CENTRAL RETINAL ARTERY OBSTRUCTION
Central retinal artery obstruction is one of the most sudden and dramatic events seen by ophthalmologists and was described as early as 1859.1 Although there have been numerous clinical and experimental studies of the pathophysiology of central retinal artery obstruction and although these studies have been augmented by the introduction of intravenous fluorescein angiography in the 1960s, the disease still has a relatively poor visual prognosis.
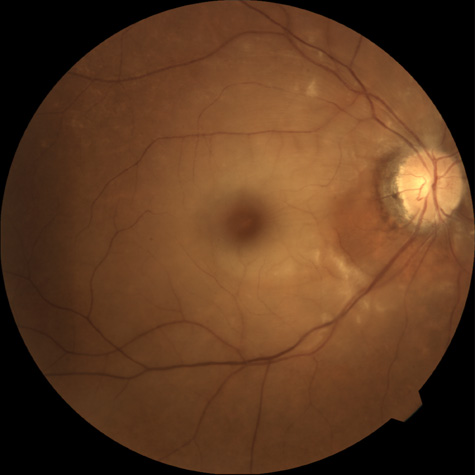
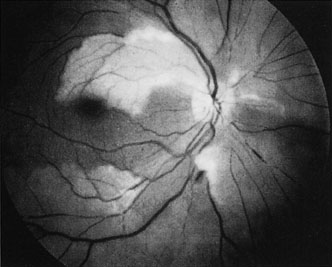
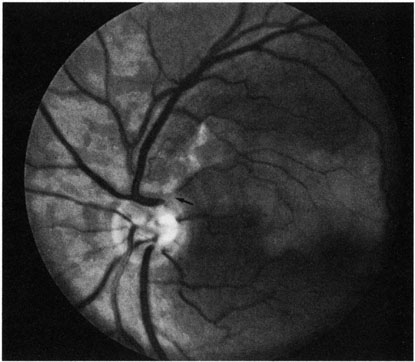
The clinical picture is typically striking. Patients present with a sudden painless loss of vision. The appearance of a cherry-red spot in the fundus is characteristic (Fig. 1).2 The cherry-red spot appears because soon after obstruction of the blood flow to the inner retina, the normally transparent retina becomes opaque and blocks the brownish-red color from the underlying choroid, which is still supplied by blood. Because the retina overlying the foveola is relatively thin, however, the normal color of the choroid is still visible in this area.3,4 Although characteristic, the cherry-red spot is not pathognomonic for central retina artery obstruction.5 Sometimes the characteristic cherry-red spot does not develop; there may be only a slight accentuation of the brownish-red color in the foveola.4 It is not known how long it takes this cherry-red spot to appear, but in a primate model, it has appeared as early as 30 minutes after obstruction.6 An afferent pupil defect is usually present.2
With obstruction, virtually all eyes have narrowing and irregularity in the arteries and there is frequently an irregularity in the caliber of the retinal veins.2,4,6,7 Segmentation (boxcarring) of the blood column frequently develops; this segmentation is particularly noticeable in the veins and can be seen as a to-and-fro movement of the blood elements.3,4 Retinal hemorrhages, however, are not characteristic of central retinal artery obstruction.7
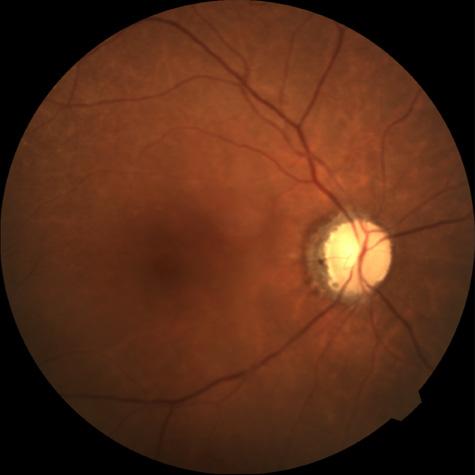
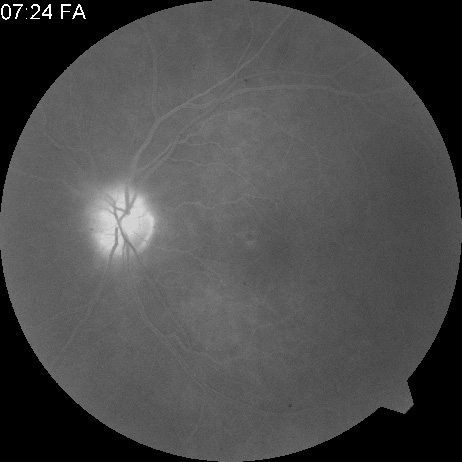
With time, the retinal opacity diminishes, generally leaving an optic nerve that is atrophic (Fig. 1). Frequently, thinned retinal arteries and veins also remain.7,8 No foveolar light reflex is evident, and a finely pigmented appearance of the macula is typical.7 In some cases, arterial collaterals develop at the optic disc.9–11 Rarely, anatomoses that exist between the central retinal artery and the ciliary arteries become visible as preretinal loops (Nettleship collaterals) after an occlusion at the edge of the disc.12,13,378In approximately 20% of patients, an embolus is evident somewhere in the arterial system.14 Emboli are discussed later.
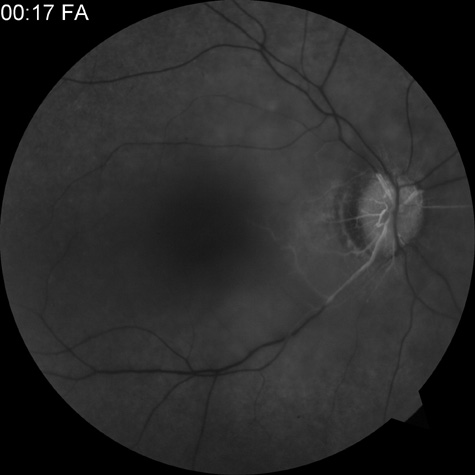
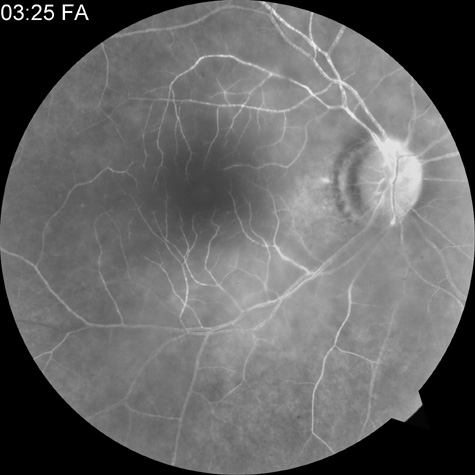
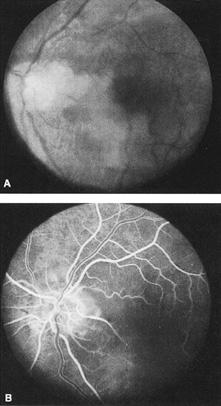
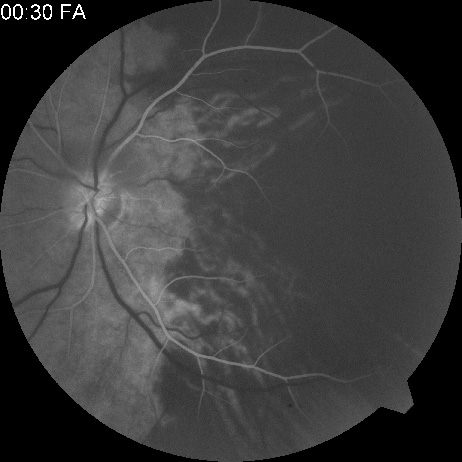
Intravenous fluorescein angiography is useful in showing the details of the abnormal circulation of a central retinal artery obstruction (Fig. 1). The principal abnormality is the delay in the appearance of the dye in the central retinal artery and its branches.2 Rather than the central retinal artery and its branches filling rapidly, considerable time may elapse before the entire arterial system is filled.15 The filling of the retinal arteries is often abnormal, with the fluorescein partially filling an artery (a dye front) or hugging the vessel wall, as in normal venous filling.15
Segmentation of the blood column is often well defined on fluorescein angiography.15 Venous filling is usually slowed and occasionally the dye does not progress beyond laminar flow during the study.14 In approximately 10% of eyes that have central retinal artery obstruction, there are abnormal choroidal filling defects, reflecting posterior circulation obstruction. These defects can occur even in patients who appear to have a typical central retinal artery obstruction.14 Leakage of dye from the vessel walls is not normally seen except at the site where an embolus lodges within a retinal artery.16 With time, the flow within the artery and its branches is reestablished and the appearance of the intravenous fluorescein angiogram may return to normal.
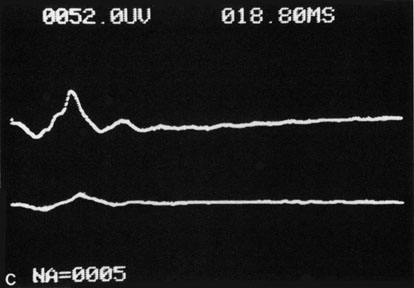
The electroretinogram (ERG) is usually abnormal in central retinal artery obstruction. The b-wave is reduced, indicating a marked abnormality in the circulation of the inner retina.17,18 The a-wave, indicating normal photoreceptor function, is preserved or accentuated. There does not appear to be a good correlation, however, between the extent of b-wave abnormality and the degree of retinal function,3 although Yotsukura and Adachi-Usami420 reported that the b-wave amplitude increased with visual improvement. The photopic negative response (PhNR) shows severe depression in this condition reflecting the significant loss of the ganglion cells and their axons.377 The electrooculogram may be either normal or abnormal. Visual field defects are usually profound but either occasionally a small portion of the temporal peripheral visual field remains or the patient has a large central scotoma.3 The onset of obstruction occurs most often between the hours of midnight and 6 AM, with the second most common period being between 6 AM and noon.2
Most patients with central retinal artery obstruction complain of a sudden loss of visual acuity in the affected eye; most of these patients present with visual acuity ranging from finger-counting to light perception.14 Only about 5% of patients have visual acuity of no light perception and most of these have involvement of the posterior ciliary circulation, which can be seen on either intravenous fluorescein angiography or electrophysiology.14 Without a partial central retinal artery obstruction or a spared cilioretinal artery (to be discussed), it is unlikely that any patient will have initial visual acuity better than finger-counting.14 With or without treatment, most eyes retain visual acuity of finger-counting to light perception. Patients with a spared cilioretinal artery do not seem to have a follow-up visual acuity that is significantly different from those without a spared cilioretinal artery.14
Central retinal artery obstruction is caused by the disruption or cessation of blood flow to the central retinal artery, its branches, and the retinal layers supplied by those vessels. The inner two thirds of the retina derives its blood supply from the central retinal artery and its branches; the outer third of the retina is supplied from the choroidal circulation.19–21 The central retinal artery and its branches function as an end artery, and with the exception of a few anastomotic connections between the retina and the ciliary circulation at the optic disc, there are no other anastomoses.19 No intrinsic retinal vessels are present in the fovea because the inner retinal layers are not present in this area.19
Histopathologically, when a central retinal artery obstruction occurs, the retina initially becomes edematous. With time, all the retinal layers supplied by the central retinal artery and the artery's branches, including the nerve fiber layer, ganglion cells, inner plexiform layers, and the inner portion of the inner nuclear layer, are lost.20 In trypsin digest preparations after obstruction of the central retinal artery, the greatest change is in the capillary bed,22 where extensive ischemic changes take place. Usually, little attempt is made to repair the retina with gliosis or neovascularization.20 The most common site of obstruction of the central retinal artery is at the level of the lamina cribrosa.20
Clinically, it is possible to examine the blood flow velocity in the central retinal artery in a noninvasive manner, using a variety of techniques.21 The most useful appears to be color Doppler imaging, providing simultaneous Doppler and B-scan ultrasound images of the optic nerve head, which primarily represents flow in the central retinal artery.23 Studies using this technique demonstrate a marked reduction of or no blood flow in the central retinal artery with an acute central retinal artery occlusion that returns to normal with time.24
Characteristics of the retinal blood flow can be measured clinically with retinal Doppler velocimetry.385 This is an instrument that can measure the blood column diameter, the centerline blood velocity, and the blood flow in major retinal vessels. However, when evaluating the blood flow in the optic nerve, choroidal, and retina, the Doppler laser flowmetry is more sensitive to the blood flow in the superficial layers than in the deeper layers.410
Masaoka and colleagues384 studied the hemodynamic changes in the retinal circulation with a scanning laser ophthalmoscope and intravenous fluorescein angiography. This technique allows the assessment of erythrocyte velocities and movement with high resolution continuous images and is a useful technique for the evaluation of hemodynamic changes in vascular occlusions.
Analysis of the retinal nerve fiber layer by one of the confocal laser scanning tomography techniques can provide information about a central retinal artery occlusion. Foroozan and co-workers383 studied patients with a central retinal artery occlusion within 1 week of occlusion with a scanning laser polarimeter. Diffuse attenuation of the retardation of the nerve fiber layer surrounding the optic nerve was seen in all eyes studied. Four patients were scanned in follow-up at intervals ranging from 1 week to 6 weeks; all had unchanged studies.383
Optic coherence tomography (OCT) of an acute central retinal artery occlusion shows a slight increase in the thickening of the retina and reflectivity of the inner layers of the retina that correspond to the opacity seen clinically (Fig. 1).409 With time, the disc becomes atrophic and the retina thinner.
Experimental studies of central retinal artery obstruction in the cat25 and the rhesus monkey26–28 have increased our understanding of arterial obstruction. The most clinically relevant finding is that there is a period in which a total lack of blood flow (ischemia) to the inner retina can be tolerated; that is, if the blood flow is not obstructed longer than this period, visual function may return to normal. This critical time in the cat eye is 90 minutes,25 and in the young, healthy rhesus monkey it is 100 minutes26–28—any longer than this and the histologic changes are irreversible and the animal has permanent visual loss. Hayreh and co-workers379,380 have repeated this experiment to determine how long the retina can survive central retinal artery occlusion in middle-aged and elderly rhesus monkeys, a situation that might be more applicable to the human situation where most occlusions occur in older patients. That study showed that an occlusion of less than approximately 100 minutes produced no permanent damage and that an occlusion of longer than 240 minutes produced massive and irreversible retinal damage. It is not known why the older monkeys had a longer retinal survival time with occlusion of the central retinal artery than younger monkeys.
Because there is no evidence that the monkey and the human have different retinas,379 the studies by Hayreh and co-workers have implications for studies that attempt to treat central retinal artery occlusion. Any treatment of this condition will almost certainly have to be initiated within 100 to 240 minutes, assuming the occlusion is total, or the patient will likely have permanent damage to the retina, regardless of treatment.
On average, central retinal artery obstruction occurs in patients who are in the fifth or sixth decades of life, with the age range varying from 17 to 84 years. Fewer than 10% of patients are younger than 30 years of age.14,29,30
In some cases, the cause of central retinal artery obstruction is relatively clear, such as when due to a visible embolus. In other cases, however, especially when found in association with a systemic disorder such as diabetes, the association is less clear. Table 1 lists some of the conditions associated with central retinal artery occlusion. Arteriosclerosis is probably the most commonly associated systemic condition. With the progressive buildup of subendothelial atheromatous material, the lumen of the central retinal artery becomes narrowed over time and eventually may become occluded.20 This association is inferred because most patients with central retinal artery obstruction are in the fifth to sixth decades of life, when atherosclerotic changes are expected.
TABLE 1. Conditions Associated With Retinal Arterial Obstructions
| Systemic |
|
| Ocular and Periocular |
The next most common systemic condition associated with central retinal artery obstruction is arterial hypertension; it can be found in as many as two thirds of all patients with central retinal artery obstruction.14,30,31 Carotid artery disease has been found in about 45% of patients who have central retinal artery obstruction,30 and hemodynamically significant stenosis occurs in approximately 20%.32 O'Farrell and Fitzgerald33 found ipsilateral carotid artery disease in 88% of patients presenting with retinal ischemia but neither study defined retinal ischemia nor indicated the ages or method of selection for patients studied. No difference was demonstrated between stenosis in the ipsilateral and the contralateral carotid artery33; this suggests that the cause of retinal artery occlusions may not be carotid artery stenosis—it may be simply associated with these occlusions or whatever process causes the carotid artery disease. Merchut and co-workers34 found no relation between the types of arterial occlusion—branch or central—and the type of underlying carotid artery disease. Wijman and colleagues35 found a high-grade internal carotid stenosis of 70% or more in 73% of patients with retinal ischemia that included transient ischemic attacks (TIAs) and arterial occlusions, both acute and chronic.
Diabetes mellitus14,28 and valvular heart disease28 are probably the next two most common associated conditions.
A variety of hematology parameters have been associated with central retinal artery occlusive disease.291–293 Walsh and co-workers291 found significant elevation of platelet coagulant activities (associated with the early stages of intrinsic coagulation) were elevated in patients with a central or branch retinal artery occlusion compared to controls. Kiesewetter and co-workers292 studied blood parameters in six patients with a central retinal artery occlusion before treatment was instituted. They found that compared to controls, the plasma viscosity, the extent of erythrocyte aggregation, and the plasma fibrinogen was significantly increased, while the erythrocyte deformation was reduced.
Sagripanti and associates389 found elevation of the prothrombin fragment (F1+2), the formation of thrombin-antithrombin III complex, and D-dimer (a stable degradation product of cross-linked fibrin) in acute central and branch retinal artery occlusions compared to controls. On follow-up examinations, the elevated F1+2 and the thrombin-antithrombin III complex significantly decreased while the D-dimer did not. The investigators conclude that increased thrombin generation may have a role in artery occlusions. However, the patients studied were treated with intravenous heparin and methylprednisolone, and the manuscript does not indicate whether their blood samples were taken prior to treatment or whether treatment could have affected the studies performed.
Greiner and co-workers388 studied 13 patients with a central retinal artery occlusion and 7 patients with a branch retinal vein occlusion for deficiencies in the protein C pathway. They concluded that the incidence of protein C deficiency in the artery occlusions they studied was not statistically significant from their control group.
Two research groups have shown a higher plasma level of homocyst(e)ine in patients with a central retinal artery occlusion, but not branch retinal artery occlusion.390,391 Wenzler and co-workers392 found hyperhomocyst(e)inemia in 2 patients: 1 patient with a central retinal artery occlusion in one eye and a branch retinal artery occlusion in the opposite eye, and 1 patient with bilateral branch retinal artery occlusions. However, the studies in the literature in regard to plasma homocyst(e)ine do not provide an answer as to whether elevated plasma homocyst(e)ine levels are an independent risk factor for retinal artery occlusive disease or are related to other vascular risk factors. Hyperhomocyst(e)inemia is an independent risk factor for atherosclerosis and atherothrombosis.416
The difference in the etiology of central retinal artery obstruction depends on the age of patients presenting with an obstruction. Central retinal artery obstruction in patients 30 years of age or younger tends to be associated with migraine, coagulant disorders, intraocular abnormalities, and trauma.29 Systemic diseases such as systemic arterial hypertension, atherosclerotic disease, and diabetes mellitus are not found in this younger age group.28,29 Some patients in the younger age group do not have a known cause for the obstruction; this was the case in as many as 12% of patients in one large series29 and in 40% of patients in a smaller series.30
Greven and associates31 studied 27 eyes in 21 patients with a retinal artery occlusion; all patients were younger than 40 years of age; emboli were identified in only 7 patients (33%) who had cardiac valvular disease, the most common cause in 4 patients (19%). The authors were able to identify various associated factors leading to a hypercoagulable state or embolic condition in 19 patients (91%).31 Despite their findings, the authors believe that the percentage of patients with ophthalmoscopically visible emboli is not indicative of the percentage of patients whose etiology for obstruction is embolic, and that most central retinal artery occlusions are embolic in origin.36
Two hundred fifty-six consecutive patients with acute unilateral retinal artery obstruction were studied using carotid Doppler ultrasonography; 18.8% had more than 60% stenosis of the internal carotid artery.37 Of those who had significant carotid stenosis, only 19 (40%) had visible retinal emboli. Thus, if ultrasound testing had been based only on visible retinal emboli, almost 60% of significant carotid artery stenotic lesions would have been missed.37 All patients at risk for extracranial carotid artery disease with acute retinal artery occlusion—not only those with visible emboli—should undergo duplex carotid Doppler ultrasonography.37
Evaluation of a patient with an arterial obstruction should be tailored somewhat to the age of the patient. A patient 20 years of age likely does not have extra-cranial carotid artery disease, whereas a 70-year-old patient clearly should be evaluated for such disease. Likewise, a 70-year-old patient with central retinal artery obstruction may have temporal arteritis, whereas this diagnosis in a 30-year-old patient is not likely.
There is probably little value, however, in determining the type of emboli if one is observed in the fundus. Sharma and co-workers38 found poor intraobserver and interobserver agreement on the quantitative assessment of retinal emboli when assessing them into groups of cholesterol, calcific, or both. Sharma and co-workers386 performed a retrospective multicenter chart review of 104 patients with both embolic and nonembolic acute retinal artery occlusion on whom transthoracic echocardiography was performed to determine the accuracy of visible retina emboli as a diagnostic test for anticoagulation or cardiac surgery. In this study, 41 patients had visible retinal emboli. The presence of a visible embolus in this review had no effect on whether a patient received anticoagulation or cardiac surgery, based on transthoracic echocardiography.
In some patients, the source of emboli from the heart can be determined with transthoracic echocardiography.39,40,412 When transthoracic echocardiography does not demonstrate the source of emboli, transesophageal echocardiography may be of more diagnostic value.41–43,381,412 Kramer and co-workers381,382 studied 18 patients with a retinal artery occlusion with transthoracic and transesophageal echocardiography. Seven of these patients had a central retinal artery occlusion. Sixty-one percent of the patients studied had at least one cardiac or aortic source of emboli detected by transesophageal echocardiography that was missed by transthoracic echocardiography. The authors recommend performing transthoracic echocardiography first, and then if the findings suggest cardiac or aortic conditions, performing the study via the esophagus: these findings include mitral valve disease, other valvular calcifications, enlargement of the left atrium, and severe left ventricular dysfunction.386
For all patients with a central retinal artery occlusion, the authors recommend an evaluation by a primary care physician or internist, to rule out systemic disease, and suggest as part of the evaluation, a Doppler study of the extracranial carotid arteries as well as a transthoracic echocardiogram. For patients with a visible embolus who have a negative carotid Doppler and normal transthoracic echocardiogram the authors generally suggest a transesophageal echocardiogram (depending on the patient's age as this is an invasive procedure). In older patients (however that is defined) with a central retinal artery occlusion we obtain a Westergren sedimentation rate and C-reactive protein to help eliminate the possibility that a patient may have temporal arteritis. Because elevated homocyst(e)ine has been associated with this disease and is an independent risk factor for atherosclerosis,416 the authors recommend testing the plasma homocyst(e)ine. Bilateral simultaneous central retinal artery occlusions413 are extremely rare and should prompt a careful search for a cause.
Thew authors' approach is more aggressive with younger patients. If a careful physical examination and search for emboli (including transesophageal echocardiography) are unrevealing and no obvious systemic cause exists for the obstruction, it may be useful to screen for hemotologic abnormalities; the most common finding is protein S deficiency.44,45 Whether this abnormality is responsible for or simply associated with retinal occlusions is unknown.
Treatment of either central retinal artery obstruction or branch retinal artery obstruction is unsatisfactory. No method has been proved to be more effective than the natural course of the disease.393,394 Although anecdotal cases abound of dramatic improvement in vision after some therapeutic intervention, no evidence is available that such intervention changes the natural course of the disease. The authors have seen one patient who presented on two different occasions with typical central retinal artery obstruction and severely decreased vision, who regained normal vision with no therapeutic intervention. Other clinicians have seen similar spontaneous recovery.399
The theoretical goal of treatment is to restore the retinal blood flow as soon as possible. Lowering intraocular pressure is one of the principal methods of treatment because the pressure load may perfuse the retina or dislodge an embolus.6,47 Paracentesis may be the fastest way to accomplish this.48,49 There is no documented evidence, however, that it is possible to improve arterial circulation with paracentesis or that paracentesis dislodges emboli in humans. Experimental studies in animals failed to show that paracentesis results in a significant increase in retinal perfusion.50–52 At least one case has been reported of endophthalmitis after paracentesis for central retinal artery obstruction.53 Reduction of intraocular pressure can also be accomplished with intravenous acetazolamide47 or topical glaucoma medications. Some investigators advocate continuation of acetazolamide for up to 2 weeks.54
Ocular massage also appears to be a useful technique for treatment of central retinal artery obstruction.395 Raising intraocular pressure for 30 seconds by pressure on the eye can result in both dilation of retinal arteries55–57 and increased blood flow volume.47,50 The change in arterial diameter with decreased perfusion (retinal arterial reactivity) decreases with the age of the patient.58 Digital massage also acts to lower intraocular pressure; it is easy to perform and can be done by the patient while awaiting other treatment.
A variety of gases have been suggested for use in treatment of central retinal artery obstruction, including oxygen (either delivered at standard atmospheric pressure or hyperbaric oxygen)59 and a mixture of 95% oxygen and 5% carbon dioxide (carbogen).54 Theoretically, it may be possible to deliver enough oxygen to perfuse the outer retina of a patient with central retinal artery obstruction by delivering 100% oxygen at atmospheric pressure. It has been found, however, that administration of 100% oxygen leads to vasoconstriction and decreased blood flow.60 The rationale for the addition of carbon dioxide to oxygen is that carbon dioxide leads to vasodilation,61 and the combination may allow delivery of the 100% oxygen to the retina without causing vasoconstriction.
Experimentally, the results of oxygenization to the retina have been variable and in some cases contradicting. Flower and Patz62 were not able to restore retinal function by delivering 100% oxygen at 1 atm of pressure to either the cat or monkey retina after retinal artery obstruction. Landers,63 however, was able to show in similar experiments that ventilation with 100% oxygen at 1 atm was capable of restoring inner retinal function, even though retinal circulation was occluded. Berkowitz64 showed in the adult and newborn rat that breathing carbogen oxygenates the inner retina better than 100% oxygen does. Birol and co-workers405 found that hyperoxia was preferable to room air in the cat model of arterial occlusion for preserving the b-wave amplitude of the electroretinogram. Deutsch and associates65 demonstrated in young, healthy human volunteers that there is no significant difference between vasoconstriction of retinal arteries with either 100% oxygen or a mixture of 95% oxygen and 5% carbon dioxide.
Experiments in healthy subjects have shown that carbogen breathing increases the retinal blood flow when studied with the blue field entopic phenomena66 and the scanning laser ophthalmoscope,68 but the retinal blood flow is not increased when tested with laser Doppler velocimetry. Schmetterer and colleagues studied the effect of different mixtures of O2 and CO2 on ocular pulsations in healthy volunteers using a laser interferometer coupled to a fundus camera.417 They concluded that the addition of 5% CO2 to oxygen is appropriate to maintain pulsatile ocular blood flow in the macula and optic disc at a level comparable to breathing air.417
Harino and co-workers421 demonstrated in young healthy volunteers that rebreathing into a bag for two minutes significantly increased the leucocyte velocity in the macular capillaries.
There is no evidence that any of these treatments are efficacious in restoring vision in patients with any type of arterial obstruction. Atebara and co-workers69 examined the records of 89 consecutive Wills Eye Hospital patients who had acute central retinal artery obstruction and received both anterior chamber paracentesis and carbogen therapy, which were compared with the records of those patients who had not been treated. No statistically significant difference was demonstrated between treated and untreated groups after adjusting for initial visual acuity.69
Although it is theoretically possible for choroidal circulation to supply adequate oxygen to the inner retina with the use of hyperbaric oxygen, the quantity of oxygen required appears to be toxic to the retina in a relatively short time.70 This may be true even when 100% oxygen under standard atmospheric pressure is delivered for prolonged periods of time.71 Two separate groups, however, have shown experimentally in animals that it is possible to supply oxygen to the inner retina after vascular obstruction by means of intravitreal perfusion.72,73
A variety of drugs to induce vasodilation have been injected into the retrobulbar space in patients with central retinal artery obstruction47 but no experimental or clinical evidence is available to suggest that any of the agents delivered by retrobulbar injection are of value in restoring blood flow. In addition, it is theoretically possible that such an injection could produce a retrobulbar hemorrhage, which would further decrease retinal blood flow.47
A few patients have been treated with a technique involving surgical cannulation of the supraorbital artery and perfusion of this artery with heparin, papaverine, streptokinase, or a combination of these agents.74,75 Watson74 reported complete restoration of vision in the eyes of 4 patients in a series of 14 with central retinal artery obstruction; he also reported 2 patients who had considerable improvement of visual acuity and visual fields. No control group of patients was used in this study, however. Schmidt and colleagues76,77 treated 46 patients with either urokinase or recombinant tissue plasminogen activator (tPA) by inserting a microcatheter into the proximal segment of the ophthalmic artery. They were able to show that visual acuity improved with this treatment in patients who have “slight edema of the retina,” no progression, well-perfused perimacular arterioles, and who were treated within 14 hours of occlusion.77 It is notable, however, that there was no standardized protocol or control group, and intravenous fluorescein angiography was performed in only about three-fourths of the patients.77
Richard and co-workers treated 46 patients with a central retinal artery occlusion and seven patients with branch retinal artery occlusion treated with rTPA infused into the ophthalmic artery via a transfemoral incision and reported that vision improved in 66% of the patients.396 However, this was an uncontrolled study that did not include pre and posttreatment fluorescein angiography to either confirm the diagnosis or to evaluate the effect of treatment on retinal circulation,397–399 and the final results of treatment may not differ from the natural history of this disease.397
Seventeen patients were treated by Weber and associates78 with intraarterial urokinase placed into the proximal segment of the ophthalmic artery; these investigators believed that complete or partial improvement of visual acuity was achieved in approximately one-third. This was not a clinical trial, however, and some patients also received paracentesis or carbogen therapy.
Rumelt and co-workers400 reported the treatment of 11 patients with a central retinal artery occlusion using an arbitrary, nonsystematic regimen of ocular massage, sublingual isosorbide dinitrate, intravenous acetazolamide, intravenous mannitol, oral glycerol, anterior chamber paracentesis, intravenous methylprednisolone and streptokinase, and retrobulbar tolazoline; although not all patients received all treatments. Improvement of blood flow was determined at the end of treatment by three-mirror contact lens biomicroscopy. The authors report that 73% had improved visual acuity after these treatments.
Kattah and co-workers401 treated 12 patients with a central retinal artery or ophthalmic artery occlusion with a stroke protocol involving the intravenous injection of rTPA and reported 10 patients had improved visual acuities after treatment. Some but not all patients were treated before injection of rTPA with a paracentesis of the anterior chamber, and four of the patients treated developed neovascular glaucoma within 3 to 4 weeks of the arterial occlusion. No results of vascular perfusion pretreatment or posttreatment with intravenous fluorescein angiography were reported in this study and there was no control group.
It has been demonstrated in rats that tPA is effective when given intravenously for lysing experimental retinal arterial thrombi.79 The use of tPA in acute obstruction has probably been increased by its proved value when given intravenously as a treatment for acute ischemic stroke.13 In the case of acute ischemic stroke, tPA is administered within 3 hours of onset; the benefits of such treatment are observed 3 months after the stroke. The systemic administration of tPA is not innocuous, and whether this risk can be justified in a patient who has another normal eye has not been demonstrated.
Beatty and Au Eong418 performed a meta-analysis of the published reports of local intra-arterial fibrinolysis to treat central retinal artery occlusion. They studied 16 reports, all retrospective and nonrandomized, and concluded that there may be a marginal visual acuity benefit of local intraarterial fibrinolysis compared to conventional management, but there is currently insufficient evidence to justify the use of this intervention outside of a randomized controlled clinical trial.418
Zheng and co-workers80 treated 245 cases of retinal artery obstruction—including 168 cases of central retinal artery obstruction—by daily acupuncture treatments delivered over several 10-day courses. They reported marked visual improvement in 25% of patients and good visual improvement in 30%. They compared these results to a retrospective group of untreated patients from their clinic but the study was not performed as a controlled clinical trial or in a prospective fashion.80
Hausmann and Richard81 treated 4 patients with high-dose bolus intravenous steroids and observed that intravenous fluorescein angiography demonstrated improved circulation. These patients were also treated patients with plasma expanders, heparin, and nicoumalone, however, and no visual acuities were reported.
It is technically possible to cannulate the central retinal artery or its branches402,404 or even remove emboli from retinal arteries.111,403 Whether this will result in improvement in vision after this surgery is unknown. An animal model of transient retinal ischemia has been developed in the rat retina407 that may allow experiments with thrombolytic agents to be conducted prior to human testing.
There are no randomized, prospective clinical trials of large enough size to determine whether any of the forms of treatment of central retinal artery obstruction are better than no treatment.393 Augsburger and Magargal54 reported the visual outcome in 34 consecutive cases of treated acute central retinal artery obstruction. The treatment in their series included paracentesis, controlled ocular massage, inhalation of 95% oxygen and 5% carbon dioxide, and the administration of oral acetazolamide and aspirin. They reported that 35% of the patients had final visual acuity equal to or greater than 20/100. No control group was available in their series, however. Hayreh and Podhajsky,82 in a detailed study of 64 eyes with central retinal artery obstruction, state that no treatment is effective for central retinal artery obstruction, although they provide no details to support this statement. Some patients recover good visual acuity after central retinal artery obstruction with no treatment,83 whereas others may reestablish normal blood flow and visual acuity several days after treatment.84 The study by Atebara and co-workers,69 which did not find anterior chamber paracentesis or carbogen therapy effective for treatment of arterial occlusive disease, has been mentioned.
Two experimental studies to protect the retina from vascular obstruction after ischemia should be mentioned. Faberowski and associates85 showed that an ice pack applied to the rat eye protected it against ischemic injury after ligation of the major vessels and the optic nerve. Yoon and Marmor86 showed in the rabbit that intravenous dextromethorphan protected against retinal ischemic damage from vascular obstruction when the obstruction was produced by elevation of intraocular pressure.
Neovascularization is probably the most serious complication that can develop after central retinal artery obstruction. Duker and Brown87,88 studied 168 cases of central retinal artery obstruction and found neovascularization of the disc developed in 1.8% of eyes; neovascularization of the iris occurred in 16.6% of eyes. Hayreh and Podhajsky82 reported neovascular glaucoma in 16% of 64 eyes with central retinal artery obstruction. These authors believe that in most patients, central retinal artery obstruction is actually caused by neovascular glaucoma secondary to severe carotid artery disease.82 There is, however, at least one case reported in which neovascular glaucoma resulted from central retinal artery obstruction in a patient who did not have carotid artery obstructive disease.89
CENTRAL RETINAL ARTERY OBSTRUCTION WITH CILIORETINAL ARTERY SPARING
A cilioretinal artery90 is an anatomic variant that is found in approximately 30% of eyes.91 In patients with central retinal artery obstruction, approximately 25% have some degree of macular sparing as a result of a patent cilioretinal artery.92 When the cilioretinal artery makes a major contribution to perfusion of the macula, these patients may have some degree of visual preservation. Brown and Magargal14 reported on 73 eyes with acute central retinal artery obstruction in which 48 eyes had no cilioretinal sparing. None of these affected patients presented with visual acuity better than finger-counting. Of the 25 eyes with some degree of cilioretinal sparing, however, 16% had an acuity of 20/200 or better. Brown and Shields92 analyzed 107 cases of central retinal artery obstruction, of which 26% showed some degree of macular sparing because of a patent central retinal artery. They believed that with rare exceptions, patients with central retinal artery obstruction but no cilioretinal sparing had poor vision and that patients with a patent cilioretinal artery did relatively well—with or without treatment in terms of visual acuity—but were usually left with a severe peripheral visual field defect.
BRANCH RETINAL ARTERY OBSTRUCTION
In one large series of retinal artery obstructions, 57% of obstructions involved the central retinal artery, 5% involved the cilioretinal artery and the remaining 38% involved one of the branch retinal arteries.92 Branch retinal artery obstructions usually occur at bifurcations.19,93
When a branch retinal artery is obstructed, there is usually whitening of the retina in the area supplied by the artery (Fig. 2). In most eyes (62% in one series), emboli are responsible for the obstruction.94 The temporal branch arteries are involved in almost all cases that have been reported,92,94 but whether this is because nasal branch retinal artery obstructions are rare or because such obstructions are simply asymptomatic is unknown. The characteristics of a branch retinal artery obstruction on intravenous fluorescein angiography are similar to those for central retinal artery obstruction.
|
The patient describes either a sudden loss of vision if the branch retinal artery involves the macula or a visual field defect or both. Initial visual acuities can range from 20/15 to hand movements, but in one series, more than three-fourths of all patients had an initial visual acuity of 20/40 or better.94 Most patients who are seen with have branch retinal artery obstruction have unilateral obstructions, but about 8% later develop branch retinal artery obstruction in the opposite eye.94
Occasionally, patients present with a bilateral branch retinal artery obstruction.94 Some have obstructive retinal arteries without evidence of systemic abnormalities,95,96 whereas others have accompanying symptoms such as deafness.97,98 Some younger patients—usually women—have been reported to have an idiopathic syndrome of branch retinal artery obstruction, encephalopathy, and hearing loss (Susac syndrome).99–104,406 The eyes on these patients are characterized by significant systemic thromboembolic events; recurrent bilateral retinal arterial occlusions; segmental arteriolar staining by fluorescein angiography, occasionally associated with periarteriolar retinal whitening or mild vitreous cells; and a predilection for vestibuloauditory or transient sensorimotor symptoms.104 Some patients, however, present with only recurrent bilateral retinal arteriolar occlusions and never develop the associated vestibuloauditory or sensorimotor systems; whether these patients have a partial manifestation of Susac syndrome is unknown. The etiology of branch retinal artery obstruction in most cases is similar to that of central retinal artery obstruction (see Table 1). Retinal diseases (such as toxoplasmosis) can also cause branch retinal artery obstruction.
Hasegawa and co-workers411 have reported on the multifocal electroretinogram in patients with a branch retinal vein occlusion. They found that both the first and second order multifocal electroretinogram amplitudes decreased in the effected area of the retina compared with the normal retina, but the second order multifocal electroretinogram was more affected by a branch retinal artery occlusion, suggesting that the second order multifocal electroretinogram is a more sensitive indicator of inner retinal dysfunction.411
The complications of branch retinal artery obstruction can include retinal neovascularization, although this is rare.105 When neovascularization develops, it may be related to decreased blood flow to the eye, such as that due to carotid artery disease106 or diabetes mellitus.107 Neovascularization has been reported as a sequel to obstructive retinal arteriolitis.95 Vitreous hemorrhage can occur after neovascularization.87,107 Occasionally, retinal breaks occur after branch retinal artery obstruction.108
Dutton and Craig109 treated a retinal embolus causing branch retinal artery occlusion with the argon laser after other therapy had failed; they were able to make the embolus break up and disappear but without improvement in visual function.109 Ciulla and colleagues,110 however, were able to only disrupt one retinal emboli out of four in a rabbit model of artery occlusion using human atherosclerotic material treated with the argon laser. They also failed to dislodge emboli in a human with branch retinal artery obstruction, and no clinical improvement resulted.110 Peyman and Gremillion111 removed a branch retinal artery embolus in a patient with branch retinal artery occlusion using a 30-gauge needle and a pars plana vitrectomy; the patient regained vision to 20/200.
Most clinicians do not treat branch retinal artery obstruction, and there are no published clinical trials that suggest any treatment is better in terms of a final visual result than no treatment at all. An investigation into associated systemic abnormalities is important, and treatment of any systemic abnormalities that may be found is necessary in most cases. In those patients who present with retinal neovascularization, the treatment of choice is scatter photocoagulation delivered to the area of ischemic retina, with delivery similar to that used for neovascularization secondary to branch retinal vein obstruction.106,112
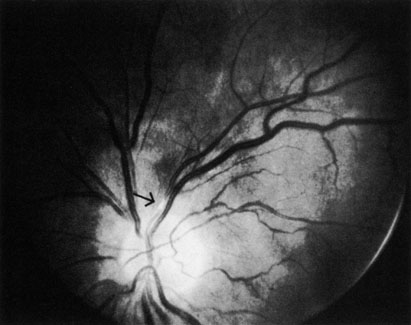
CILIORETINAL ARTERY OBSTRUCTION
Cilioretinal arteries can be selectively obstructed (Fig. 3). More commonly, however, they occur in association with central retinal vein obstruction.113 When they supply a significant portion of the macula, decreased visual acuity is common. Prognosis for visual outcome in such patients is good. Brown and co-workers114 studied 10 eyes having an isolated cilioretinal artery obstruction. Of those, 60% had an initial visual acuity of 20/40 or better and on follow-up examination, all eyes but one had a best-corrected visual acuity of 20/40 or better.114
|
OBSTRUCTION OF THE OPHTHALMIC ARTERY, POSTERIOR CILIARY ARTERIES, OR ARTERIES SUPPLYING THE CHORIOCAPILLARIS
Symptoms that involve obstruction of the ophthalmic artery, posterior ciliary arteries, or arteries supplying the choriocapillaris depend on which artery is affected. In some patients, the location of the obstruction must be inferred because it is not always possible to visualize it, even with intravenous fluorescein angiography. The ophthalmic artery is a branch of the carotid artery that branches into the central retinal artery and the posterior ciliary arteries.115 There are usually two main ciliary arteries; each one supplies about half of the choroid. The medial posterior ciliary artery supplies the nasal half, and the lateral posterior ciliary artery supplies the temporal half, with a watershed zone in between. The branches of the medial and lateral posterior ciliary arteries (and occasionally a superior ciliary artery) become the long or short posterior ciliary artery.116,117 Each lobule of the choriocapillaris supplied by the terminal choroidal arteriole forms one of the branches of the posterior ciliary artery, and each lobule is drained by a venule.116,118There is no anastomotic communication between lobules.
OPHTHALMIC ARTERY OBSTRUCTION
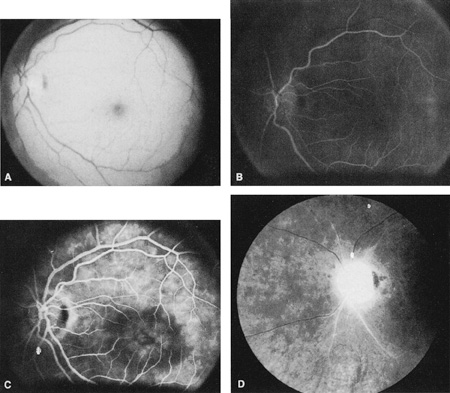
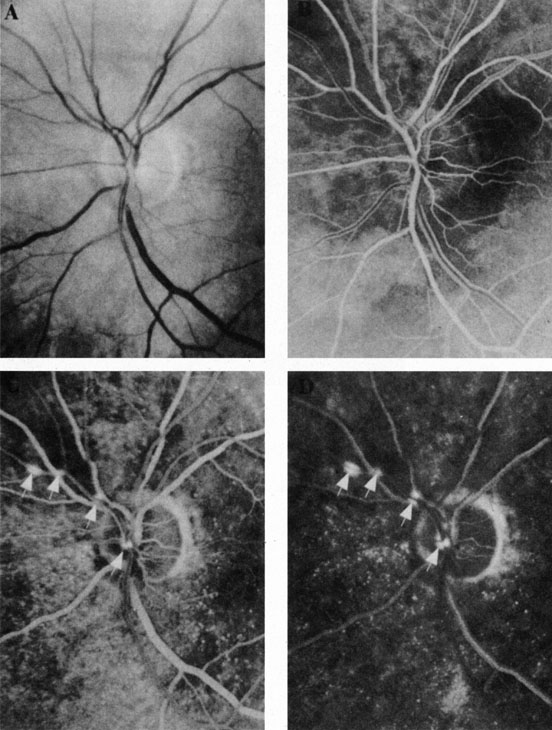
Ophthalmic artery obstruction is characterized by an initial opacification of the entire retina in a manner similar to that of central retinal artery obstruction (Fig. 4A). The cherry-red spot may or may not be present, however.119 The difference between an ophthalmic artery obstruction and central retinal artery obstruction is that in an ophthalmic artery obstruction, with time, optic atrophy develops, as does varying amounts of pigmentation due to the lack of perfusion of the retinal pigment epithelium (see Fig. 4D). This pigment is generally diffusely scattered throughout the posterior pole but it may also be seen in greater amounts in the periphery.
|
Intravenous fluorescein angiography generally shows moderate to marked abnormalities in the filling of the choroid, in addition to a delay in filling or even nonfilling of retinal vessels (see Fig. 4B and 4C). Staining of the retinal pigment epithelium can also be seen; this staining either can occur locally in the macular area or it can be diffuse.119 The electroretinogram shows abnormalities of both a- and b-waves, reflecting ischemia to both the inner and outer retina. Almost all reported patients have had an initial visual acuity of no light perception; virtually no patients can be expected to have a final visual acuity better than that.119 An acuity of no light perception is a clue to the presence of an ophthalmic artery obstruction. Because relatively few patients with central retinal artery obstruction have an initial visual acuity of no light perception, no light perception usually suggests the likelihood of some obstruction of the choroidal circulation.14
The causes of ophthalmic artery obstruction vary. In the 10 eyes reported by Brown and co-workers,119 the cause in 2 patients was carotid artery disease. In 1 patient each, the following causes were found: embolization from an arterial myxoma, atrial fibrillation, external compression of the orbit and globe, trauma (a knife wound), orbital mucormycosis, and one of unknown cause.119 Because the ophthalmic artery makes an acute bend in the orbit, it is likely that emboli could lodge at that location.115,120 The site of this type of obstruction must be speculative, however, because there is no way to know that a simultaneous obstruction of the retinal and the choroidal circulation has not occurred distal to the ophthalmic artery.
Two of the cases reported by Brown and coworkers119 were bilateral. One occurred in a patient with bilateral mucormycosis and the other was in a patient experiencing atrial fibrillation with accompanying thyroid fever.119 Patients with a presumed ophthalmic artery obstruction require careful and complete systemic evaluation in the search for possible causes.
POSTERIOR CILIARY ARTERY OBSTRUCTION
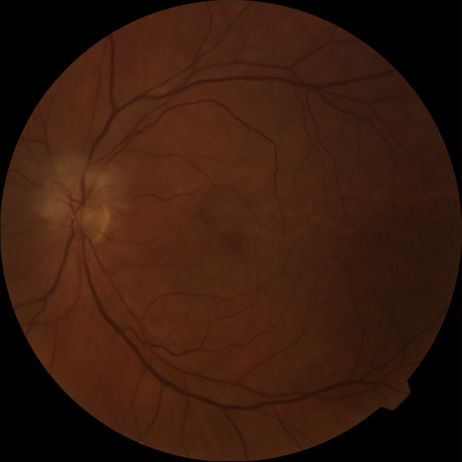
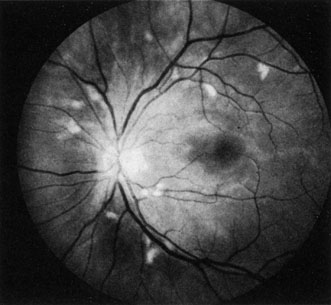
Obstruction of one of the posterior ciliary arteries may result in a whitening of the area of the retina supplied by the involved posterior ciliary artery and the subsequent appearance of patchy pigmentation in the same area. However, in patients with anterior ischemic optic neuropathy secondary to temporal arteritis the retina may appear normal (Fig. 5). In the case of either the medial posterior ciliary artery or the lateral posterior ciliary artery, the area involved is the size of approximately half of the choroid.121–123 In the case of a long posterior ciliary artery, the temporal side of the eye including the macula is involved.121
|
We have seen several patients with anterior ischemic optic neuropathy with suspected temporal arteritis where the Westergren sedimentation rate and C-reactive protein were normal and the intravenous fluorescein angiogram helped confirm the diagnosis (Fig. 5).
CHOROIDAL OBSTRUCTION
The appearance of a lesion involving the arterial supply to the choroid varies, depending on the size of the vessel occluded. Obstruction of a larger choroidal artery produces a whitening of the retina in a wedge shape, with the apex positioned toward the posterior pole.124–126 With time, pigmentation occurs in this area.
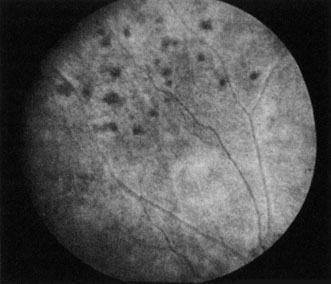
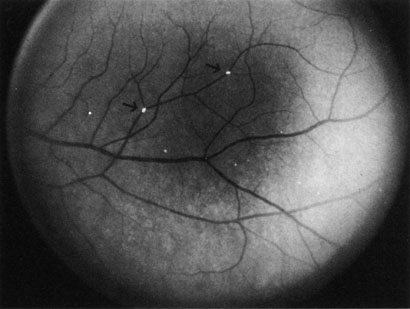
Obstruction of smaller terminal choroidal arterioles produces only small patchy areas of whitening, with overlying pigmentation (Elschnig spots, Fig. 6).127 The obstruction of smaller arterioles may occur in multiple sites in some cases.128
The syndrome of serous retinal detachments in various systemic diseases, such as arterial hypertension,129 eclampsia (toxemia of pregnancy),130 Goodpasture's syndrome,131 thrombotic thrombocytopenic purpura,132 periarteritis nodosa,133 and disseminated intravascular coagulation,134 are probably due to obstruction of the choroidal vessels affecting the pigment epithelium, with breakdown of the chorioretinal barrier but without frank infarction.135 Experimental work involving the injection of microspheres into the posterior ciliary arteries supports this concept.136 Some patients have resolution of the serous retinal detachment in addition to their systemic disease; depending on the severity of the ischemia produced, they may be left with decreased visual function and scarring, whereas others may be left with normal vision.135 Some investigators believe that acute multifocal posterior placoid pigment epitheliopathy (which Hayreh calls multifocal acute ischemic choroidopathy) and geographic (helicoid or serpiginous) choroidopathy are both manifestations of acute choroidal ischemia.135–137
A syndrome that appears to affect the choroidal and outer retinal circulation has been reported in a few patients after phacoemulsification138,139 and has been termed the outer retinal ischemic infarction syndrome.139,140 After uneventful phacoemulsification, such patients are observed to have loss of central and paracentral vision and diffuse and patchy whitening of the outer retinal layer in the posterior fundus. They also have an unusual polygonal pattern of fluorescein angiography and staining of the retinal pigment epithelium and outer retinal layers. They experience clearing of the retinal whitening that is later replaced by mottled pigmentation of the retinal pigment epithelium and partial recovery of the central visual field.139 The optic nerve does not become pale, and the vessel caliber is normal. The cause is postulated to be an elevation of the intraocular pressure that temporarily obstructs the blood flow to the choroid (and probably also to the retina). This obstruction then causes a temporary obstruction of the blood supply of the retinal pigment epithelium and outer retinal layers but results in sparing of both the macula and the periphery.139,140 This syndrome has been reported to occur also in patients undergoing pars plana vitrectomy with a scleral buckling procedure.141
OBSTRUCTION OF A RETINAL ARTERIOLE
Obstruction of a retinal arteriole produces cotton-wool spots, which have also been called white patches or soft exudates (Fig. 7). Cotton-wool spots are caused by focal hypoxia, which obstructs axoplasmic flow, resulting in accumulations of axoplasmic debris in the nerve fiber layers of the retina.142–144 Cotton-wool spots may be either single or multiple. On intravenous fluorescein angiography, they correspond to areas of capillary nonperfusion.6 The patients are often asymptomatic but may experience severe visual loss if the areas of arteriolar obstructions are extensive (Fig. 8).145
|
|
There are multiple causes for cotton-wool spots. Brown and co-workers146 studied 24 consecutive patients who presented with a cotton-wool spot; any patient with known diabetes mellitus was excluded. In this series, 23 of the 24 patients were found to have one or more associated systemic diseases. The disease entities associated with cotton-wool spots are listed in Table 2.
TABLE 2. Conditions Associated with Cotton-Wool Spots
| Diabetes mellitus |
| Systemic arterial hypertension |
| Collagen vscular diseases |
| Systemic lupus erythematosus |
| Dermatomyositis |
| Polyarteritis nodosa |
| Scleroderma |
| Giant cell arteritis |
| Cardiac valvular disease |
| Mitral valve prolapse |
| Rheumatic heart disease |
| Endocarditis |
| Acquired immunodeficiency syndrome (AIDS) |
| Leukemia |
| Trauma |
| Radiation retinopathy |
| Central retinal artery obstruction |
| Retinal vein obstruction |
| Metastatic carcinoma |
| Leptospirosis |
| Rocky Mountain spotted fever |
| High altitude retinopathy |
| Severe anemia |
| Acute blood loss |
| Papilledema |
| Papillitis |
| Carotid artery obstruction |
| Dysproteinemias |
| Septicemia |
| Aortic arch syndrome (pulseless disease) |
| Intravenous drug abuse |
| Acute pancreatitis |
| Onchocerciasis |
Adapted from Brown GC, Brown MM, Hiller T, et al: Cotton-wool spots. Retina 5:206, 1985.
The most common cause of cotton-wool spots is diabetes mellitus, and most of these patients are already aware of their disease. The second most likely cause is either acquired immunodeficiency syndrome or systemic arterial hypertension. Occasionally, a healthy patient has a cotton-wool spot and on systemic evaluation is found to be entirely normal. This was the case with one of Brown and co-workers146 patients, and we have encountered similar patients. However, we have also seen an occasional patient with no history of systemic disease who presented with a single cotton wool spot and subsequently was diagnosed with diabetes.
COMBINED CENTRAL RETINAL ARTERY AND CENTRAL RETINAL VEIN OBSTRUCTION
Occasionally, patients present who have an obstruction of both the central retinal artery and the central retinal vein.56 Such patients have fundus characteristics of both conditions. Generally, there is a whitening of the retina associated with a cherry-red spot and in addition, optic disc edema, retinal hemorrhages, and venous engorgement. On follow-up examination, these patients develop optic atrophy accompanied by extreme narrowing of the retinal vessels. Subsequent visual acuity tends to be poor, although rare patients have been reported to have a good return of visual function.147 In the largest series reported in the literature, one-third of patients developed neovascular glaucoma.56