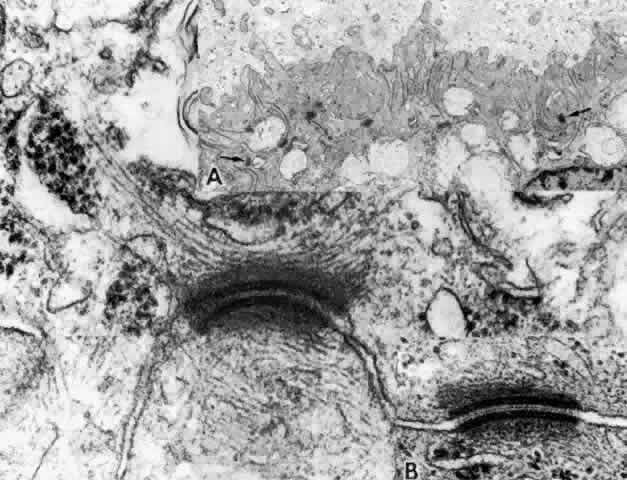 Fig. 15. Anterior pars plicata. Inset A. The basement membrane does not enter the
extensive surface infoldings and lateral interdigitations of the nonpigmented
epithelium. Note large numbers of desmosomal intercellular junctions (arrows). Main
figure. A desmosome cut slightly obliquely showing
intermediate filaments of the cell's cytoskeleton inserting
into the dense plaque material. Inset B. Central dense core and plasmalemmal
unit membranes of desmosome cut perpendicularly. (Main figure and
inset B, X 104,000; inset A, X 10,800) Fig. 15. Anterior pars plicata. Inset A. The basement membrane does not enter the
extensive surface infoldings and lateral interdigitations of the nonpigmented
epithelium. Note large numbers of desmosomal intercellular junctions (arrows). Main
figure. A desmosome cut slightly obliquely showing
intermediate filaments of the cell's cytoskeleton inserting
into the dense plaque material. Inset B. Central dense core and plasmalemmal
unit membranes of desmosome cut perpendicularly. (Main figure and
inset B, X 104,000; inset A, X 10,800)
|
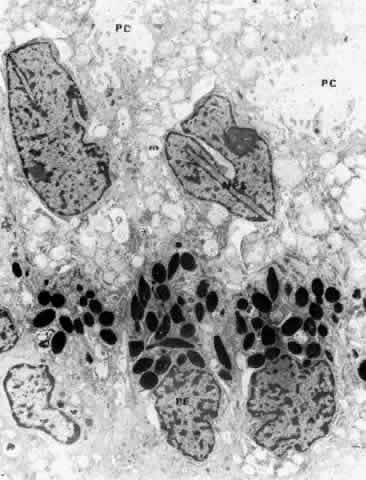 Fig. 16. Ciliary epithelium in the anterior pars plicata of a 19-year-old. Posterior
chamber surface (PC) of the nonpigmented epithelium (NPE) has a sawtooth
pattern seen irregularly throughout this layer with aging. Mitochondria (m) are
large, plentiful, and show artifactual hydropic change. Rough
endoplasmic reticulum is present below the nuclei (asterisk). Note
that the apices of the pigmented epithelial cells (PE) are conical, with
prolongations of NPE between them. (X 8320) Fig. 16. Ciliary epithelium in the anterior pars plicata of a 19-year-old. Posterior
chamber surface (PC) of the nonpigmented epithelium (NPE) has a sawtooth
pattern seen irregularly throughout this layer with aging. Mitochondria (m) are
large, plentiful, and show artifactual hydropic change. Rough
endoplasmic reticulum is present below the nuclei (asterisk). Note
that the apices of the pigmented epithelial cells (PE) are conical, with
prolongations of NPE between them. (X 8320)
|
In the child's eye, the basement membrane is a simple thin layer over
the base of the NPE cells, not extending into the surface or lateral
infoldings (Fig. 17, Inset A). As is typical for thin basement membranes, it is a 30-nm granular
layer separated from the plasmalemma by a 50-nm lucent zone. Beginning
in the first decade, the basement membrane undergoes a remarkable
thickening of the multilaminar reticulated type. This change has been
identified by the age of 3 years,17 beginning in the valleys of the posterior half of the pars plicata. From
this region, the thickening by multiple intertwining thin layers of
reticular basement membrane extends posteriorly and up onto the lateral
walls of the ciliary processes, involving almost all of the ciliary
epithelium in those over the age of 50 years. This thick basement membrane
may reach 2 μm with an increasing quantity of membrane-bound
vesicles and granular material, apparently debris of cellular metabolism (see Fig. 17). Seen from the inner surface, the basement membrane has a fibrogranular
texture resembling the loose superficial lens capsule in the zonula
lamellar region (Fig. 17, Inset B). Zonular bundles are seen in close apposition to the basement
membrane and pass superficially within it in the ciliary valleys, to
which they have a very firm attachment.15  Fig. 17. Changes in the ciliary nonpigmented epithelial basement membrane that occur
with aging.Main figure shows multilaminar pattern of basement membrane
proliferation filled with vesicles and granular debris at age 20. (X 54,600) Inset
A. Thin basement membrane with patchy multilaminar
change at age 7. (X 15,000) Inset B. Scanning electron micrograph showing
fibrogranular mossy appearance of basement membrane surface over sides
of anterior ciliary processes at age 20. (X 10,200) Fig. 17. Changes in the ciliary nonpigmented epithelial basement membrane that occur
with aging.Main figure shows multilaminar pattern of basement membrane
proliferation filled with vesicles and granular debris at age 20. (X 54,600) Inset
A. Thin basement membrane with patchy multilaminar
change at age 7. (X 15,000) Inset B. Scanning electron micrograph showing
fibrogranular mossy appearance of basement membrane surface over sides
of anterior ciliary processes at age 20. (X 10,200)
|
In the pars plana, the tall NPE cells have numerous intermediate filaments
and granules that give the cells a more electron-dense appearance
than anteriorly (Fig. 18A) and are profusely supplied with tubules of smooth-surfaced endoplasmic
reticulum (Fig. 18B). The intermediate filaments in the NPE of the pars plicata over the ciliary
crests and in the pars plana are strongly immunoreactive for vimentin, with
fainter staining for cytokeratin 18, just the reverse of
the staining pattern in the ciliary PE.26 The vimentin-positive intermediate fibers attach the cytoskeleton to adherens
junctions and often indicate cells subject to tractional or cell-shape
stresses. Fine actin filaments are present in the cytoskeleton
of both epithelial cell types, without regional differences. Extensive
cystic dilatations of the intercellular spaces between the NPE cells
commonly occur in the posterior pars plana of adult eyes. Fine and Zimmerman2 showed that these spaces contain hyaluronidase-sensitive acid mucopolysaccharide
and suggested it might be hyaluronic acid intended for the
vitreous. An increased number of Golgi complexes in this region also suggests
production of some glycoprotein.22 Immunostaining for the membrane-bound enzyme hyaluronan synthase is positive
on the membranes of the primate posterior pars plana NPE cells
and hyalocytes, consistent with local hyaluronan secretion.3 Interestingly, staining is equally intense over the crests of the ciliary
processes. 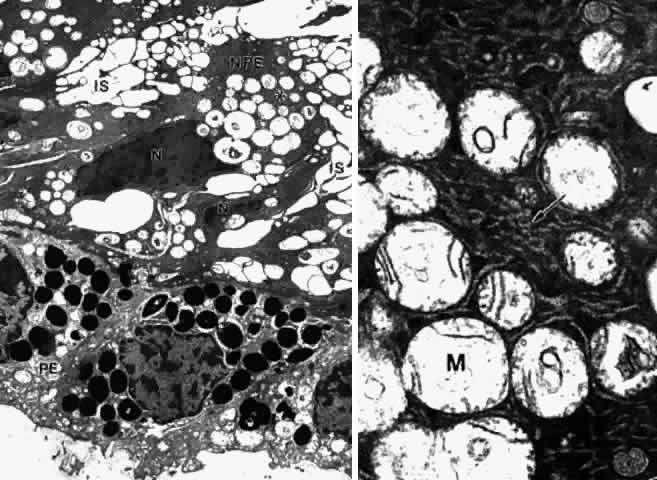 Fig. 18. Posterior pars plana. A. Nonpigmented epithelial (NPE) cells appear tilted
and compressed with dense cytoplasm. The extensive cystic dilatation
of the intercellular spaces (IS) around these cells is characteristic
of this region. Only the nucleus (N) and the many hydropic mitochondria
are visible in the cytoplasm. The basement membrane under the pigment
epithelium (PE) is multilaminarand thick. (X 5700) B. Higher magnification
of asterisked region in A shows that the cytoplasm is full of
branching and curved profiles of smooth endoplasmic reticulum (arrow), which
is also typical of this region. M, mitochondrion. (X 22,000) Fig. 18. Posterior pars plana. A. Nonpigmented epithelial (NPE) cells appear tilted
and compressed with dense cytoplasm. The extensive cystic dilatation
of the intercellular spaces (IS) around these cells is characteristic
of this region. Only the nucleus (N) and the many hydropic mitochondria
are visible in the cytoplasm. The basement membrane under the pigment
epithelium (PE) is multilaminarand thick. (X 5700) B. Higher magnification
of asterisked region in A shows that the cytoplasm is full of
branching and curved profiles of smooth endoplasmic reticulum (arrow), which
is also typical of this region. M, mitochondrion. (X 22,000)
|
There are a large number of intercellular junctions between the ciliary
epithelial cells, each giving important data about the specific functions
of these cells (see Fig. 11). Toward the base of the NPE cells their lateral sides are joined by desmosomes (see Fig. 15). At their apical ends they are connected by typical tight junctional
complexes consisting of a zonula occludens and zonula adherens (Fig. 19). These tight junctions represent the primary blood-aqueous barrier in
the ciliary body. When large tracer molecules such as horseradish peroxidase
are injected intravenously into primates,20,21 the tracer has an easy passage through the fenestrated capillaries of
the ciliary processes, but does not pass beyond the apices of the NPE
cells (see Fig. 19). 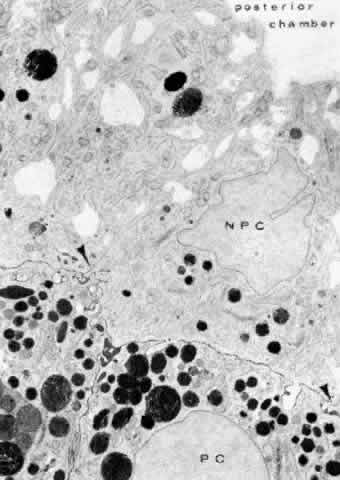 Fig. 19. Evidence of tight junctional complexes in the anterior ciliary epithelium
of Macaca mulatta. The pigmented epithelial cells (PC) are outlined
by a thin black line that is the reaction product of horeseradish peroxidase, a
tracer substance injected intravenously in vivo. The tracer
has entered the intercellular spaces of the nonpigmented epithelium (NPC) but
is held up by occluding junctions (arrowheads), preventing further
progress into the posterior chamber. (X 8450; Courtesy of Dr. Guiseppina Raviola) Fig. 19. Evidence of tight junctional complexes in the anterior ciliary epithelium
of Macaca mulatta. The pigmented epithelial cells (PC) are outlined
by a thin black line that is the reaction product of horeseradish peroxidase, a
tracer substance injected intravenously in vivo. The tracer
has entered the intercellular spaces of the nonpigmented epithelium (NPC) but
is held up by occluding junctions (arrowheads), preventing further
progress into the posterior chamber. (X 8450; Courtesy of Dr. Guiseppina Raviola)
|
The zonula occludens is the primary component of the blood-aqueous barrier “tight
junction.” It appears as a focal area at which
the bilayered plasmalemmal membranes of each cell surface are tightly
joined (Fig. 20). Zonular adherens junctions occur adjacent to occludens junctions on
the basal side. They show a 12- to 15-nm space between the adjoining cells, with
filamentous matrix material clinging to the cell membranes
on either side. By the freeze-fracture technique, the zonula occludens
consists of branching anastomosing strands on the cytoplasmic side of
the plasmalemmal membrane (P-face) and matching grooves on the external
side (E-face), giving a quilted effect (Fig. 21). The variation in number of strands seen from area to area in the ciliary
zonula occludens region23 is consistent with physiologic evidence that the NPE is leaky to ions
and small molecules, rather than being an absolute barrier like that between
the endothelial cells of the retinal vessels. Ohnishi and Kuwabara24 found the tight junctions of the anterior pars plicata had the fewest
strands, explaining why this region is so sensitive to leakage after paracentesis
in several species. 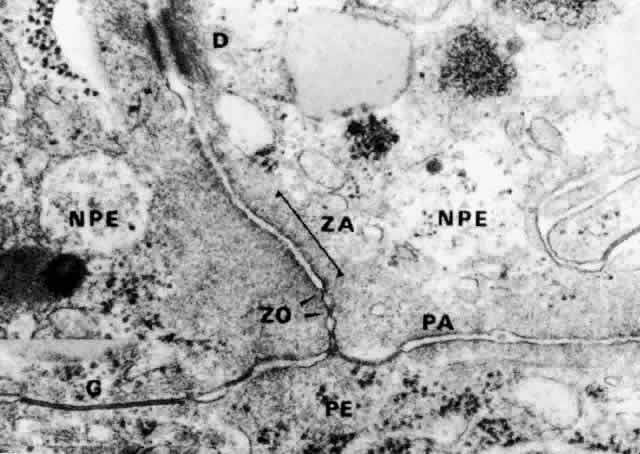 Fig. 20. Apical junction of two nonpigmented epithelial cells (NPE) showing focal
zonula occludens (ZO) junctions (arrows), an adjacent zonula adherens (ZA), and
desmosome (D), puncta adherentes (PA), and gap (G) junctions. PE, pigmented
epithelium. (X 58,700) Fig. 20. Apical junction of two nonpigmented epithelial cells (NPE) showing focal
zonula occludens (ZO) junctions (arrows), an adjacent zonula adherens (ZA), and
desmosome (D), puncta adherentes (PA), and gap (G) junctions. PE, pigmented
epithelium. (X 58,700)
|
 Fig. 21. A zonula occludens “tight” junctional area (TJ) between nonpigmented
epithelial cells in Macaca mulatta as seen by the freeze-fracture
technique. It shows a network of anastomosing grooves (arrows) on
the outer plasmalemmal leaflet, giving a quilted effect. A few fragments
of the complementary strands from the inner leaflet (P-face) are
still adherent. (X 60,500; Courtesy of Dr. Guiseppina Raviola) Fig. 21. A zonula occludens “tight” junctional area (TJ) between nonpigmented
epithelial cells in Macaca mulatta as seen by the freeze-fracture
technique. It shows a network of anastomosing grooves (arrows) on
the outer plasmalemmal leaflet, giving a quilted effect. A few fragments
of the complementary strands from the inner leaflet (P-face) are
still adherent. (X 60,500; Courtesy of Dr. Guiseppina Raviola)
|
Gap junctions occur in “extraordinary numbers” in the ciliary
epithelium and may be found even among the strands of zonula occludens.23 At gap junctions, the surface membranes of the two cells run a very straight
course. They are separated only by a 2- to 3-nm cleft, which becomes
filled with reaction product in tracer experiments (Fig. 22, Inset). In freeze-fractured specimens, gap junctions are easily recognized
as rounded patches of 8- to 9-nm particles arranged in crystalline-like
array on the inner plasmalemmal leaflet (see Fig. 22), matched by pits on the outer surface. The plethora of gap junctions
in the ciliary epithelium indicates that the cells are closely coupled
for electrical and metabolic cooperative work across these sites of low
ionic resistance. 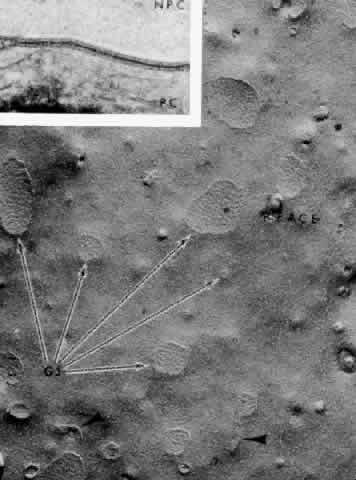 Fig. 22. Inset. A gap junction between pigmented and nonpigmented epithelial cells (NPC) in
Macaca mulatta. The tracer horseradish peroxidase, injected
in vivo, has filled the small gap between the cell plasma membranes. Main
figure. Gap junctions (GJ) at apex of nonpigmented epithelial cells
are rounded aggregates of 8- to 9-nm particles (arrows) on the inner
leaflet (P-face) of the plasmalemmal membrane. Zonula occludens-like
strands are frequently seen at the periphery (arrowheads). (Freeze-fracture, X 34,000; Inset, X 168,000; Courtesy of Dr. Guiseppina
Raviola) Fig. 22. Inset. A gap junction between pigmented and nonpigmented epithelial cells (NPC) in
Macaca mulatta. The tracer horseradish peroxidase, injected
in vivo, has filled the small gap between the cell plasma membranes. Main
figure. Gap junctions (GJ) at apex of nonpigmented epithelial cells
are rounded aggregates of 8- to 9-nm particles (arrows) on the inner
leaflet (P-face) of the plasmalemmal membrane. Zonula occludens-like
strands are frequently seen at the periphery (arrowheads). (Freeze-fracture, X 34,000; Inset, X 168,000; Courtesy of Dr. Guiseppina
Raviola)
|
Another structural feature in the ciliary epithelium that may be related
to its secretory activity is the frequent attachment of mitochondria
to desmosomal junctions in these cells.29 This relationship has been noted in many other secretory cells in the
body. As the mitochondrion can sequester calcium, its proximity was suggested
to protect against uncoupling of the desmosomal junction, when
needed to control intercellular volume. JUNCTION OF NONPIGMENTED AND PIGMENTED CILIARY EPITHELIAL CELLS The junctions between the NPE and the PE are of great importance because
these cells must work in metabolic concert and also overcome the intraocular
stresses that tend to separate them. The first requirement is
met by the presence of the largest number of gap junctions in the ciliary
epithelium and the second by desmosomes as well as unusual junctions
called puncta adherentes.23 The latter junction resembles the zonula adherens but is a focal adhesion, rather
than a band around the cell (see Fig. 20). Like the zonula adherens, it has a loose mat of filmy filaments on either
side and poorly seen matrix in the intercellular cleft. Both junctions
lack the dense plaques present on each side of the desmosomal junction, with
their attaching large (10-nm) intermediate filaments and
central band in the intercellular cleft (see Fig. 15). Fine 4- to 6-nm contractile actin filaments may attach to the cell membrane
at the puncta adherentes junctions23 and lend strength to intercellular junctions as well.26 According to Ober and Rohen,25 puncta adherentes occur in larger numbers in the ciliary valleys than
over their crests, possibly to counteract the pull of zonular attachments
in the valleys. The great frequency of desmosomes between the posterior
pars plana cells may have a similar function. The gap junctions
have a strong tendency to invaginate into the pigment epithelial cells, producing
fingerlike prolongations. Another unusual structural differentiation between NPE and PE cells is
the “ciliary channel,”16 (Fig. 23) an explanation for which has not yet been offered. These channels are
small foci of dilated intercellular space that often contain fine granular
material and have microvilli from the cell surfaces protruding into
them. 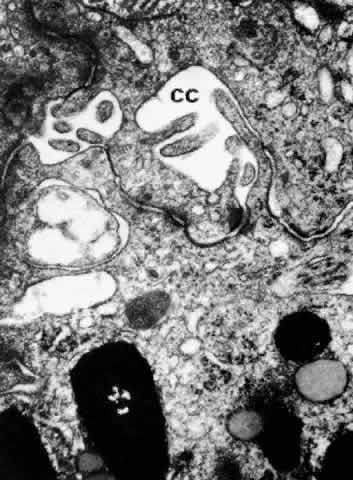 Fig. 23. Ciliary channels (CC) between the pigmented and nonpigmented epithelium
in the anterior pars plicata. Microvilli from the cell surfaces extend
into these spaces. (X 35,000) Fig. 23. Ciliary channels (CC) between the pigmented and nonpigmented epithelium
in the anterior pars plicata. Microvilli from the cell surfaces extend
into these spaces. (X 35,000)
|
PIGMENTED CILIARY EPITHELIUM The ciliary PE is in most regions a single layer from the ora serrata to
the iris. The cells have a similar cuboidal shape in all parts of the
ciliary body, from 10 to 12 μm in height and about 10 μm in width.4 Scattered irregularly through the pars plicata region and more regularly
in the pars plana are small nodules of PE cells protruding more deeply
into the stroma. These nodules of cells have small round nuclei and
no lumina, thus not appearing to be glandular in nature (Fig. 24). It is suggested they may provide a stronger anchorage against tractional
stresses in these regions.30 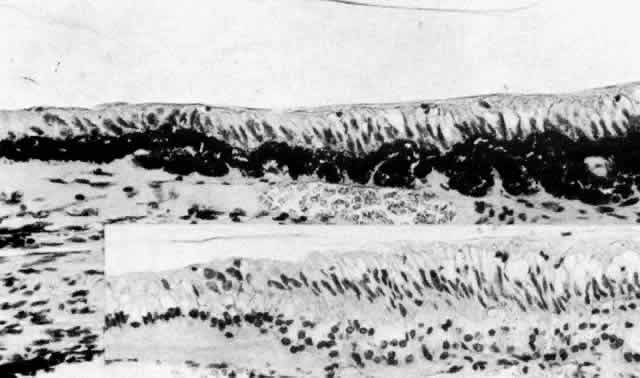 Fig. 24. Proliferation of the pigmented epithelium in the posterior pars plana region
of a 29-year-old patient. Inset. An adjacent section after pigment
bleaching reveals the multinodular pattern of this proliferation. (hematoxylin-eosin, X 500) Fig. 24. Proliferation of the pigmented epithelium in the posterior pars plana region
of a 29-year-old patient. Inset. An adjacent section after pigment
bleaching reveals the multinodular pattern of this proliferation. (hematoxylin-eosin, X 500)
|
The lateral margins of the PE cells are straight, with the same junctions
seen between NPE and PE cells, that is, gap junctions, desmosomes, and
puncta adherentes.22,23 No zonula occludens is found, so this layer has no barrier function. However, in
the most posterior pars plana adjacent to the retina, the PE
cells appear to contain both carbonic anhydrase and Na+ /K+ -ATPase.27,28 Flugel and Lutgen-Drecoll28 suggest this stronger pump could prevent retinal edema in the region where
the tight junction site switches from the apical NPE of the ciliary
body to the apical PE of the retina. The cytoplasm of the PE cell is more electron-dense than that of the NPE
cell, containing many free ribosomes, actin filaments, and predominantly
cytokeratin-containing intermediate filaments (Fig. 25).26 The mitochondria are smaller and fewer in number than in the NPE, as is
the RER, and the Golgi apparatus is present irregularly. There are large
numbers of rounded and some elliptical melanin pigment granules of
the 0.8 μm to 2 μm size characteristic of neuroepithelium, and
three to four times larger than those of the stromal pigment cells. A
decrease in pigment granules occurs in the PE over the crests of the
ciliary processes from the fourth decade of life. Lipid droplets and complex
lipopigment granules are seen within the cytoplasm during aging (see Fig. 25). The base of the PE cell is markedly infolded, lying on a basement membrane
that with aging develops a thick, multilaminar pattern generally
denser than that over the NPE cells but also containing vesicular, fibrillar, and
granular inclusions (Fig. 26). The basement membrane of the PE is often very close to or almost continuous
with that of the fenestrated capillaries in the pars plicata region (Fig. 27). 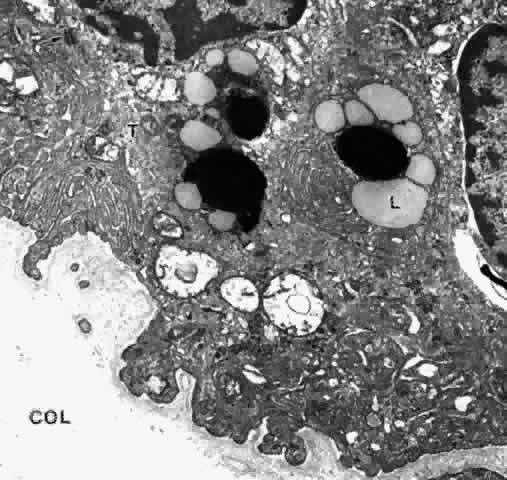 Fig. 25. Ciliary pigmented epithelial cells in mid pars plana of a young adult. The
cytoplasm is electron-dense with many tonofilaments (T) (intermediate
filaments). Lipid droplets (L) are present around dark lysosomal residual
bodies. Desmosomes connect the cells (arrow). The basal surface
and intercellular junctional areas are markedly infolded, and the basement
membrane is moderately thick. Negative images of collagen fibers (COL) are
seen below, in the dense stroma typical of this region (X 17,500) Fig. 25. Ciliary pigmented epithelial cells in mid pars plana of a young adult. The
cytoplasm is electron-dense with many tonofilaments (T) (intermediate
filaments). Lipid droplets (L) are present around dark lysosomal residual
bodies. Desmosomes connect the cells (arrow). The basal surface
and intercellular junctional areas are markedly infolded, and the basement
membrane is moderately thick. Negative images of collagen fibers (COL) are
seen below, in the dense stroma typical of this region (X 17,500)
|
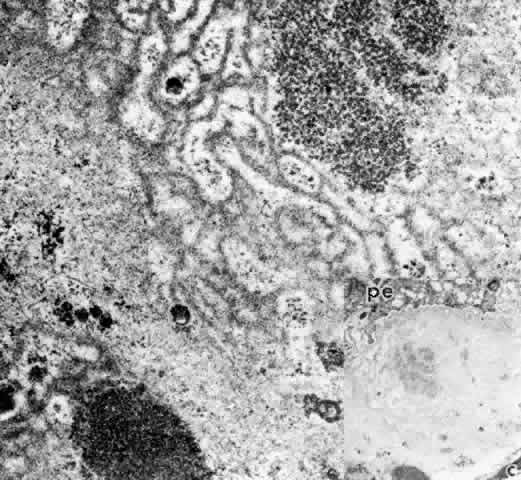 Fig. 26. Inset. Greatly thickened basal lamina under pigmented epithelial cell in (pe) pars
plicata of 80-year-old patient. c, capillary. Higher magnification
of area in inset shows mixed multilaminar and solid basement
membrane with fine filaments, vesicles, and granular clumps, after fixation
with Alcian blue to preserve glycosaminoglycans. (X 41,600; inset, X 10,330) Fig. 26. Inset. Greatly thickened basal lamina under pigmented epithelial cell in (pe) pars
plicata of 80-year-old patient. c, capillary. Higher magnification
of area in inset shows mixed multilaminar and solid basement
membrane with fine filaments, vesicles, and granular clumps, after fixation
with Alcian blue to preserve glycosaminoglycans. (X 41,600; inset, X 10,330)
|
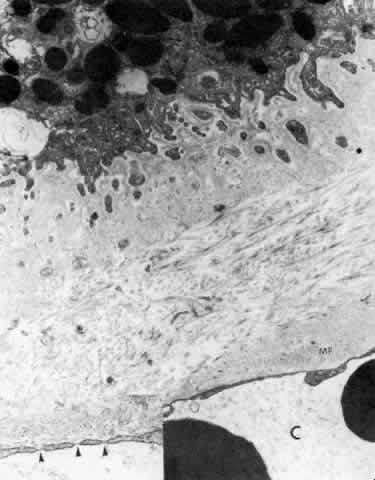 Fig. 27. Ciliary body stroma under the processes, anterior pars plicata. Even at
age 19, the basement membrane is thick. Collagen and other fine filaments
fill the narrow space between epithelium and fenestrated capillary
wall (C). Inset. Arrowheads indicate fenestrae in wall. Clumps of tubular
microfibrils (MF) of the elastic system are closely associated with
the capillary wall. (X 17,300; inset, X 43,000) Fig. 27. Ciliary body stroma under the processes, anterior pars plicata. Even at
age 19, the basement membrane is thick. Collagen and other fine filaments
fill the narrow space between epithelium and fenestrated capillary
wall (C). Inset. Arrowheads indicate fenestrae in wall. Clumps of tubular
microfibrils (MF) of the elastic system are closely associated with
the capillary wall. (X 17,300; inset, X 43,000)
|
CILIARY BODY STROMA The stroma of the ciliary body contains all the usual components of extracellular
matrix including collagens, elastic system fibers, and small
matrix molecules such as proteoglycans. The cellular components are
melanocytes, fibroblasts, blood vessels, and nerves, besides the large
quantity of smooth muscle comprising the bulk of this tissue. In the
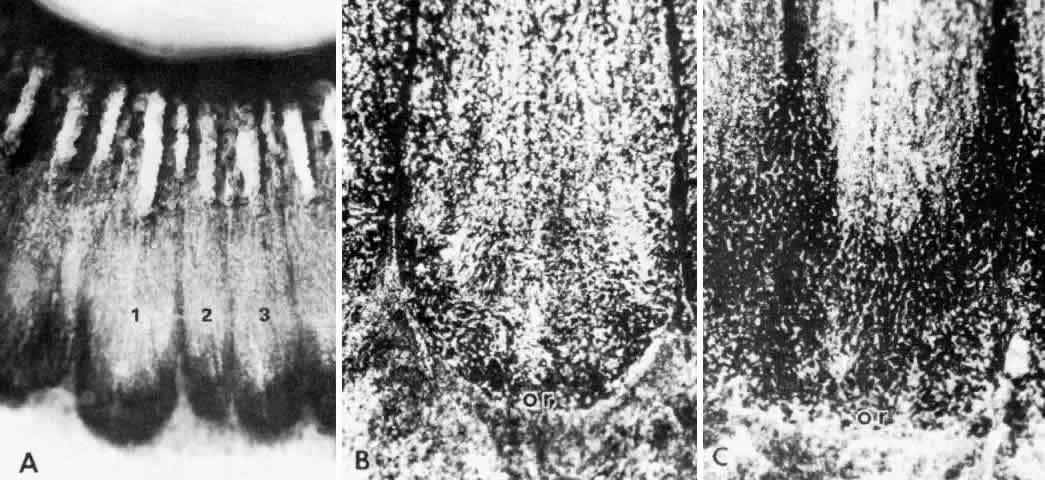
first one and one-half decades the nonvascular connective tissue in the
ciliary processes is scanty, resulting in the thin, underdeveloped appearance
of the juvenile ciliary processes (Fig. 28A). Their vessels are primarily fenestrated capillaries and veins, forming
plexi (see later section on blood supply). The subepithelial tissue
in the processes and plicae becomes very much thickened by collagenous
and hyaline material with aging (Fig. 28B and C), extending down to the ciliary muscle itself. In the deeper stroma, capillaries
are usually not fenestrated and show intermittent pericytes
outside the endothelial cell layer, surrounded by basement membrane that
merges with that of the endothelial cells (Fig. 29). The ciliary processes are essentially vascular structures and do not
contain extensions of the ciliary muscle, so the muscle has the same
thickness under the processes as under the ciliary valleys (Fig. 30). 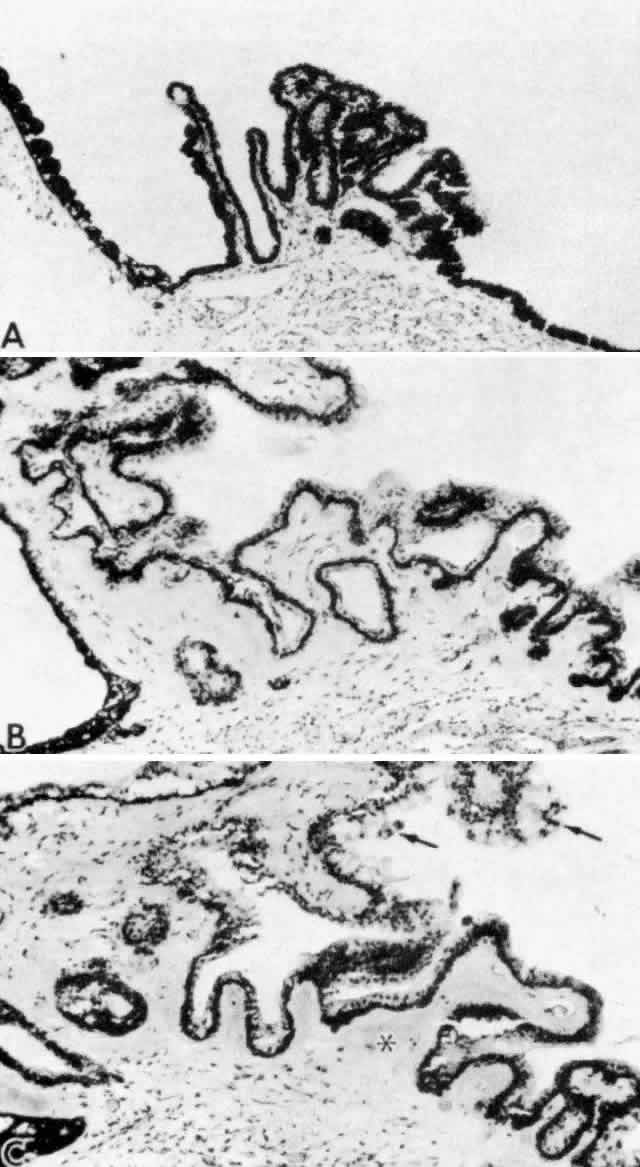 Fig. 28. Increase in cellularity and collagen density of ciliary process stroma
with age (A, 2 years; B, 49 years; C, 72 years). Note focal proliferation
of nonpigment epithelial cell caps (arrows) and increasing hyalinization
of the plicae (asterisk). (All hematoxylin-eosin, X 200) Fig. 28. Increase in cellularity and collagen density of ciliary process stroma
with age (A, 2 years; B, 49 years; C, 72 years). Note focal proliferation
of nonpigment epithelial cell caps (arrows) and increasing hyalinization
of the plicae (asterisk). (All hematoxylin-eosin, X 200)
|
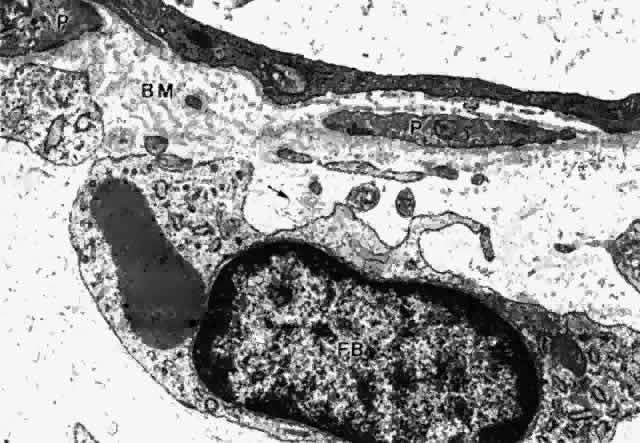 Fig. 29. Intermittent pericyte (P) coverage and lack of fenestrae in the endothelial
cell wall characterize capillaries of the deeper stroma in the pars
plicata. Basement membrane (BM) is multilayered. Fibroblast (FB) has
active rough endoplasmic reticulum and a large cisterna with granular
material. A small clump of elastic system microfibrils is seen at its
upper edge (arrow). (X 20,900) Fig. 29. Intermittent pericyte (P) coverage and lack of fenestrae in the endothelial
cell wall characterize capillaries of the deeper stroma in the pars
plicata. Basement membrane (BM) is multilayered. Fibroblast (FB) has
active rough endoplasmic reticulum and a large cisterna with granular
material. A small clump of elastic system microfibrils is seen at its
upper edge (arrow). (X 20,900)
|
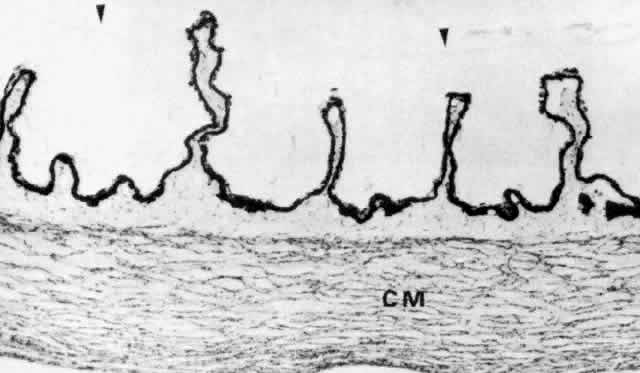 Fig. 30. Pars plicata of the ciliary body cut coronally, perpendicular to the usual
plane. Ciliary muscle (CM) does not extend into the ciliary processes, but
has the same thickness under processes and valleys. The anterior
hyaloid membrane is visible above the processes (arrowheads). (hematoxylin-eosin, X 120) Fig. 30. Pars plicata of the ciliary body cut coronally, perpendicular to the usual
plane. Ciliary muscle (CM) does not extend into the ciliary processes, but
has the same thickness under processes and valleys. The anterior
hyaloid membrane is visible above the processes (arrowheads). (hematoxylin-eosin, X 120)
|
The choriocapillaris does not continue forward into the ciliary body from
the choroid, but a thin layer of elastica continuous with Bruch's
membrane does (Fig. 31D). In the ciliary body, the elastica quickly becomes separated from the
basement membrane of the ciliary PE by the interposition of a dense and
then looser connective tissue (Fig. 31C). The elastic layer remains close to the underlying thin-walled pars plana
veins (Fig. 31A), becoming increasingly discontinuous (Fig. 31B) with wider branching, and is finally lost under the pars plicata. 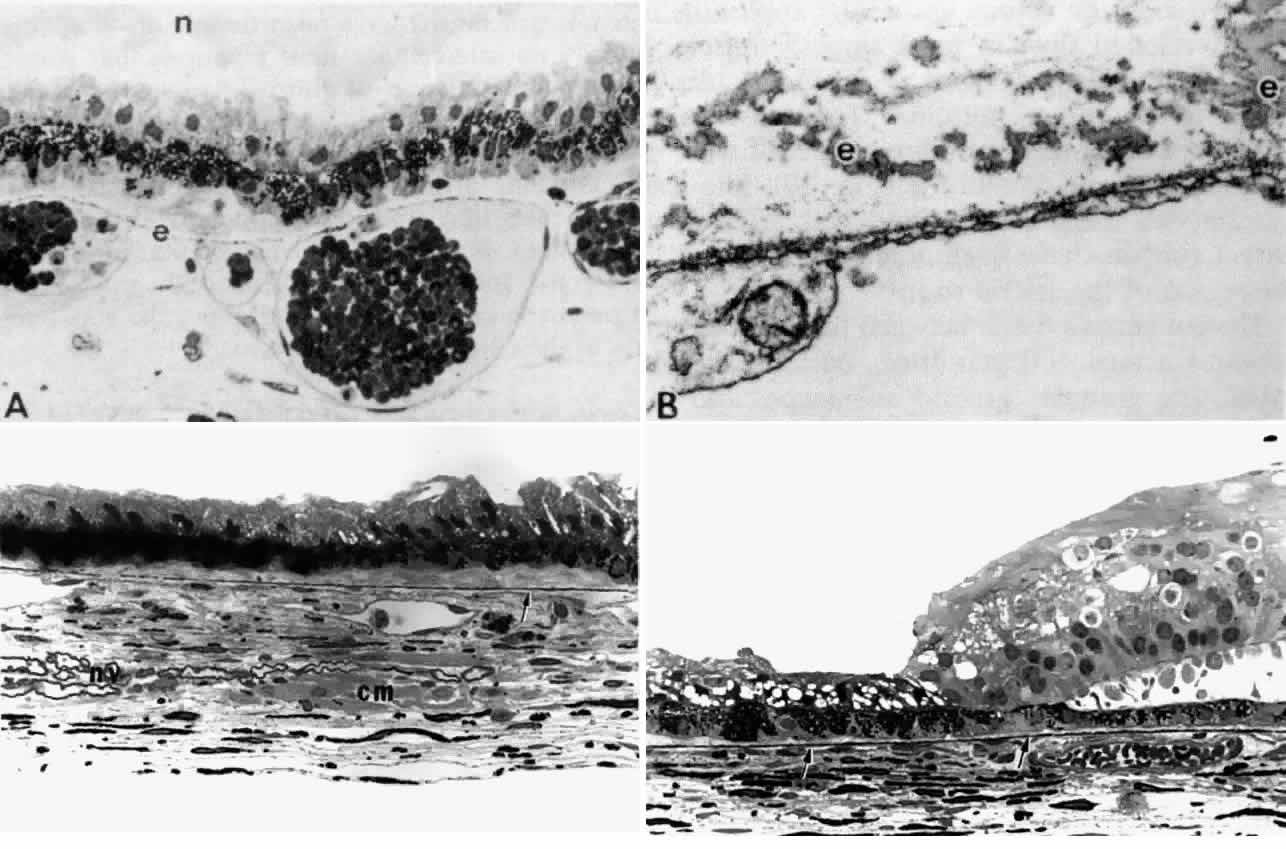 Fig. 31. Bruch's membrane in the ciliary body. A. In mid pars plana, Bruch's
elastica is a dotted thin line (e), appearing continuous with vein
walls. n, zonular bundles. (Toluidine blue, X 400) B. The dotted line
is a network of small elastic fibers with homogeneous elastin cores (e), outside
the fenestrated capillary wall. (Alcian blue, X 24,500) C. Elastica
becomes thicker and more complete (arrow) further posteriorly
in pars plana. Note nerve bundles (nv) and ciliary muscle bundles (cm) in
stroma. (Toluidine blue, X 433) D. Elastica continues smoothly
from end of pars plana (left arrow) into peripheral choroid (right arrow). (Toluidine
blue, X 433) Fig. 31. Bruch's membrane in the ciliary body. A. In mid pars plana, Bruch's
elastica is a dotted thin line (e), appearing continuous with vein
walls. n, zonular bundles. (Toluidine blue, X 400) B. The dotted line
is a network of small elastic fibers with homogeneous elastin cores (e), outside
the fenestrated capillary wall. (Alcian blue, X 24,500) C. Elastica
becomes thicker and more complete (arrow) further posteriorly
in pars plana. Note nerve bundles (nv) and ciliary muscle bundles (cm) in
stroma. (Toluidine blue, X 433) D. Elastica continues smoothly
from end of pars plana (left arrow) into peripheral choroid (right arrow). (Toluidine
blue, X 433)
|
Elastic fibers are composed of two major components, the 10- to 12-nm elastic
system microfibrilswith 12-nm microperiodicity, and the homogeneous
electron-lucent core of elastin. The elastic microfibril is tubular
in cross section, beaded on rotary shadowing, and belongs to the same
family as the zonular fibril.31,32 When aggregated together without elastin, these microfibrils are called
oxytalan fibers (Fig. 32A). Elastin molecules are laid down on this microfibrillar template during
elastogenesis (Fig. 32B), completely obscuring the microfibrillar substructure in mature elastic
fibers (Fig. 32C). Nonelasticized oxytalan fibers are ubiquitous throughout most connective
tissues and are plentiful throughout the ciliary body. They serve
as connecting or anchoring fibers between basement membranes of epithelia, vessels, muscle
bundles, and nerves, and link together different
portions of the elastic fiber system in the ciliary body33 (see Figs. 27, 29, 33) as in other tissues.34 Incompletely elasticized fibers with many remaining microfibrils (elaunin
fibers) are also seen (Fig. 32D).32 Besides the subepithelial elastica continuous with Bruch's membrane, mature
elastic fibers are not frequent in the ciliary body except
at the muscular insertions (see next section). 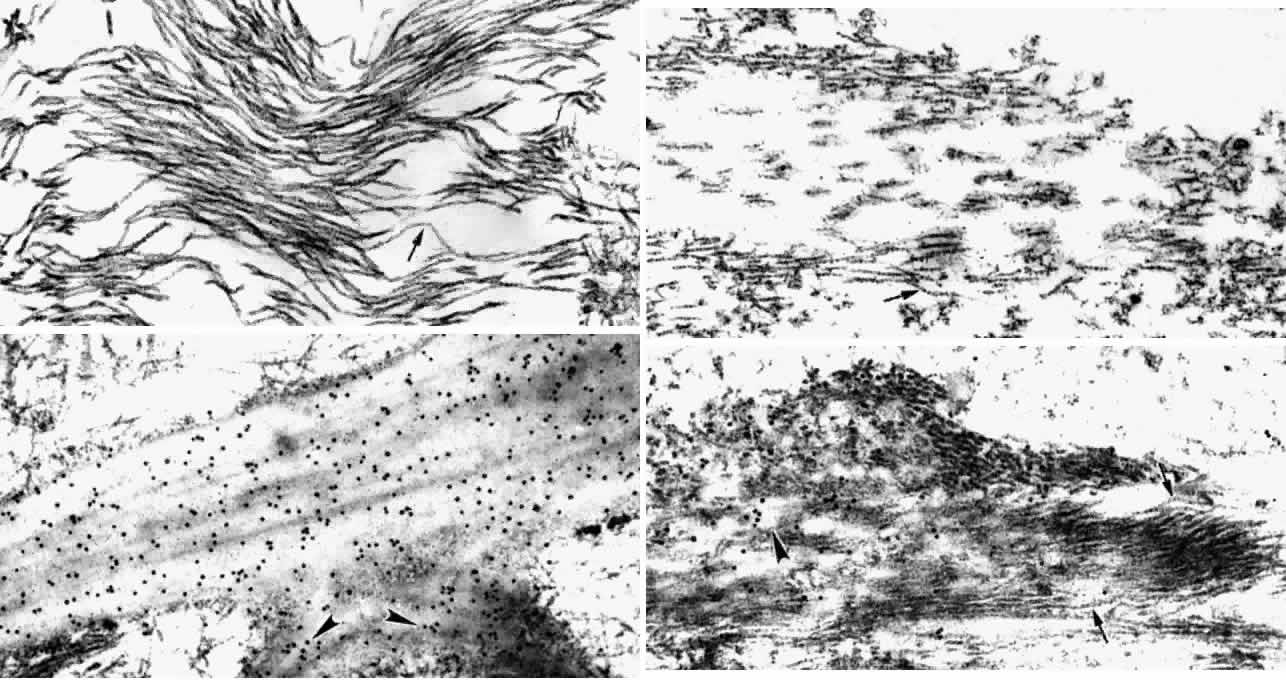 Fig. 32. Elastic fiber system. A. Bundles of elastic microfibrils (oxytalan) in
zonule, showing 12-nm microperiodicity (arrow). (X 51,900) B. Developing
elastic fiber with cords of amorphous electron-lucent elastin being
laid down on the microfibrillar template. Orbit of 14-month-old child. Note
same 12-nm microperiodicity (arrow). (X 51,700) C. Large mature
elastic fiber (Bruch's elastica, tangential cut) at site of two
branches (arrowheads). Scanty attachment microfibrils peripherally. Gold-labeled
with elastin antibody. (X 51,700) D. Predominantly microfibrillar (arrows) elastic
fibers (elaunin type) in trabeculum. Elastin-antibody
binding to central white elastin cords (arrowhead) in 2-month-old
infant. (X 51,700) Fig. 32. Elastic fiber system. A. Bundles of elastic microfibrils (oxytalan) in
zonule, showing 12-nm microperiodicity (arrow). (X 51,900) B. Developing
elastic fiber with cords of amorphous electron-lucent elastin being
laid down on the microfibrillar template. Orbit of 14-month-old child. Note
same 12-nm microperiodicity (arrow). (X 51,700) C. Large mature
elastic fiber (Bruch's elastica, tangential cut) at site of two
branches (arrowheads). Scanty attachment microfibrils peripherally. Gold-labeled
with elastin antibody. (X 51,700) D. Predominantly microfibrillar (arrows) elastic
fibers (elaunin type) in trabeculum. Elastin-antibody
binding to central white elastin cords (arrowhead) in 2-month-old
infant. (X 51,700)
|
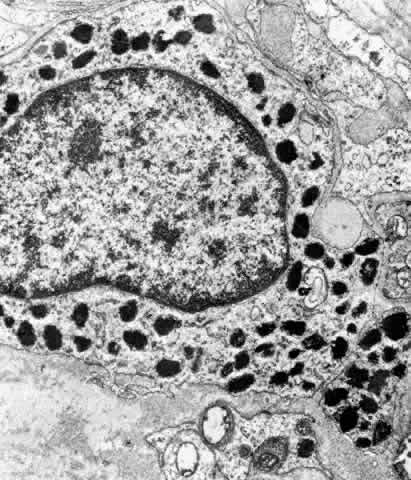 Fig. 33. Melanocyte with dense melanosomes in the ciliary stroma surrounded by collagen
fibers and many bundles of elastic system microfibrils (MF), (oxytalan). A
Schwann cell (SC) enveloping small nerve fibers is present
below. (X 26,299) Fig. 33. Melanocyte with dense melanosomes in the ciliary stroma surrounded by collagen
fibers and many bundles of elastic system microfibrils (MF), (oxytalan). A
Schwann cell (SC) enveloping small nerve fibers is present
below. (X 26,299)
|
The interstitial cells of the ciliary stroma are fibroblasts, melanocytes, and
scattered mast cells, along with a large number of myelinated
and unmyelinated nerves (see Fig. 33). The fibroblasts have scanty cytoplasm, many long, thin processes, and
elongated nuclei with finely clumped chromatin. Organelles are rather
scanty, with many free ribosomes and cilia. Segments of RER and Golgi
apparatus are the primary organelles. The multiprocessed melanocytes
are scanty except in melanosis oculi or in darkly pigmented individuals
where they can be diffuse, containing the small 0.3 to 0.8 μm uveal
type of melanosome (see Fig. 33). Between the stromal cells, collagen fibrils of medium size (45-nm to 60-nm
diameter, banded at 64 nm) are dispersed in a faint granular matrix. Types
I, III, and VI collagen have been reported in the ciliary stroma, besides
the type IV collagen in basement membranes.35,36 Type VI collagen fibrils are present mostly among the larger collagen
bundles of the stroma around the ciliary muscle, between the muscle bundles, and
as a component of the banded material associated with the anterior
insertions of the ciliary muscle. In the loose connective tissue
just behind the origin of the root of the iris lies the major arterial
circle of the iris (See Blood Supply section). Branches of this system
as yet unidentified are vulnerable to tearing in contusive injury
to the globe when the iris root is suddenly displaced posteriorly, resulting
in traumatic hyphema. CILIARY MUSCLE The ciliary muscle has a complex architecture, and its three dimensional
organization and function have been difficult to visualize. Traditionally, the
muscle is divided into three portions (Fig. 34): an outer longitudinal or meridional portion ( Brücke's muscle), a
middle oblique portion (also called reticular or radial), and
an inner circular component ( Müller's muscle). These regions
are so interconnected that they were recognized early as designed to
function like a single muscle mass when stimulated.37 Experimental evidence in humans, primates, and other mammals supports
the view that the contracting ciliary muscle undergoes a shortening with
anterior traction on the ora serrata region, and an inward and posterior
pull on the scleral spur and trabeculum.6,38–40 Contraction of the oblique and circular portions in particular contributes
a strong anterior and inward movement of the processes. The result
is a well coordinated anterior-inward squeezing effect, displacing the
processes toward the lens equator, and resulting in relaxation of zonular
pull on the lens capsule. This inward movement of the ciliary processes
has been dramatically shown by cinematography in primates after
iridectomy.41 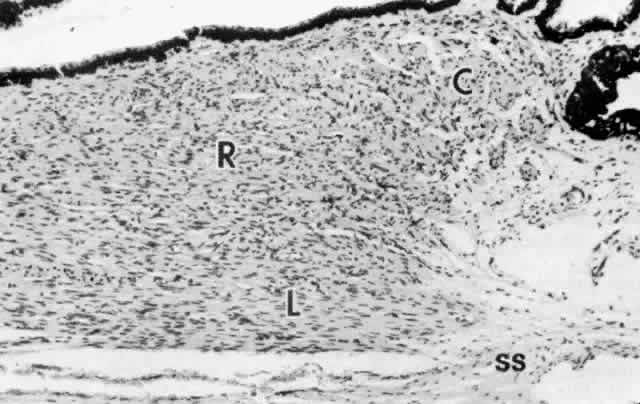 Fig. 34. Ciliary muscle showing circular (C), radial (R), and longitudinal (L) divisions
and their relation to the scleral spur (ss) at age 2. A rare
muscle bundle is present in the iris root. (hematoxylin-eosin, X 250) Fig. 34. Ciliary muscle showing circular (C), radial (R), and longitudinal (L) divisions
and their relation to the scleral spur (ss) at age 2. A rare
muscle bundle is present in the iris root. (hematoxylin-eosin, X 250)
|
Several investigators have used somewhat different schemata based on muscle
dissection to illustrate the ciliary muscle fiber topography that
allows such a complex yet coordinated muscle movement. The muscle fibers
are visualized as each arising by two heads in interdigitating V patterns (Fig. 35).37,42,43 The two heads are close together in the longitudinal muscle so the fibers
pass in an almost straight anteroposterior direction. For the oblique
muscle fibers, the angle between the two heads is wider and for the
circular muscle fibers is obtuse, allowing the latter to function in
an almost purely circular plane. 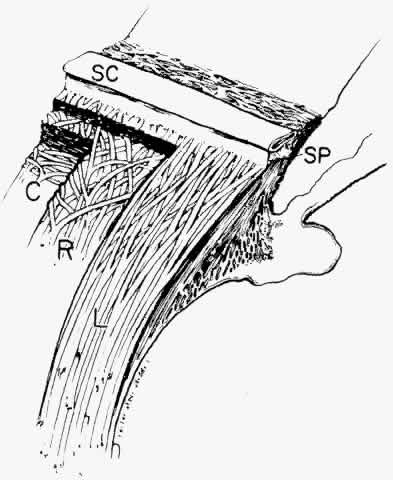 Fig. 35. Diagram of ciliary muscle divisions: circular (C), radial (R), and longitudinal (L). Anterior
attachments to the collagenous scleral spur (SP) and
the trabecular beams are indicated. SC, Schlemm's canal. (Modified from Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human
Eye. Philadelphia, WB Saunders, 1971; and Rohen JW: Der Ziliarkorper
als functionelles System. Morph Jahrbuch 92:415, 1952) Fig. 35. Diagram of ciliary muscle divisions: circular (C), radial (R), and longitudinal (L). Anterior
attachments to the collagenous scleral spur (SP) and
the trabecular beams are indicated. SC, Schlemm's canal. (Modified from Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human
Eye. Philadelphia, WB Saunders, 1971; and Rohen JW: Der Ziliarkorper
als functionelles System. Morph Jahrbuch 92:415, 1952)
|
The great bulk of the ciliary muscle lies in the anterior two thirds of
the ciliary body (see Fig. 5). At the light microscopic level in the child, the longitudinal muscle
shows a primary attachment to the scleral spur and to the outer corneoscleral
and uveal trabecular meshwork, while the oblique radial fibers
have more connection to the inner uveal meshwork (see Fig. 34). The circular muscle attachments are primarily to the adjacent ciliary
and iris root stroma. During aging the addition of significant fibrous
tissue and hyaline greatly increases the bulk of the radial and circular
muscles (Fig. 36) but not the longitudinal muscle. Tamm, Tamm, and Rohen44 found that connective tissue comprised about half of the oblique muscle
in the 50- to 60-year-old age group. The circular muscle was also significantly
increased in area and partially separated from the oblique
muscle by this connective tissue, which was continuous with hyaline in
the processes. The overall effect of aging on the ciliary muscle made
it shorter in length and greater in area with a prominence of the circular
muscle, resulting in a forward and inward configuration resembling
the accommodated state. Others have described some atrophy of the
muscle with decreased nuclei, particularly in the circular muscle and
over the age of 40 years.45 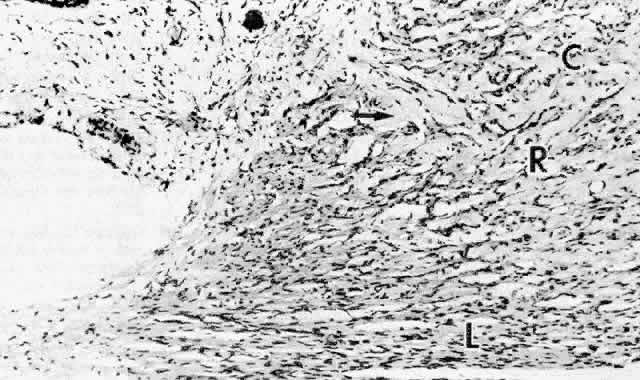 Fig. 36. Dense pale hyaline deposition (arrow) between the circular (C) and radial (R) but
not the longitudinal (L) muscle bundles of a 64-year-old patient. Compare
with Figure 34 from 2-year-old child. (hematoxylin-eosin, X 250) Fig. 36. Dense pale hyaline deposition (arrow) between the circular (C) and radial (R) but
not the longitudinal (L) muscle bundles of a 64-year-old patient. Compare
with Figure 34 from 2-year-old child. (hematoxylin-eosin, X 250)
|
In the young eye, connective tissue is scanty between the muscle bundles
and much like that of the stroma previously described, with similar
collagen types, fine granular ground substance, small numbers of unfenestrated
capillaries, and occasional melanocytes, but a great increase
in myelinated and unmyelinated nerve fibers. Elastic fibers are few in
most of the muscle, but many clumps of oxytalan microfibrils are associated
with muscle, nerve, and vascular basement membranes (see Fig. 33), where they appear to serve as anchoring structures. The ultrastructure of the ciliary muscle fibers resembles that of smooth
muscle elsewhere, with a few interesting differences. The muscle bundles
are surrounded by a sheath of flattened fibrocytes rather than primarily
by collagen fibers (Fig. 37),46–48 showing that they belong to the multiunit family of smooth muscles instead
of the syncytial family.49 Each fiber is covered by a continuous basement membrane and has many pinocytotic
vesicles or caveolae on the plasmalemmal membrane. The fiber
is filled with 60- to 70-nm myofibrils that show the usual attachment
densities among them, as well as focally where they attach to the basement
membranes (Fig. 38). These myofibrils are the intermediate filaments of the cell and contain
the protein desmin, used to identify smooth or skeletal muscle cells
by immunohistochemistry. A less specific protein, smooth muscle actin, is
also present but characterizes myofibroblasts as well. Mitochondria
and endoplasmic reticulum are more numerous and Golgi apparatus better
developed than in most smooth muscle cells. Occasional desmosomes
interconnect the cells but no gap junctions. Studies of muscle enzymes
have suggested that there may be functional differences between the
longitudinal muscle and the radial-circular muscle complex.50 The longitudinal muscle cells, particularly their anterior tips, are heavily
fibrillar with fewer mitochondria than the other muscles and have
enzyme characteristics somewhat like those of skeletal rapid twitch
fibers. It is hypothesized that their multiunit structure might allow
the muscle tips to react first in accommodation, stiffening them to counteract
the posterior pull of the remaining muscle on the scleral spur. 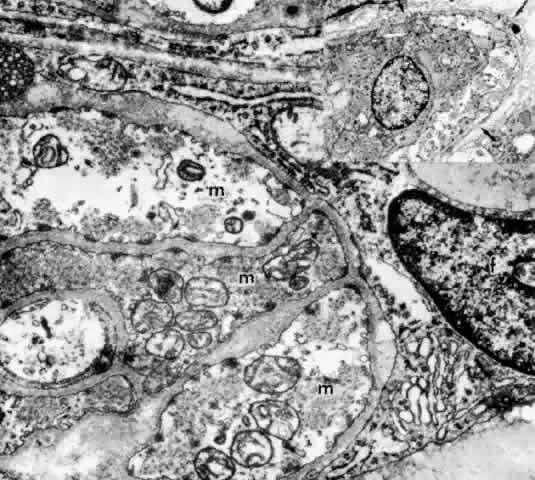 Fig. 37. Inset. Thin fingers of fibrocyte cytoplasm (arrows) surround a bundle of
ciliary muscle fibers. Main figure. Three muscle fibers (m) surrounded
by processes of a fibrocyte (f). Thick basement membrane around each
muscle fiber and several nerve terminals are visible. (X 33,800; Inset, X 8500) Fig. 37. Inset. Thin fingers of fibrocyte cytoplasm (arrows) surround a bundle of
ciliary muscle fibers. Main figure. Three muscle fibers (m) surrounded
by processes of a fibrocyte (f). Thick basement membrane around each
muscle fiber and several nerve terminals are visible. (X 33,800; Inset, X 8500)
|
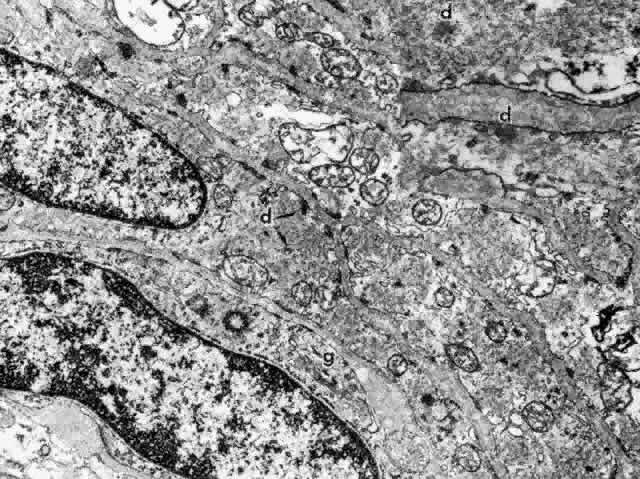 Fig. 38. Elongated radial muscle fibers surrounded by basement membranes have large
mitochondria, Golgi apparatus (g), and many central and peripheral
densities (d). (X 21,400) Inset. The plentiful smooth muscle myofibrils
in the cytoplasm attach to the densities in both central and peripheral
locations (d). (X 70,000) Fig. 38. Elongated radial muscle fibers surrounded by basement membranes have large
mitochondria, Golgi apparatus (g), and many central and peripheral
densities (d). (X 21,400) Inset. The plentiful smooth muscle myofibrils
in the cytoplasm attach to the densities in both central and peripheral
locations (d). (X 70,000)
|
The ciliary muscle is richly innervated with large numbers of cholinergic
nerve terminals. These show primarily the small agranular vesicles
characteristic of cholinergic neuromuscular junctions51 (Fig. 39), consistent with the virtually complete inhibition of ciliary muscle
contraction resulting from use of atropine. Most investigators have described
three types of neuromuscular junctions in the ciliary muscle.46–48,51–53 The most common synaptic junction has an indirect muscle cell contact, with
basement membrane intervening; direct contacts are less frequent (see Fig. 39). 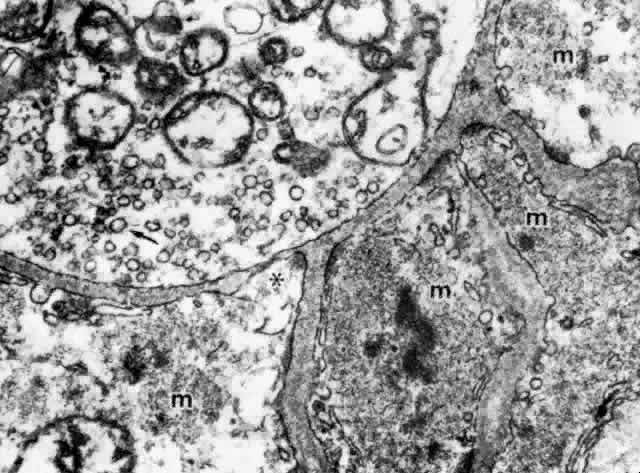 Fig. 39. Dense and lighter muscle fibers (m) around an unmyelinated nerve fiber
terminal that contains many mitochondria and small agranular synaptic
vesicles (arrow), indicating that this is aneuromuscular parasympathetic
junction. Indirect contacts with intervening basement membrane and
one (asterisk) site of direct cell-to-cell contact are seen. (X 53,000) Fig. 39. Dense and lighter muscle fibers (m) around an unmyelinated nerve fiber
terminal that contains many mitochondria and small agranular synaptic
vesicles (arrow), indicating that this is aneuromuscular parasympathetic
junction. Indirect contacts with intervening basement membrane and
one (asterisk) site of direct cell-to-cell contact are seen. (X 53,000)
|
Lipofuscin deposition in the muscle cells usually begins after the age
of 50,54 as well as an increase in lysosomal vacuoles, occasional lipid droplets, and
membranous whorls that may derive from degenerate endoplasmic reticulum
or myofibrils no longer anchored to their densities.54,55 THE ANTERIOR INSERTION OF THE CILIARY MUSCLE The possibility that the ciliary muscle fibers insert anteriorly in the
region of the scleral spur via “elastic tendons” was proposed
by Rohen several decades ago.37 He and his colleagues have made continual progress in understanding the
anterior muscle insertion and more recently the posterior insertion, adding
a new hypothesis about the cause of accommodative loss with aging. Three
types of anterior tendons are described.56 One attaches the tapering longitudinal muscle bundles to the anterior
sclera and scleral spur, and the second anchors in the trabecular meshwork. Both
consist of fibers described as elastic-like, showing extensive
connections to the elastic fibers of the scleral spur and the juxtacanalicular
elastic system (“cribriform plexus”56) as well as to the trabecular meshwork (Fig. 40). The fibers were called elastic-like because they do not resemble normal
elastic fibers and are not completely digested by elastase. Ultrastructurally
in the infant they contain a relatively small amount of elastin
in unfused cords with large numbers of elastic system microfibrils, like
an elaunin fiber (see Fig. 32D). However, the microfibrils become obscured by 50-nm granular banded “sheath” material by the second decade, and later an outer
layer of 100-nm banded material. This coating is reported to contain collagen
VI and chondroitin sulfate.57 The banded material increases markedly with age and in chronic open-angle
glaucoma. The origin of the third type of tendons is less clear, but
they are broad collagenous bands that cross the meshwork to insert
in the peripheral corneal stroma.  Fig. 40. Elastic fibers and insertion of anterior ciliary muscle. Tangential section
showing fine black elastic fibers (arrows) extending from the tapering
ends of the longitudinal ciliary muscle (CM), blending into the
circumferential elastica of the scleral spur (SS) and the corneoscleral
meshwork (CSM). (Verhoeff's stain X 500) Fig. 40. Elastic fibers and insertion of anterior ciliary muscle. Tangential section
showing fine black elastic fibers (arrows) extending from the tapering
ends of the longitudinal ciliary muscle (CM), blending into the
circumferential elastica of the scleral spur (SS) and the corneoscleral
meshwork (CSM). (Verhoeff's stain X 500)
|
During the posterior-inward movement of the scleral spur during ciliary
muscle contraction, the tendons cause fanning of the trabecular tissues, opening
the intertrabecular spaces and pores more widely.6,40 This mechanical action may facilitate filtration, by aiding wash-out of
trabecular debris. It is thought to be the basis for pilocarpine's
effect on outflow in glaucoma and is abolished in primates by disinserting
the ciliary muscle from the scleral spur.58 Ultrastructurally, the anterior ends of the ciliary muscle fibers taper
toward their attachment at the scleral spur and the trabecular meshwork, associated
with a plethora of elastic microfibrils and small banded
elastic fibers running parallel to them (Fig. 41). In areas of elastic fiber contact, there are dense focal bands on the
muscle cell membrane to which intracellular actin filaments are attached. This
kind of contact is similar to elastic tendons connecting the
arrector pili smooth muscle fibers to hair follicles, where the tendons
are composed of oxytalan and elaunin fibers.59 However, there has rarely been reference to the unique 50-nm banding on
these nonocular elastic fibers. Accompanying collagen fibers running
along the ciliary muscle in the same direction may be part of the tendon. With
aging, the tendons are enveloped by extensive fibrogranular
elastotic debris besides banded material (Fig. 42). 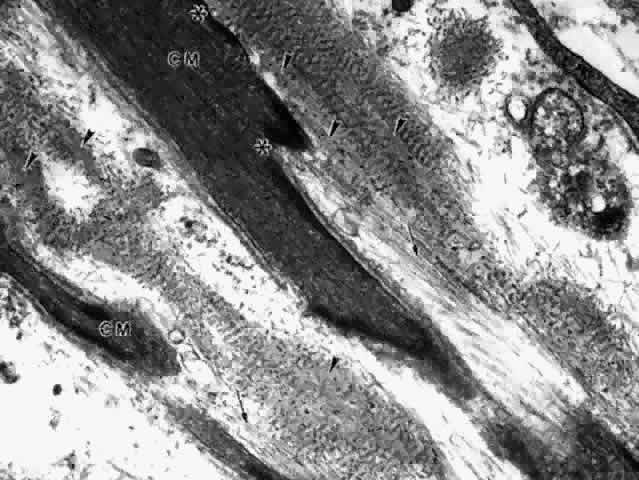 Fig. 41. Insertion of the anterior ciliary muscle (CM). Muscle fiber tips are densely
fibrillar and surrounded by basement membrane, except between areas
where elastic tendons attach (asterisks). The tendons are composed
of long bundles of elastic microfibrils (arrows) and banded elastic fibers
attaching near dense areas on the muscle cell wall. Cords of amorphous
elastin (arrowheads) in the banded fibers are identified by gold-labeled
elastin antibody. (X 37,200) Fig. 41. Insertion of the anterior ciliary muscle (CM). Muscle fiber tips are densely
fibrillar and surrounded by basement membrane, except between areas
where elastic tendons attach (asterisks). The tendons are composed
of long bundles of elastic microfibrils (arrows) and banded elastic fibers
attaching near dense areas on the muscle cell wall. Cords of amorphous
elastin (arrowheads) in the banded fibers are identified by gold-labeled
elastin antibody. (X 37,200)
|
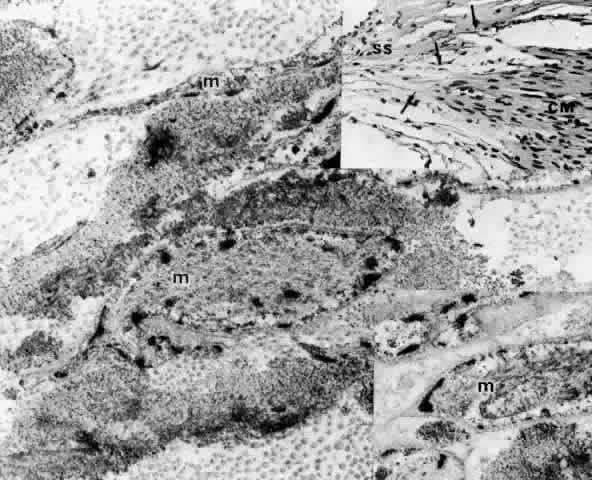 Fig. 42. Inset, top right. Tendinous attachments (arrows) of ciliary muscle fibers (CM) to
the scleral spur (SS) and trabecular beams in a 70-year-old. (hematoxylin-eosin, X 500) Main figure and bottom right inset. Tapering
ciliary muscle fibers (m) surrounded by granular elastotic debris
and collagen in the scleral spur region of same eye. (X 26,300) Fig. 42. Inset, top right. Tendinous attachments (arrows) of ciliary muscle fibers (CM) to
the scleral spur (SS) and trabecular beams in a 70-year-old. (hematoxylin-eosin, X 500) Main figure and bottom right inset. Tapering
ciliary muscle fibers (m) surrounded by granular elastotic debris
and collagen in the scleral spur region of same eye. (X 26,300)
|
The association of the anterior elastic tendons with the circumferential
elastic system in the scleral spur has acquired new importance with
the finding that the scleral spur cells associated with this system are
myofibroblasts.60 They express muscle-specific actin and vimentin but not desmin, and show
many ultrastructural differences from true muscle fibers, such as the
presence of gap junctions and incomplete basement membranes. Like other
myofibroblasts, they are thought to be contractile61 and appear to be innervated at least in part by adrenergic fibers. If
so, they could be responsive to epinephrine and have an effect on aqueous
outflow complementary to that of the ciliary muscle.60 THE POSTERIOR ATTACHMENTS OF THE CILIARY MUSCLE The posterior attachments of the ciliary muscle have been studied extensively
in young and old primates (rhesus monkeys). Their elastic tendons
have many associated elastic microfibrils as in the anterior tendons, but
their elastin content is greater, with broader areas of more mature
fibers (Fig. 43A).62 The tendons have connections to the elastica surrounding the pars plana
vessels (Fig. 43B), and both have connections with the elastica of Bruch's membrane. These
elastic structures are connected to each other as well as to the
basement membranes of the ciliary epithelium and vascular walls by
oxytalan fibers (elastic microfibrils), so that the whole complex can
function as a unit.33,62,63 What percent of the tendons have direct attachments to Bruch's elastica
is still somewhat uncertain, as it is difficult to follow the elastic
fibers in their three-dimensional course, but the elastic network
is extensive enough to support the concept of a coordinated action. 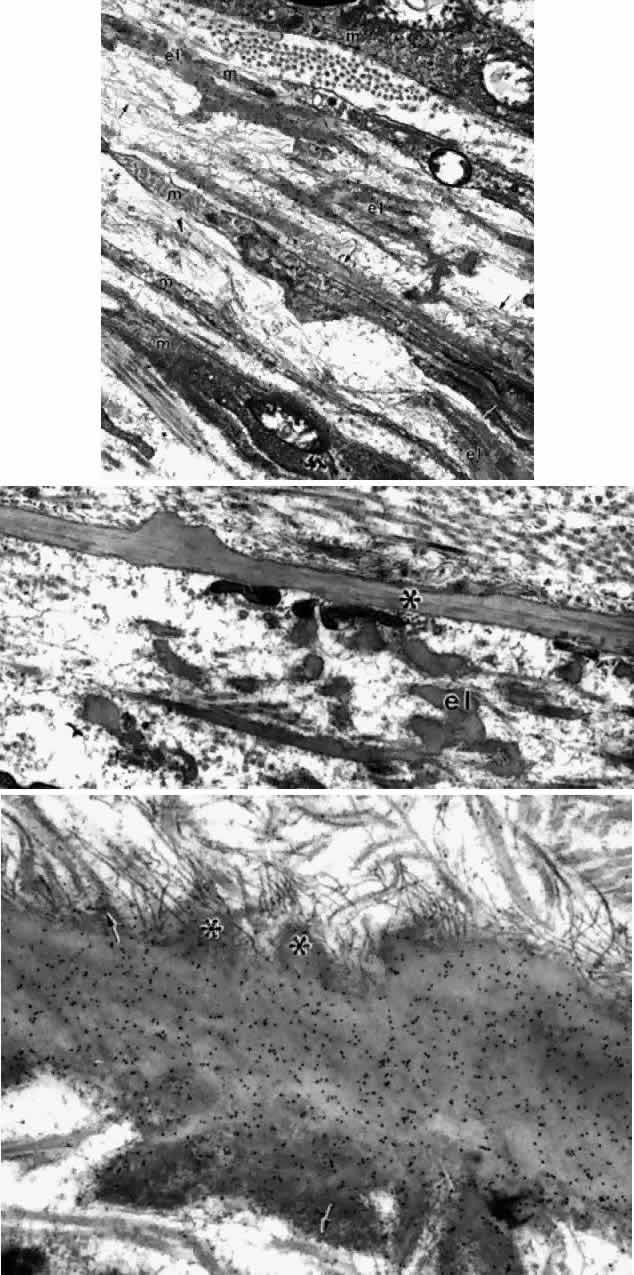 Fig. 43. Insertion of the posterior ciliary muscle (m) in the pars plana. A. Elastic
tendons are seen associated with the three central thin muscle tips. The
tendons are networks of elastic fibers (el), some aggregated oxytalan
fibers (arrows), infrequent small elaunin fibers (arrowhead), interconnected
by diffuse elastic microfibrils. Basement membranes are
interrupted where tendons attach to the cells. Stained with gold-labeled
elastin antibody. (X 30,700) B. Perivascular elastica (el) showing
microfibrillar interconnections adjacent to a knobby Bruch's membrane (asterisk). (X 19,600) C. Bruch's membrane cut parallel to
its surface, showing excrescences with attaching elastic microfibrils (asterisks) and
closely associated collagen fibrils (arrows). A-C, at
age 17. Fig. 43. Insertion of the posterior ciliary muscle (m) in the pars plana. A. Elastic
tendons are seen associated with the three central thin muscle tips. The
tendons are networks of elastic fibers (el), some aggregated oxytalan
fibers (arrows), infrequent small elaunin fibers (arrowhead), interconnected
by diffuse elastic microfibrils. Basement membranes are
interrupted where tendons attach to the cells. Stained with gold-labeled
elastin antibody. (X 30,700) B. Perivascular elastica (el) showing
microfibrillar interconnections adjacent to a knobby Bruch's membrane (asterisk). (X 19,600) C. Bruch's membrane cut parallel to
its surface, showing excrescences with attaching elastic microfibrils (asterisks) and
closely associated collagen fibrils (arrows). A-C, at
age 17.
|
The posterior tendons resemble large elastic tendons elsewhere,59 showing invagination of the terminal ends of the muscle fibers, and dense
plaques on the plasmalemma with interruption of the basement membrane
where the cells attach closely to elastic tissue. Aging changes in
these posterior tendons, however, are quite different from those in the
anterior tendons. By the second decade there are knobby excresences
of elastin and dense granular material on Bruch's elastica (see Fig. 43B), some at branching sites. Other protrusions suggest attachment sites
for oxytalan and elastic fibers or collagen (Fig. 43C), connecting Bruch's elastica to the pigment epithelial basement
membrane and to the stromal elastica. The whole insertion region in the
inner pars plana stroma increasingly becomes filled with large collagen
fibers and electron-dense amorphous and granular material of a hyaline
degenerate type, rather than of the banded and elastotic type seen
anteriorly. This fibrotic hyalinized tissue is hypothesized to be a
major cause of accommodative loss with age, as it would tend to “fix” the
muscle in place, preventing forward movement, and also
to reduce the amount of elastic tension available for a return to the
nonaccommodated state.62 SUPRACILIARIS No unusual histologic characteristics of the junction between the ciliary
body and sclera (lamina fusca, supraciliaris) have been described and
specifically no intercellular tight junctions that would impede the
passage of fluid between these two tissues. The accumulation of fluid
in the supraciliaris when the ciliary body is detached demonstrates the
lamellar arrangement of connective tissue in this area. An important
uveoscleral route for aqueous outflow passes posteriorly through the
loose stroma of the anterior ciliary face and between the fibers of the
longitudinal ciliary muscle, back along the supraciliaris, to enter
the ciliary and vortex vein systems.63,64 Passage along the scleral canals into the episcleral veins also occurs. The
percent of daily aqueous flow exiting via this extrascleral route
varied from 30% in young eyes when measured by fluorophotometry,64 to 11% in 60-year-old eyes as measured directly.65 |