COLLAGENS
As is true for connective tissues everywhere, collagen is an important structural protein in vitreous. Gross1 initially claimed that vitreous collagen fibrils are morphologically distinct from collagen in other connective tissues. However, Swann and coworkers2 demonstrated that the amino acid composition of the insoluble residue of vitreous is similar to that of cartilage collagen and later identified that it is most similar to cartilage collagen composed of alpha 1, type II chains.3 Comparisons of the arthrogenic and immunologic properties of collagens from bovine articular cartilage (type II) and vitreous showed that the two were indistinguishable by these assays.4 Subsequent studies5 demonstrated that, although vitreous collagen contains an alpha 1, type II chain similar to cartilage collagen, there is a lower alanine content. Furthermore, these studies found that vitreous collagen has additional peptides present as uncleaved extension chains containing an amino acid composition different from the alpha chain component. The investigators concluded, however, that the overall similarities in amino acid composition and in the types of cyanogen bromide cleavage peptides indicate that the fibers of the central and posterior peripheral regions of the vitreous are composed of a collagen that should be classified as type II. Linsenmayer and collaborators6 measured in vivo synthesis of types I and II collagen in chick embryo vitreous by radioimmunoprecipitation after tritiated proline labeling and found that more than 90% of the labeled material in the vitreous was type II collagen. Snowden7 provided further physicochemical evidence in support of the similarities between vitreous and cartilage collagens. This may explain why certain clinical phenomena, such as inborn errors of type II collagen metabolism in arthro-ophthalmopathies, manifest phenotypic expression in each of these two tissues.
There are, however, distinct differences in the chemical composition of vitreous and cartilage collagens that are only partly due to the presence of terminal peptide constituents in vitreous collagen.5 Swann and Sotman8 have demonstrated that the carbohydrate content of pepsin-solubilized vitreous alpha chains is significantly greater than cartilage alpha chains, indicating that the carbohydrate side chains of vitreous collagen are largely composed of disaccharide units similar to those found in basement membrane collagen. They proposed that these distinct chemical features are related to the special structure of the mature vitreous fibrils in vivo. Liang and Chakrabarti9 have shown that there are differences between bovine cartilage and vitreous with respect to collagen fibril growth, melting temperature, and fluorescence with a hydrophobic fluorescent probe. These investigators and others10 proposed that vitreous collagen should be considered a “special” type II collagen. Schmut and associates11 employed differential salt precipitation of pepsin-solubilized collagen from bovine vitreous and found that type II collagen is the major component of native vitreous fibers.
Gloor12 pointed out that the collagen content is highest where the vitreous is a gel. Ayad and Weiss13 studied bovine vitreous collagen to determine whether the gel-like structure of vitreous could be explained on the basis of chemical composition. Their findings demonstrated that type II is the major vitreous collagen, but collagens composed of α1, α2, and α3 chains as well as C-PS disulfide-bonded collagen were present in concentrations similar to those in cartilage. In contrast to cartilage, however, vitreous type II collagen was significantly more hydroxylated in the lysine and proline residues. The α1, α2, and α3 collagen chains were interpreted by Van der Rest14 to represent type IX collagen, although Eyre and colleagues15 felt that there was evidence to indicate the presence of type V collagen in vitreous. Furthermore, with respect to the disulfide-bonded collagen, vitreous had three times more C-PS1 and C-PS2 collagens than cartilage, although the molar ratio of C-PS 1 to C-PS2 in both was 1:1, suggesting that in both tissues these collagens are components of a larger molecule. Other studies14 demonstrated that these disulfide-linked triple-helix fragments were actually derivatives of type IX collagen. In this regard vitreous is again similar to cartilage, insofar as both contain type IX collagen,15 although the two tissues differ in the sizes of type IX collagen chains.16 Hong and Davison17 have identified a procollagen in the soluble fraction of rabbit vitreous that was identified as type II by segment-long-spacing banding patterns. Detection of a propeptide extension only at the N-terminus prompted these investigators to conclude that this was a novel type II procollagen. Such distinctive characteristics are possibly related to the unique physiologic roles of vitreous, in particular, its mechanical function.18
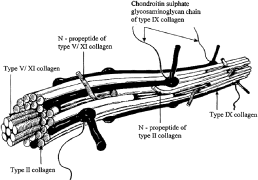
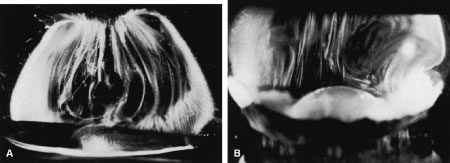
Individual vitreous collagen fibrils are organized as a triple helix of three alpha chains. The major collagen fibrils of the corpus vitreus are heterotypic, consisting of more than one collagen type. Recent studies of pepsinized forms of collagen confirm that the corpus vitreus contains collagen type II, a hybrid of types V/XI, and type IX (Fig. 1).
Type II Collagen
Type II collagen, a homotrimer composed of three identical alpha chains designated as [α1 (II)]3, comprises 75% of the total collagen content in vitreous. When first synthesized as a procollagen and secreted into the extracellular space, type II collagen is highly soluble. The activity of N-proteinase and C-proteinase enzymes reduces the solubility and enables type II collagen molecules to cross-link covalently in a quarter-staggered array. Within this array are likely to be N-propeptides, which probably extend outward from the surface of the forming fibril.11 This may influence the interaction of the collagen fibril with other components of the extracellular matrix. Recent studies19 combined immunolocalization with Western blot analysis of macromolecules extracted from bovine vitreous collagen fibrils and found that the pN-type IIA procollagen is located on the surface of the vitreous collagen fibril. The finding20 that type IIA procollagen propeptides specifically bind transforming growth factor-beta 1 (TGFβ1) and BMP-2 supports the concept that, in certain circumstances, these growth factors and cytokines interact with vitreous fibrils to promote the cell migration and proliferation that result in proliferative diabetic retinopathy and proliferative vitreoretinopathy.
Type IX Collagen
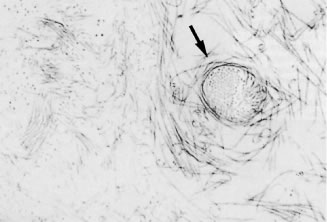
Type IX collagen is a heterotrimer that is disulfide-bonded with an [α1 (IX) α2 (IX) α3 (IX)] configuration. This heterotrimer is orientated regularly along the surfaces of the major collagen fibrils in a “D periodic” distribution, in which it is cross-linked onto the fibril surface. Type IX is not a typical collagen but is a member of the fibrillar-associated collagens with interrupted triple helixes (FACIT) group of collagens. It contains collagenous regions described as COL1, COL2, and COL3 interspersed between noncollagenous regions called NC1, NC2, NC3, and NC4.21,22 In vitreous (as opposed to cartilage) the NC4 domain is small and, therefore, not highly charged and not likely to exhibit extensive interaction with other extracellular matrix components.23 In vitreous, type IX collagen always contains a chondroitin sulfate glycosaminoglycan chain,21,22 which is linked covalently to the α2 (IX) chain at the NC3 domain; this enables the molecule to assume a proteoglycan form. Electron microscopy of vitreous stained with cationic dyes enables visualization of the chondroitin sulfate chains of type IX collagen. In some of these studies sulfated glycosaminoglycans are found distributed regularly along the surface of vitreous collagen fibrils24 and often bridge between neighboring collagen fibrils. Duplexing of glycosaminoglycan chains from adjacent collagen fibrils may result in a ladder-like configuration.25
Type V/XI Collagen
Ten percent of vitreous collagen is a hybrid V/XI collagen, which is believed to comprise the central core of the major collagen fibrils of vitreous.26 Type V/XI is a heterotrimer that contains α1 (XI) and α2 (V) in two chains, but the nature of the third chain is presently not known.15 Along with type II collagen, type V/XI is a fibril-forming collagen. Although the interaction of the fibril with other extracellular matrix components is probably influenced by a retained N-propeptide that protrudes from the surface of the fibril in cartilage,26 it is not known whether this is the case in vitreous.27
Type VI Collagen
Although there are only small amounts of type VI collagen in vitreous, the ability of this molecule to bind both type II collagen and hyaluronan (HA) suggests that it could be important in organizing and maintaining the supramolecular structure of vitreous gel.
GLYCOSAMINOGLYCANS
Glycosaminoglycans are polysaccharides of repeating disaccharide units, each consisting of hexosamine (usually N-acetyl glucosamine or N-acetyl galactosamine) glycosidically linked to either uronic (glucuronic or iduronic) acid or galactose. The nature of the predominant repeating unit is characteristic for each glycosaminoglycan and the relative amount, molecular size, and type of glycosaminoglycan are said to be tissue specific.28 Glycosaminoglycans do not normally occur as free polymers in vivo but are covalently linked to a protein core; the ensemble is called a proteoglycan. A sulfated group is attached to oxygen or nitrogen in all glycosaminoglycans except HA. Balazs29 first documented the presence of sulfated galactosamine-containing glycosaminoglycans in bovine vitreous (less than 5% of total vitreous glycosaminoglycans), and others30,31 identified these as chondroitin-4-sulfate and undersulfated heparan sulfate. Studies in the rabbit32 found a total vitreous glycosaminoglycans content of 58 ng with 13% chondroitin sulfate and 0.5% heparan sulfate.
Hyaluronan
Although HA is present throughout the body, it was first isolated from bovine vitreous. In humans, HA first appears after birth and then becomes the major vitreous glycosaminoglycan. Although it has been proposed that hyalocytes synthesize HA,18 other plausible candidates are the ciliary body and retinal Müller cells. Whereas the synthesis of HA seems to continue at a constant rate in the adult while no extracellular degradation occurs, HA levels are in a steady-state because the molecule escapes via the anterior segment of the eye.29 Laurent and Fraser33 showed that the passage of HA from the vitreous to the anterior segment is strongly molecular-weight dependent, indicating a diffusion-controlled process. In contrast, disappearance of HA from the anterior chamber is independent of molecular weight, suggesting that this is controlled by bulk flow.
HA is a long, unbranched polymer of repeating glucuronic acid β-1,3-N,N-acetylglucosamine disaccharide moieties linked by β 1–4 bonds.5 It is a linear, left-handed, three-fold helix with a rise per disaccharide on the helix axis of 0.98 nm.34 Rotary shadowing electron microscopy of human and bovine vitreous detected lateral aggregates of HA that form an anastomosing three-dimensional network.35 This periodicity, however, can vary depending on whether the helix is in a compressed or extended configuration.36 Changes in the degree of extension of HA could be important in the role vitreous plays in retinal disease. Indeed, the volume of the unhydrated HA molecule is about 0.66cm3/g, whereas the hydrated specific volume is 2000 to 3000 cm3/g.29 Thus, the degree of hydration has a significant influence on the size and configuration of the HA molecular network. Although there is no definitive evidence that adjacent HA chains bind to one another, Brewerton and Mayne37 first proposed such an arrangement. Recent rotary shadowing electron microscopy studies38 of bovine and human vitreous found lateral aggregates of HA that formed three-dimensional lattice-like networks. HA also interacts with the surrounding mobile ions and can undergo changes in its conformation that are induced by changes in the surrounding ionic milieu.39 A decrease in surrounding ionic strength can cause the anionic charges on the polysaccharide backbone to repel one another, resulting in an extended configuration of the macromolecule. An increase can cause contraction of the molecule and, in turn, the entire vitreous body. As a result of HA's entanglement and immobilization within the vitreous collagen fibril matrix, this mechanical force can be transmitted by collagen fibrils to the retina, optic disc, and other structures such as neovascular complexes. In this way, changes in the ionic milieu of vitreous may be converted into mechanical energy via extension or contraction of the HA macromolecule. This can be important in certain pathologic conditions that feature fluctuations in ionic balance and hydration, such as diabetes.40
The sodium salt of HA has a molecular weight of 3 to 4.5 × 106 Da in normal human vitreous.29 Laurent and Granath41 used gel chromatography and found the average molecular weight of rabbit vitreous to be 2 to 3 × 106 Da and of bovine vitreous to be 0.5 to 0.8 × 106 Da. In these studies there were age-related differences in the bovine vitreous, in which HA molecular weight varied from 3 × 106 Da in the newborn calf to 0.5 × 106 Da in old cattle. Furthermore, there may be several species of HA within vitreous that have polysaccharide chains of different lengths42 with a variable distribution in different topographic regions within the corpus vitreous.43
An important property of HA is steric exclusion.44 With its flexible linear chains and random coil conformation, HA occupies a large volume and resists the penetration of this volume by other molecules to a degree dependent on their size and shape.36 This excluded volume effect can influence equilibria between different conformational states of macromolecules and alter the compactness or extension of these molecules.44 Steric exclusion also causes an excess of osmotic pressure when such compounds as albumin and HA are mixed, because the resultant osmotic pressure is greater than the sum of the two components. This could be important in diabetes, in which vascular incompetence can increase vitreous concentrations of serum proteins such as albumin. These osmotic effects can induce contraction and expansion of the corpus vitreus similar to the foregoing description of hydration and ion-induced changes within vitreous and can similarly play an important role in neovascularization and diabetic vitreous hemorrhage.40 An increase in the chemical activity of a compound because of steric exclusion can also cause its precipitation if the solubility limit is reached. This could be important in the formation of pathologic vitreous opacities, such as asteroid hyalosis and amyloid bodies.18
Chondroitin Sulfate
Vitreous contains two chondroitin sulfate proteoglycans. The minor type is actually type IX collagen, which was described above. Most vitreous chondroitin sulfate is in the form of versican.38 This large proteoglycan has a globular N-terminal that binds HA via a 45 kDa link protein.38 Thus, in human, but not bovine vitreous, versican is believed to form complexes with HA, as well as with microfibrillar proteins such as fibulin-1 and fibullin-2.45
Heparin Sulfate
This sulfated proteoglycan is normally found in basement membranes and on cell surfaces throughout the body. It was first detected in bovine vitreous in 197746 and in chick vitreous (as agrin) in 1995.47 However, it is not clear whether heparan sulfate is a true component of vitreous or a contaminant from adjacent basement membranes, such as the internal limiting lamina (ILL) of the retina.48 As pointed out by Bishop45 this may also be the case for nodogen-1, the aforementioned fibulins, and fibronectin.
NONCOLLAGENOUS STRUCTURAL PROTEINS
Fibrillins
Fibrillin-containing microfibrils are more abundant in vitreous than the type VI collagen microfibrils described above. They are the found in vitreous gel, as well as in the zonules of the lens. This fact explains why in Marfan's syndrome the defects in the gene encoding fibrillin-1 (FBN1 on chromosome 15q21) result in both ectopia lentis and vitreous liquefaction.45 The latter probably plays a role in the frequent occurrence of rhegmatogenous retinal detachment in these patients.
Opticin
The major noncollagenous protein of vitreous is a leucine-rich repeat (LRR) protein, which is bound to the surface of the heterotypic collagen fibrils, known as opticin.49 Formerly called vitrican, opticin is believed to be important in collagen fibril assembly and in preventing the aggregation of adjacent collagen fibrils into bundles. Thus, a breakdown in this property or activity may play a role in age-related vitreous degeneration50 (see later discussion below).
VIT 1
Another novel vitreous protein is VIT1, a collagen-binding macromolecule.51 Because of its propensity to bind collagen, this highly basic protein may play an important role in maintaining vitreous gel structure.
MISCELLANEOUS MOLECULAR COMPONENTS
Amino Acids and Proteins
Free amino acids are present in the vitreous, but at one fifth of the levels found in plasma.52 An amino acid concentration gradient is present within the vitreous body, with anterior vitreous concentrations greater than posterior levels. This may be due to uptake and utilization of amino acids by the retina, a consideration that led Reddy53 to propose that the vitreous acts as a metabolic repository for retinal protein metabolism. Other studies54 found that the soluble proteins of vitreous resemble serum proteins with isoelectric points less than 6.0, leading investigators to conclude that the soluble proteins of vitreous derive from plasma and are constantly renewed. Flood and Balazs55 studied 920 human eyes and found the following age-related protein concentrations: ages 10 to 50, 400 to 600 μg/ml; ages 50 to 80, 700 to 800 μg/ml; older than 80 years of age, about 1000 μ/ml. These age-related findings may be due to increased leakage of plasma proteins from the intravascular compartment into the vitreous, resulting from decreased tight-junction integrity with aging of the retinal and ciliary body vasculature and epithelia.
Glycoproteins
Glycoproteins are heteropolysaccharide macromolecules, as opposed to the homogeneous, repeating disaccharide units of glycosaminoglycans that are mostly proteinaceous and contain only a minor carbohydrate component (5% to 10% by weight). According to Balazs29 the most important difference between vitreous and serum proteins is the high content of glycoproteins in the vitreous, because these constitute 20% of the total noncollagenous protein content of vitreous. Sialic acid-containing glycoproteins are believed to be synthesized by hyalocytes.56 Other studies57 have led to the postulate that the inner layer of the ciliary epithelium is responsible for vitreous glycoprotein synthesis. Cartilage oligomeric protein (COMP), an acidic glycoprotein with a characteristic five-armed structure, is present in vitreous, but its function is currently unknown.
Ascorbic Acid
Ascorbic acid concentrations in vitreous are about 0.43 mmol/kg.58 Thus, the vitreous-plasma ratio for ascorbic acid is 9:1. Vitreous levels that are much higher than plasma concentrations are believed to be due to active transport by the ciliary body epithelium.59 The purpose of having high concentrations of ascorbic acid in the vitreous may relate to the abilities of this compound to absorb ultraviolet light and to serve as a free-radical scavenger,60 which may protect not only the retina and lens but also vitreous from the untoward effects of metabolic and light-induced61 singlet oxygen generation.
Lipids
Swann and coinvestigators62 found that the residual fraction of vitreous contained a significant quantity of lipid. Later studies63 found evidence of active lipid metabolism in vitreous of dogs and humans, but in the latter species no significant changes in vitreous lipid composition between the ages of 37 and 82 years.
SUPRAMOLECULAR ORGANIZATION
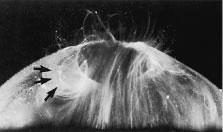
As described by Mayne,65 vitreous is organized in a dilute meshwork of collagen fibrils interspersed with extensive arrays of long HA molecules. The collagen fibrils provide a scaffold-like structure that is inflated by the hydrophilic HA. If collagen is removed, the remaining HA forms a viscous solution; if HA is removed, the gel shrinks39 but is not destroyed. Early physiologic observations66 suggested the existence of an interaction between HA and collagen that stabilizes collagen. Biomechanical studies67 of vitreous viscoelasticity suggested similar effects on HA. Early investigators68 observed reversible complexes of an electrostatic nature between solubilized collagen and various glycosaminoglycans and suggested that collagen-HA interaction occurs on a physicochemical rather than chemical level. Others69 later demonstrated that the sulfate group of a glycosaminoglycan is largely responsible for such interactions with the guanidino groups of arginine and epsilon-amino groups of lysine in collagen. Comper and Laurent39 proposed that in vitreous electrostatic binding occurs between negatively charged polysaccharides and positively charged proteins. These authors extensively reviewed the existing data characterizing the electrostatic properties of glycosaminoglycans and the factors influencing their electrostatic interactions with different ions and molecules.
Balazs29 hypothesized that the hydroxylysine amino acids of collagen mediate polysaccharide binding to the collagen chain via O-glycosidic linkages. These polar amino acids are present in clusters along the collagen molecule, which may explain why proteoglycans attach to collagen in a periodic pattern.24 Hong and Davison70 identified a type II procollagen in the soluble fraction of rabbit vitreous and proposed a possible role for this molecule in mediating collagen-HA interaction. In cartilage, “link glycoproteins” have been identified that interact with proteoglycans71 and HA.72 Supramolecular complexes of these glycoproteins are believed to occupy the interfibrillar spaces. In Asakura's24 studies of bovine vitreous stained with ruthenium red there are amorphous structures located on collagen fibrils at 55 to 60 nm intervals along the fibrils and filaments connecting these amorphous masses to adjacent collagen fibrils. These filaments may represent “link” structures of either a glycoprotein or proteoglycan nature. HA is known to interact with link proteins, as well as with an HA-binding glycoprotein called hyaluronectin.73 In the cornea, chondroitin sulfate and keratan sulfate bridge the interfibrillar spaces and keep the fibrils at specified distances to achieve transparency.74 The protein cores of these proteoglycans could be the linkage sites to collagen fibrils.
Bishop27 has proposed that an understanding how vitreous gel is organized and stabilized requires an understanding of what prevents collagen fibrils from aggregating and by what means the collagen fibrils are connected to maintain a stable gel structure. Studies75 have shown that the chondroitin sulfate chains of type IX collagen bridge adjacent collagen fibrils in a ladder-like configuration spacing them apart. This arrangement might account for vitreous transparency, because keeping vitreous collagen fibrils separated by at least one wavelength of incident light would minimize light scattering, allowing unhindered transmission of light to the retina for photoreception. However, depolymerizing with chondroitinases does not destroy the gel, suggesting that chondroitin sulphate side chains are not essential for vitreous collagen spacing. Complexed with HA, however, the chondroitin sulfate side chains might space the collagen fibrils,25,74,75 apart but Bishop believes that this form of collagen-HA interaction is “very weak.” Instead, he proposes that the LRR protein opticin is the predominant structural protein in short-range spacing of collagen fibrils. Concerning long-range spacing, Scott25 and Mayne and coworkers77 have claimed that HA plays a pivotal role in stabilizing the vitreous gel via this mechanism. However, studies76 using HA lyase to digest vitreous HA demonstrated that the gel structure was not destroyed, suggesting that HA is not essential for the maintenance of vitreous gel stability, leading to the proposal that collagen alone is responsible for the gel state of vitreous.27