HISTORY AND CLASSIC FINDINGS
MEWDS was first described by Jampol and associates1 and in the same year by Takeda and coinvestigators.2 The first cases involved acute unilateral visual loss and scotomas in 12 young women and 3 men. All had multiple white lesions at the level of the retinal pigment epithelium and approximately half had a previous flu-like syndrome. Macular granularity was seen commonly in these cases.
EPIDEMIOLOGY
Most affected persons tend to be young women in their 20s and thirties with an average age of 26 years although there have been reports of a female who was 14 years old and several in their sixties.2-4 Males are less often affected in a ratio of 3:1.
PATHOPHYSIOLOGY
The etiology of this form of white dot syndrome is unknown. There is a history of a precedent upper respiratory tract infection in 25% to 50% of cases.5 It could be that in certain predisposed individuals, exposure to a certain infectious organism can trigger an immune reaction to the choriocapillaris or the overlying retinal pigment epithelium. There is one case of murine typhus associated with MEWDS-like fundus findings.6 There is also a case with MEWDS-like findings that occurred after hepatitis B vaccination.7
INITIAL CLINICAL FINDINGS
Presenting Symptoms
The most common presenting symptoms are unilateral loss of vision and photopsias. These photopsias are described as flashing colored lights. Occasionally they may complain of a unilateral blind spot. Rarely do they have bilateral symptoms.
There may be a history of a preceding upper respiratory tract infection. Usually this is not volunteered by the patient because if it had been present, it was mild and so the patients do not consider it related to the present problem.
Signs
VISION.
The vision is usually only mildly diminished although the vision may range from 20/20 to 20/400. Color vision may be slightly or moderately diminished as well.
PUPILS AND FIELDS.
The visual fields are variable but most often they show an enlarged blind spot. There may be an afferent papillary defect as well.
BIOMICROSCOPY.
Iritis is rare but has occasionally been described. Vitreitis is more common although if it is present, it tends to be very mild.
FUNDUS FINDINGS.
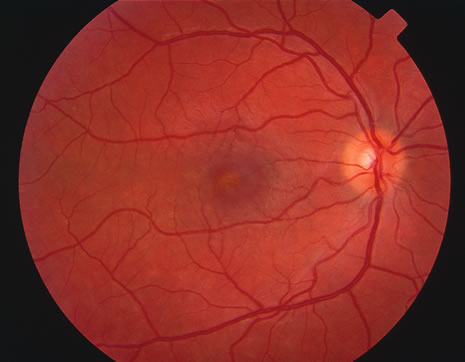
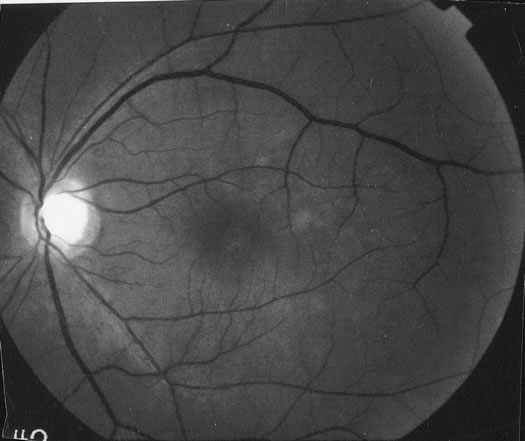
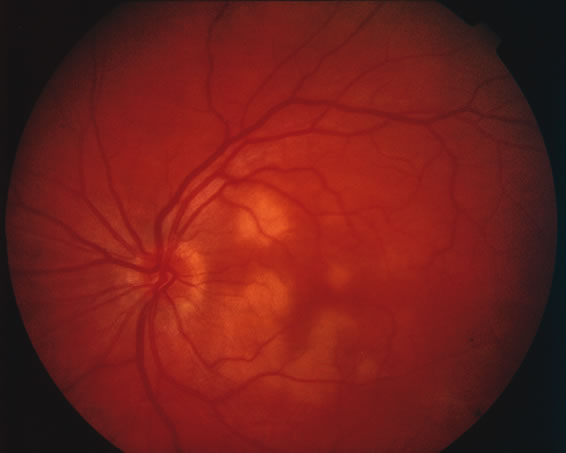
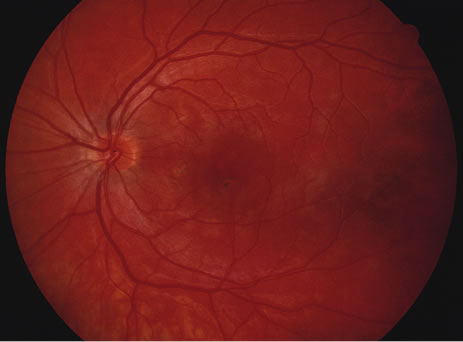
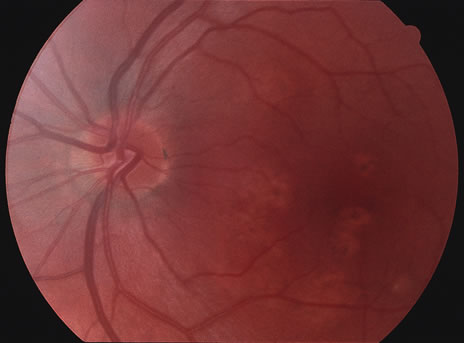
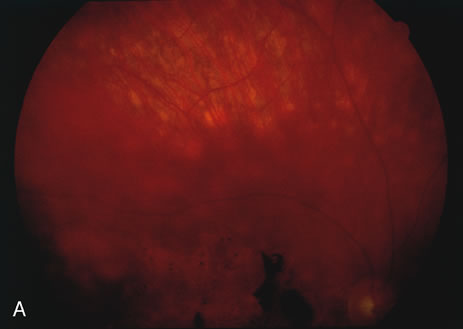
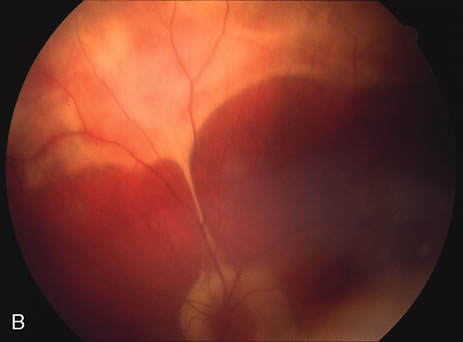
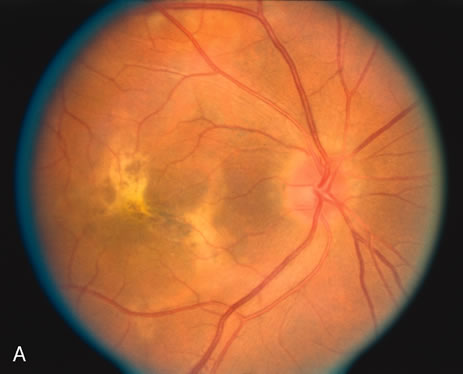
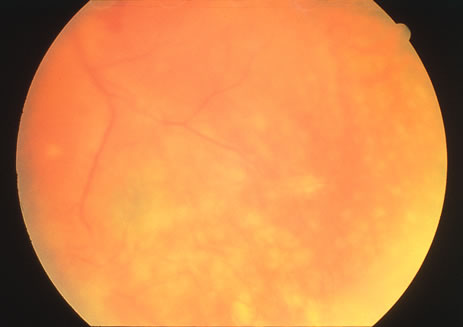
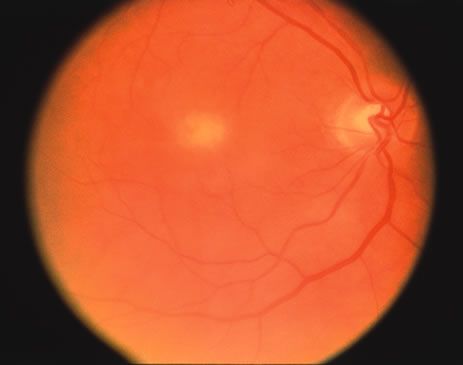
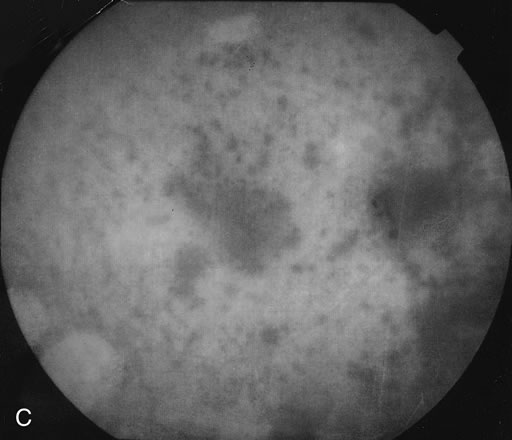
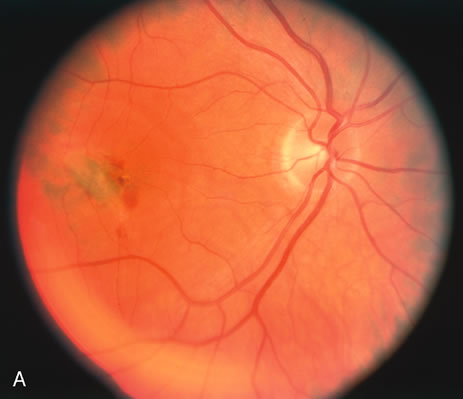
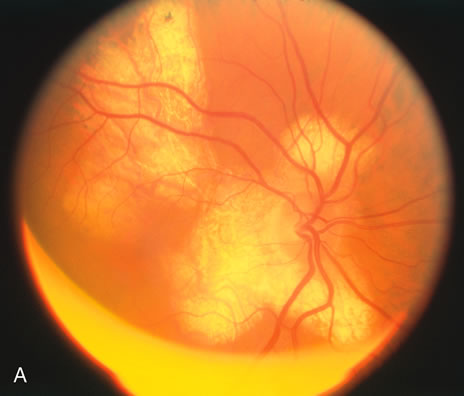
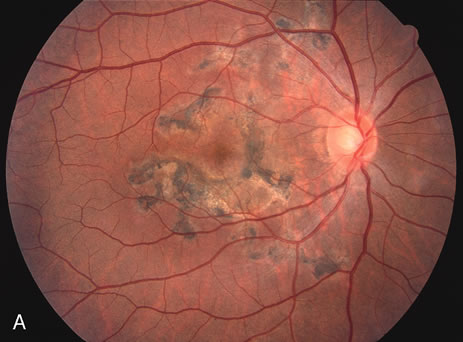
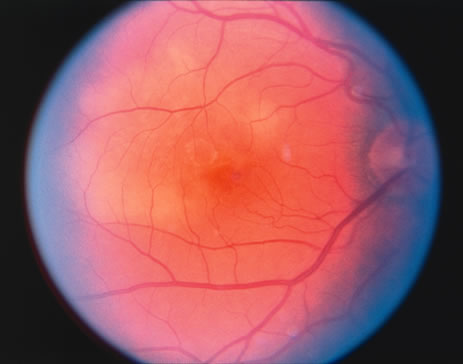
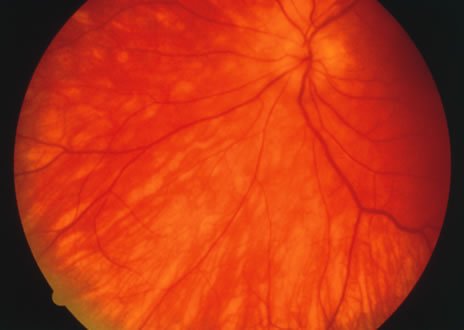
The classic fundus findings are small (500 μm), ill-defined, and sometimes difficult to see grayish-white dots that are dispersed throughout the posterior pole. These spots rarely may become confluent and if this occurs to spots that are around the disc, it may present as a case of giant blind spot syndrome or of an entity that probably is a variant of MEWDS called AZOOR.8 In addition to the dispersed small spots, there is a fine granularity of the fovea. This finding is pathognomonic for this disease, and it can exist in the absence of the white dots (Figs. 1 and 2).
|
|
Usually there is resolution of the fundus findings, although careful fundus evaluation sometimes shows some residual fine retinal pigment epithelial mottling. In rare cases, there may be long-standing pigment clumping with surrounding pigment atrophy similar to the lesions seen in multifocal choroiditis or punctate inner choroidopathy although that has been contended. The foveal granularity may take longer to resolve than the white spots.
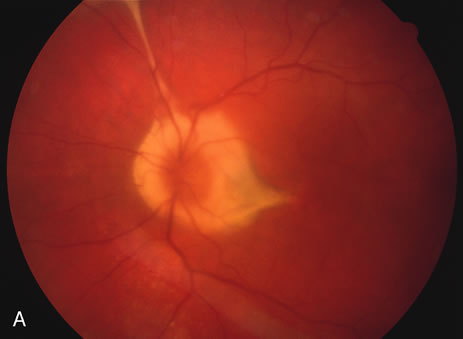
There may be mild optic disc swelling. This may lead someone unfamiliar with this disease to consider that the cause of visual loss is optic neuritis. There also may be mild retinal phlebitis that may be more easily seen by fluorescein angiography than by ophthalmoscopy. Choroidal neovascularization rarely develops.9
ANCILLARY TESTING
Fluorescein
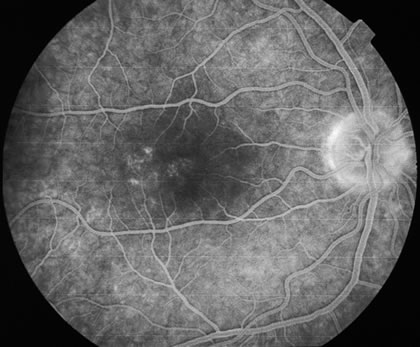
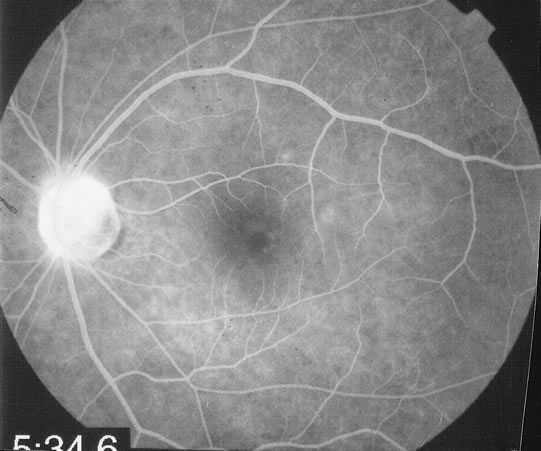
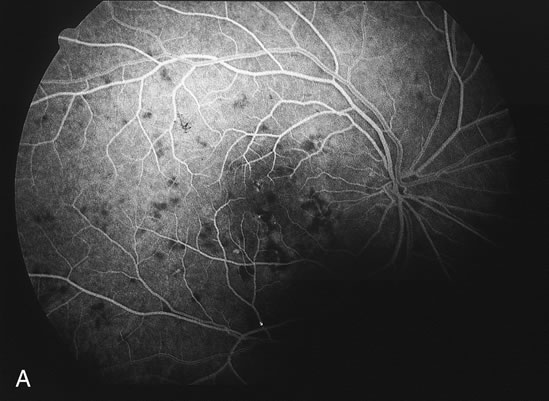
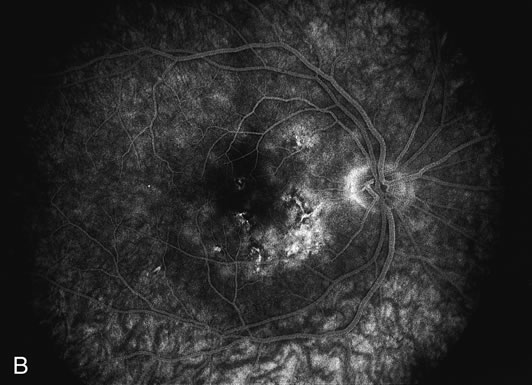
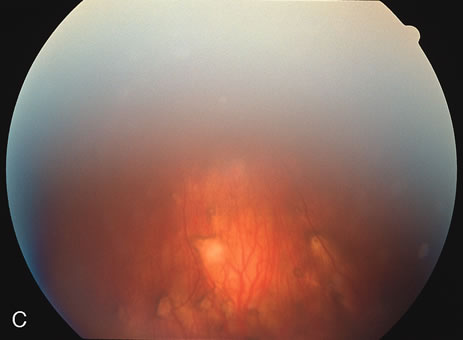
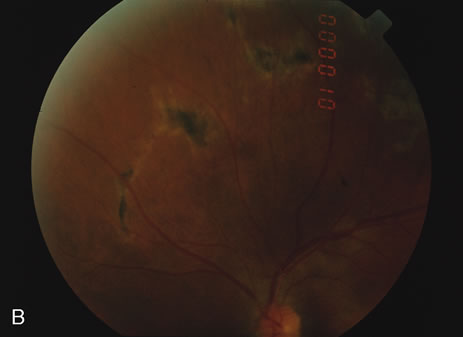
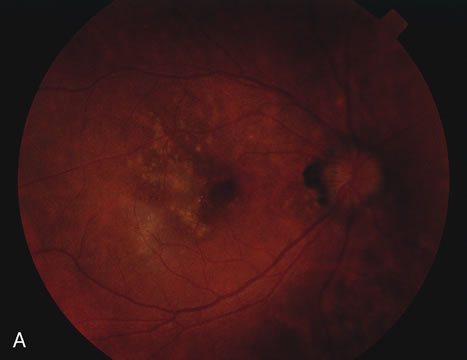
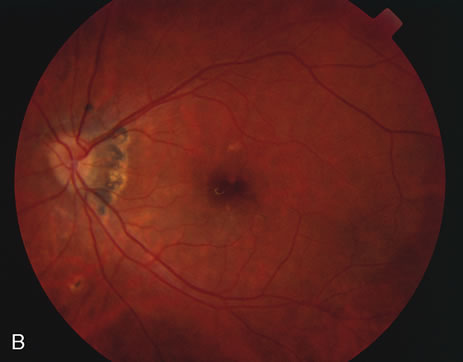
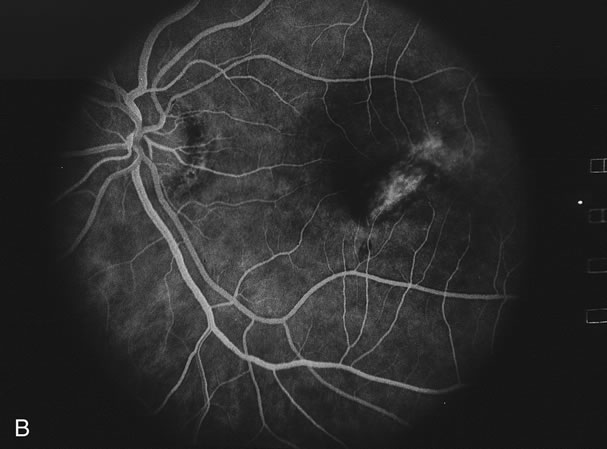
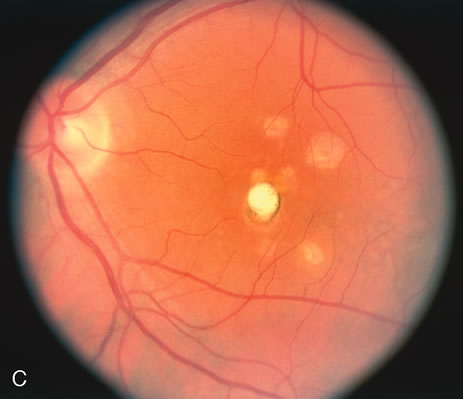
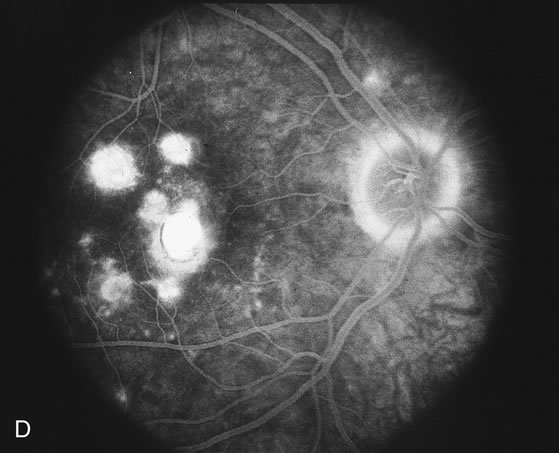
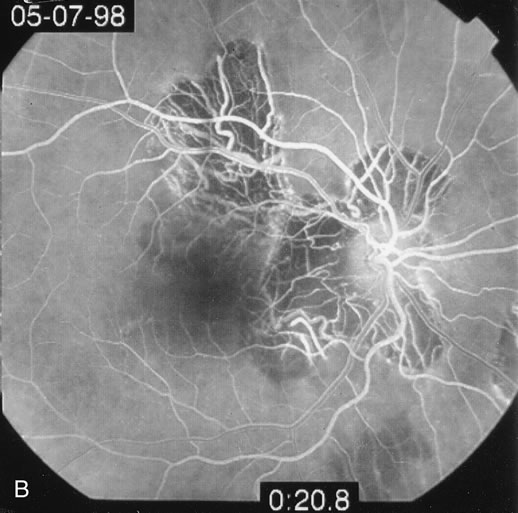
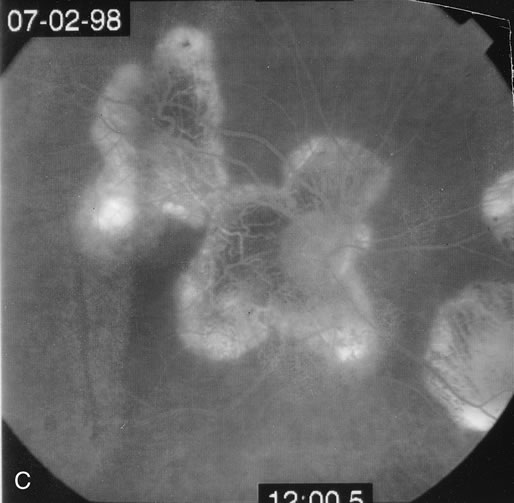
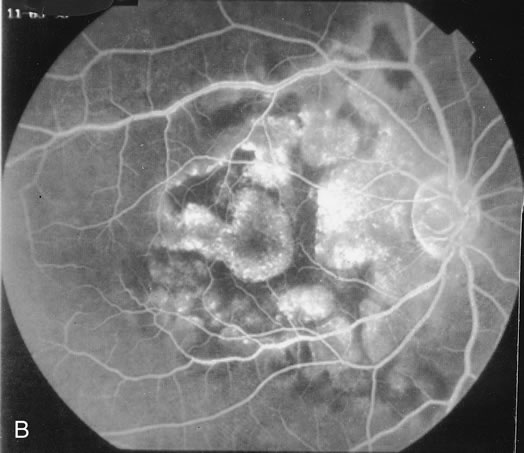
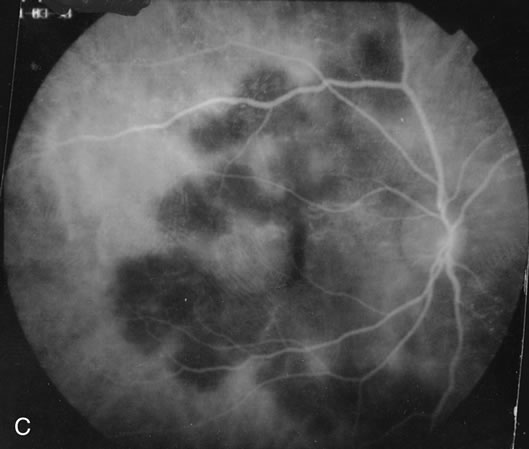
The fluorescein angiograms of the white dots show a fine halo appearance in a wreath-like swirl configuration to some of the white dots, and this halo diffuses in the later stages of the angiogram, first developing a stippled pattern of fluorescence and then becoming a diffuse white spot that leaks only mildly. There may be leakage of fluorescein from the optic nerve and occasionally, there may be late staining of retinal vessels because of a mild vasculitis (Figs. 3 and 4).
|
|
Indocyanine Green
In the early stages of the angiogram, the indocyanine green (ICG) angiogram may not show any distinct abnormalities. In the late phases, ICG angiography can show more lesions than can be seen by ophthalmoscopy or by fluorescein angiography. These lesions tend to block or show absence of fluorescence.10,11 In addition, there can be many hypofluorescent lesions around the disc. Sometimes the spots are so extensive that they become confluent. It is thought that it is a confluence of these small lesions that cause the blind spot enlargement seen on visual field testing. At the peripheral edge of the confluent spots however, it is still possible to see single spots that are characteristic with the stippled halo-like fluorescence.12 The hypofluorescent spots noted by ICG can last longer than the symptoms, or ophthalmoscopic or fluorescein angiographic findings.13
Electroretinography
Electroretinography (ERG) shows a diffuse loss of the early receptor potential and a decrease of the a wave, indicating that the disease appears to affect the retinal pigment epithelium-photoreceptor complex.14 Multifocal ERG shows reduced a and b waves in the areas that correspond to the visual field scotomas as well as showing areas of dysfunction where the visual field does not seem affected.15 In addition, the reduced amplitudes may be noted months after the visual fields and visual acuity have returned to normal. In patients with acute idiopathic blind spot enlargement (AIBSE) syndrome, a disease that may be related to MEWDS, the multifocal ERG is similarly affected in the peripapillary area.16 These patients may actually have had MEWDS in later stages of resolution because the white lesions may resolve much earlier than the scotomatous changes.17 Electrooculography may also show a diminution of the dark peak to light trough ratio.
Laboratory Tests/Immune Testing
There is a small study that showed a higher incidence of the HLA B51 haplotype in persons with MEWDS compared to the normal population. There is a relative risk of 5.86. This study needs to be confirmed in a larger cohort.18 It is interesting to note that this haplotype is also seen more frequently in patients with Behçet's disease, but in Behçet's disease the relative risk is almost twice as high as in MEWDS.
Testing for immunoglobulin staining in patients with different causes of outer retinopathy have not shown reactivity with the retina. This does not necessarily mean that the disease does not involve an immune response to the retina. It means that the immune reaction does not involve autoantibodies. Cell-mediated immunity may still be present.19
Differential Diagnosis/Mimics
The differential diagnosis tends to involve the other causes of the white dot syndromes. Most commonly the diagnoses that are most often confused with MEWDS are APMPPE, birdshot retinochoroidopathy, multifocal choroiditis, and retinal pigment epitheliopathy. In addition, acute macular neuroretinopathy and diffuse unilateral subacute neuroretinitis are sometimes considered in the differential diagnosis. None of the other white dot syndrome diseases have the peculiar foveal granularity that is seen with MEWDS.
APMPPE is usually a bilateral disease that affects both young men and women. The creamy lesions tend to be larger than those seen in MEWDS, and fluorescein angiography reveals classic early blocked fluorescence and late staining of the lesions.
Birdshot chorioretinopathy usually affects older women between 40 and 60 years of age. It causes deep creamy lesions the borders of which are sometimes difficult to discern. In the late phases of fluorescein angiography some of the lesions show hyperfluorescence while others do not show up on the angiograms. The optic nerve tends to be swollen and shows leakage by fluorescein angiography. Cystoid macular edema may be present.
Many times the lesions in multifocal choroiditis show lesions in different stages of scarring. Choroidal neovascularization, bilaterality, and recurrences are common.
Acute retinal pigment epitheliopathy shows a few small spots that are noted to have pigment clumping in their center with surrounding hypopigmentation. Fluorescein angiography shows window defect with a central spot of blocked fluorescence. The electroretinogram is unaffected.
Acute macular neuroretinopathy tends to be unilateral. There is a mild diminution of vision associated with a reddish discoloration centered under the fovea. This pattern is most easily seen with red free photography.
Diffuse unilateral subacute neuroretintis is caused by a nematode. It can be associated with creamy-white small lesions in the macula. This is a progressive disorder unless the nematode is identified in the subretinal space and treatment is given.
There are three cases of two men and one woman all in their 50s who had whitish spots that were consistent with those seen in MEWDS. The age was atypical and there was progressive vision loss in these cases. These patients had primary intraocular large-cell (non-Hodgkin's) lymphoma.20 In no case were there the pathognomonic foveal changes seen in MEWDS.
Natural History
The symptoms usually resolve within 6 to 15 weeks although some may have the blind spot enlargement and photopsias for up to 1 year after the initial presentation.5,21 Recurrence is rarely noted and choroidal neovascularization is also a rare occurrence. Punched-out lesions, similar to those seen in multifocal choroiditis, can rarely be seen in a few cases.
Treatment and History with Treatment
Treatment is not indicated because the disease spontaneously improves. Laser photocoagulation or possibly photodynamic therapy may be of help in cases that develop choroidal neovascularization.