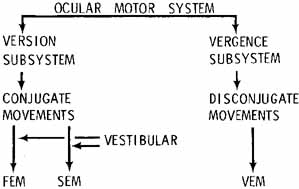
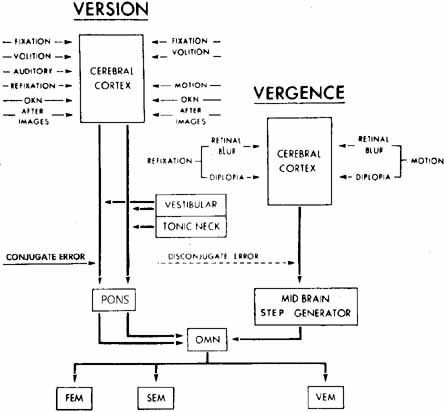
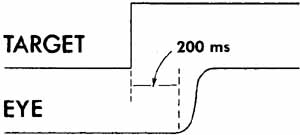
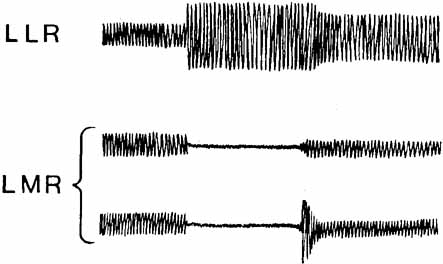
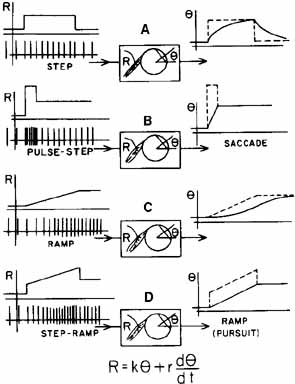
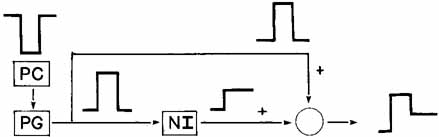
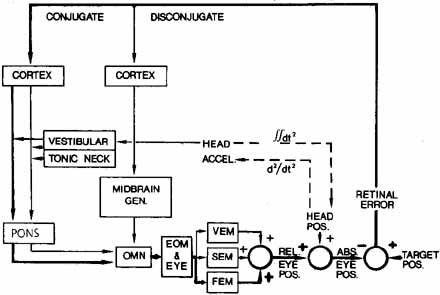
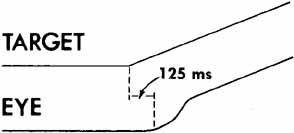
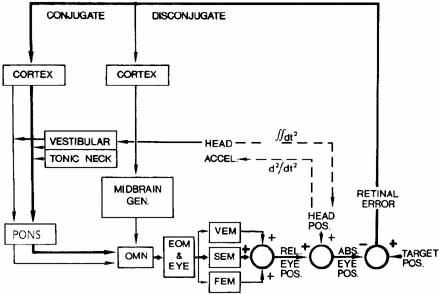
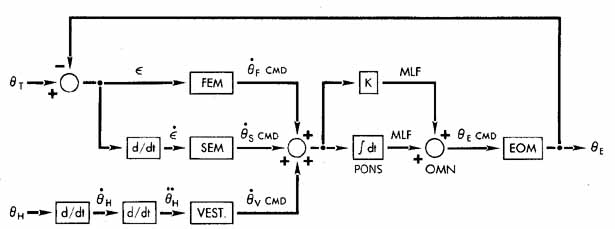
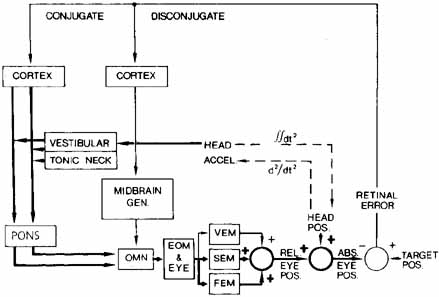
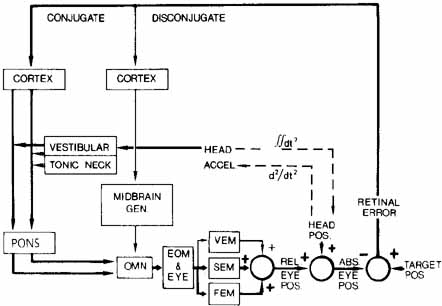
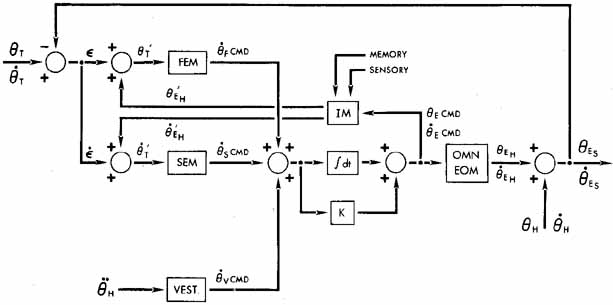
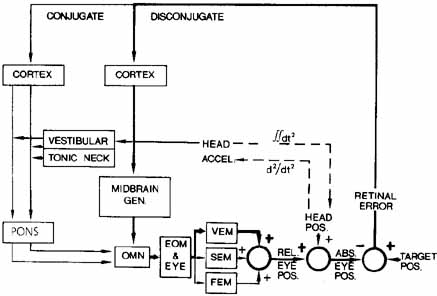
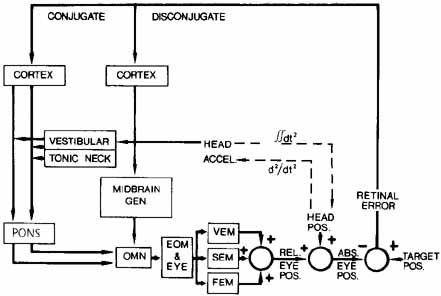
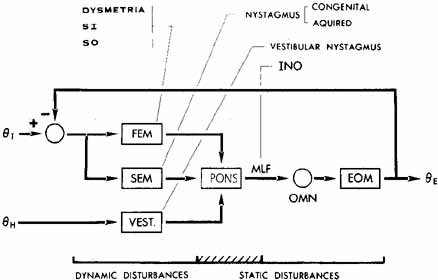
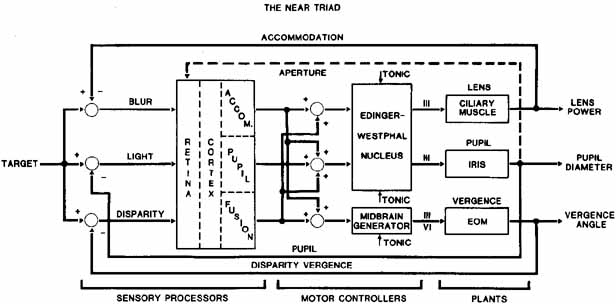
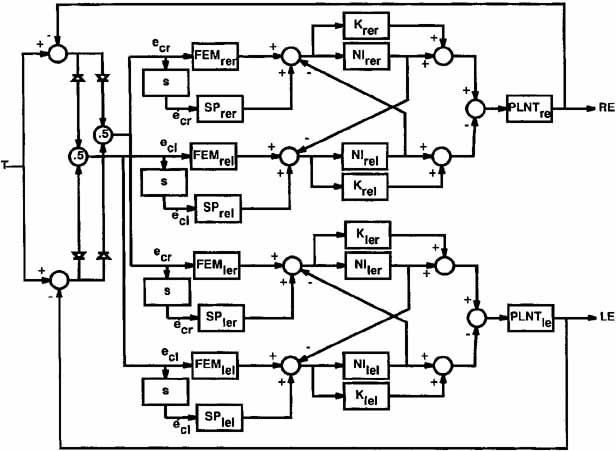
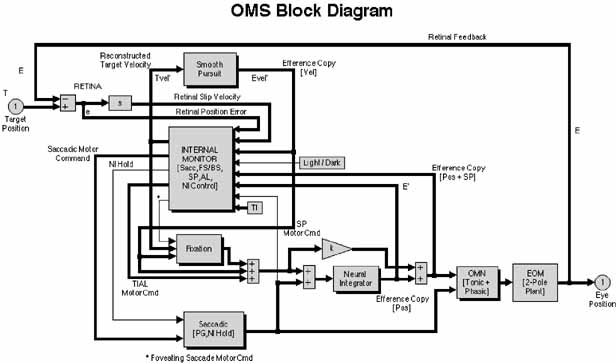
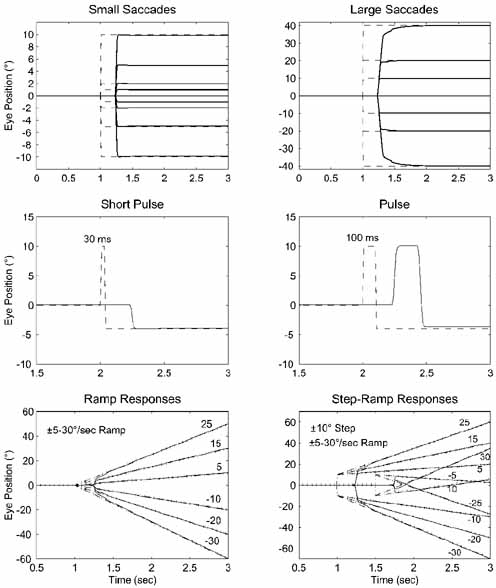
1. Shaunak S, O'Sullivan E, Kennard C: Eye movements. J Neurol Neurosurg Psychiat 59:115, 1995 2. Dell'Osso LF, Daroff RB: Functional organization of the ocular motor system. Aerospace Med 45:873, 1974 3. Keller EL, Robinson DA: Abducens unit behavior in the monkey during vergence movements. Vision Res 12:369, 1972 4. Carpenter RHS: Movements of the eyes. 2nd ed. London: Pion, 1988:1 5. Leigh RJ, Zee DS: The neurology of eye movements (Contemporary Neurology Series). 4th ed. New York: Oxford University Press, 2001:1 6. Dell'Osso LF: Nystagmus basics. Normal models that simulate dysfunction. In Hung GK, Ciuffreda KJ (eds): Models of the visual system. New York: Kluwer Academic / Plenum Publishers, 2002:711 7. Robinson DA, Gordon JL, Gordon SE: A model of smooth pursuit eye movements. Biol Cyber 55:43, 1986 8. Dell'Osso LF: Fixation characteristics in hereditary congenital nystagmus. Am J Optom Arch Am Acad Optom 50:85, 1973 9. Dell'Osso LF, Van der Steen J, Steinman RM, et al: Foveation dynamics in congenital nystagmus I: Fixation. Doc Ophthalmol 79:1, 1992 10. Dell'Osso LF, Van der Steen J, Steinman RM, et al: Foveation dynamics in congenital nystagmus II: Smooth pursuit. Doc Ophthalmol 79:25, 1992 11. Luebke AE, Robinson DA: Transition dynamics between pursuit and fixation suggest different systems. Vision Res 28:941, 1988 12. Epelboim J, Kowler E: Slow control with eccentric targets: evidence against a position-corrective
model. Vision Res 33:361, 1993 13. Tusa RJ, Zee DS, Hain TC, et al: Voluntary control of congenital nystagmus. Clin Vis Sci 7:195, 1992 14. Skavenski AA, Steinman RM: Free headed monkeys make saccades at very high frequencies in a novel laboratory
environment. Invest Ophthalmol Vis Sci 36:S354, 1995 15. Abel LA, Dell'Osso LF, Daroff RB: Analog model for gaze-evoked nystagmus. IEEE Trans Biomed Engng BME 25:71, 1978 16. Dell'Osso LF, Daroff RB: Clinical disorders of ocular movement. In Zuber BL (ed): Models of oculomotor behavior and control. West Palm Beach: CRC Press Inc, 1981:233 17. Scudder CA: A new local feedback model of the saccadic burst generator. J Neurophysiol 59:1455, 1988 18. Keller EL: Participation of medial pontine reticular formation in eye movement generation
in monkey. J Neurophysiol 37:316, 1974 19. Fischer B, Weber H: Express saccades and visual attention. Behav Brain Sci 16:553, 1993 20. Waitzman DM, Ma TP, Optican LM, et al: Superior colliculus neurons provide the saccadic motor error signal. Exp Brain Res 72:649, 1988 21. Van Gisbergen JAM, Van Opstal AJ: Models. In Wurtz RH, Goldberg ME (eds): The neurobiology of saccadic eye movements. Reviews of Oculomotor Research, Vol. 3. Amsterdam: Elsevier, 1989:69 22. Munoz DP, Wurtz RH: Saccade-related activity in monkey superior colliculus. I. Characteristics
of burst and buildup cells. J Neurophysiol 73:2313, 1995 23. Munoz DP, Wurtz RH: Saccade-related activity in monkey superior colliculus. II. Spread
of activity during saccades. J Neurophysiol 73:2334, 1995 24. Optican L: Control of saccade trajectory by the superior colliculus. In üttner U, et al (eds): Contemporary ocular motor and vestibular research: A tribute to David A. Robinson. Stüttgart: Thieme, 1994:98 25. Optican LM: A field theory of saccade generation: Temporal-to-spatial
transform in the superior colliculus. Vision Res 35:3313, 1995 26. Kaneko CRS: Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye
movements in rhesus macaques. J Neurophysil 75:2229, 1996 27. Fukushima K, Kaneko CRS, Fuchs AF: The neuronal substrate of integration in the oculomotor system. Prog Neurobiol 39:609, 1992 28. Pola J, Robinson DA: An explanation of eye movements seen in internuclear ophthalmoplegia. Arch Neurol 33:447, 1976 29. Schmidt D, Dell'Osso LF, Abel LA, et al: Myasthenia gravis: Dynamic changes in saccadic waveform, gain and velocity. Exp Neurol 68:365, 1980 30. Hendriks AW, Enright JT: Eye movements become slower with increasing demand for accuracy. Invest Ophthalmol Vis Sci 36:S355, 1995 31. Enright JT, Hendriks AW: Cognitive influence on the relationship between peak velocity and amplitude
of saccades. Invest Ophthalmol Vis Sci 36:S597, 1995 32. Demer JL, Miller JM, Poukens V, et al: Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci 36:1125, 1995 33. Zee DS, Optican LM, Cook JD, et al: Slow saccades in spinocerebellar degeneration. Arch Neurol 33:243, 1976 34. Chase R, Kalil RE: Suppression of visual evoked responses to flashes and pattern shift during
voluntary saccades. Vision Res 12:215, 1972 35. Mitrani L, Mateef ST, Yakimoff N: Is saccadic suppression really saccadic? Vision Res 11:1157, 1971 36. Koerner F, Schiller PH: The optokinetic response under open and closed loop conditions in the monkey. Exp Brain Res 14:318, 1972 37. Abel LA, Schmidt D, Dell'Osso LF, et al: Saccadic system plasticity in humans. Ann Neurol 4:313, 1978 38. Meyer CH, Lasker AG, Robinson DA: The upper limit of human smooth pursuit velocity. Vision Res 25:561, 1985 39. Van Den Berg AV, Collewijn H: Human smooth pursuit: Effects of stimulus extent and of spatial and temporal
constraints of the pursuit trajectory. Vision Res 26:1209, 1986 40. Yasui S, Young LR: Perceived visual motion as effective stimulus to pursuit eye movement system. Science 190:906, 1975 41. Yarbus AL: Eye movements and vision. New York: Plenum Press, 1967:1 42. Krauzlis RJ, Lisberger SG: A control systems model of smooth pursuit eye movements with realistic
emergent properties. Neural Computation 1:116, 1989 43. Watamaniuk SNJ, Heinen SJ: Is the visual system's insensitivity to acceleration also evident
in the smooth pursuit system? Invest Ophthalmol Vis Sci 36:S205, 1995 44. Pola J, Wyatt HJ: Target position and velocity: The stimuli for smooth pursuit eye movements. Vision Res 20:523, 1980 45. Pola J, Wyatt HJ: Active and passive smooth eye movements: Effects of stimulus size and location. Vision Res 25:1063, 1985 46. Wyatt HJ, Pola J: Smooth eye movements with step-ramp stimuli: The influence of attention
and stimulus extent. Vision Res 27:1565, 1987 47. Pola J, Wyatt HJ: The perception of target motion during smooth pursuit eye movements in
the open-loop condition: Characteristics of retinal and extraretinal
signals. Vision Res 29:471, 1989 48. Pola J, Wyatt HJ: Offset dynamics of human smooth pursuit eye movements: Effects of target
presence and subject attention. Vision Res 37:2579, 1997 49. Barnes GR, Asselman PT: The mechanism of prediction in human smooth pursuit eye movements. J Physiol 439:439, 1991 50. Barnes GR, Asselman PT: Pursuit of intermittently illuminated targets in the human. J Physiol 445:617, 1992 51. Barnes GR: Visual-vestibular interaction in the control of head and eye movement: The
role of visual feedback and predictive mechanisms. Prog Neurobiol 41:435, 1993 52. Rubin AM, Young JH, Milne AC, et al: Vestibular-neck integration in the vestibular nuclei. Brain Res 96:99, 1975 53. Weber RB, Daroff RB: Corrective movements following refixation saccades: Type and control system
analysis. Vision Res 12:467, 1972 54. Dell'Osso LF, Troost BT, Daroff RB: Macro square wave jerks. Neurology 25:975, 1975 55. Dell'Osso LF: A dual-mode model for the normal eye tracking system and the system
with nystagmus. (Ph.D. Dissertation). In: Electrical Engineering (Biomedical. University of Wyoming, Laramie, 1968:1 56. Dell'Osso LF: A model for the horizontal tracking system of a subject with nystagmus. Proc 20th Ann Conf EMB 9:24.2, 1967 57. Dell'Osso LF: A dual-mode model for the normal eye tracking system and the system
with nystagmus. IEEE Trans Biomed Engng BME 17:87, 1970 58. Enright JT: The remarkable saccades of asymmetrical vergence. Vision Res 32:2261, 1992 59. Collewijn H, Erkelens CJ, Pizlo Z, et al: Binocular gaze movements: Coordination of vergence and version. In Ygge Y, Lennerstrand G (eds): Eye movements in reading. Wenner-Gren International Series. Oxford: Elsevier Science, 1994:97 60. Erchul DM, Jacobs JB, Dell'Osso LF: Latent nystagmus fast-phase generation [ARVO Abstract]. Invest Ophthalmol Vis Sci 37:S277, 1996 61. Zee DS, Fitzgibbon EJ, Optican LM: Saccade-vergence interactions in humans. J Neurophysiol 68:1624, 1992 62. Averbuch-Heller L, Zivotofsky AZ, Remler BF, et al: Convergent-divergent pendular nystagmus: Possible role of the vergence
system. Neurology 45:509, 1995 63. Averbuch-Heller L, Zivotofsky AZ, Das VE, et al: Investigations of the pathogenesis of acquired pendular nystagmus. Brain 118:369, 1995 64. Hung GK, Semmlow JL, Ciuffreda KJ: The near response: Modeling, instrumentation, and clinical applications. IEEE Trans Biomed Engng BME 31:910, 1984 65. St. Cyr GJ, Fender DH: The interplay of drifts an flicks in binocular fixation. Vision Res 9:245, 1969 66. Steinman RM, Haddad GM, Skavenski AA, et al: Miniature eye movement. Science 181:810, 1973 67. Doslak MJ, Dell'Osso LF, Daroff RB: A model of Alexander's law of vestibular nystagmus. Biol Cyber 34:181, 1979 68. Dell'Osso LF: Evidence suggesting individual ocular motor control of each eye (muscle). J Vestib Res 4:335, 1994 69. Abel LA, Dell'Osso LF, Schmidt D, et al: Myasthenia gravis: Analogue computer model. Exp Neurol 68:378, 1980 70. Dell'Osso LF, Jacobs JB: A robust, normal ocular motor system model with congenital nystagmus (CN) including
braking and foveating saccades. In Sharpe JA (ed): Neuro-ophthalmology at the beginning of the new millennium. Englewood: Medimond Medical Publications, 2000:107 71. Dell'Osso LF, Jacobs JB: A robust, normal ocular motor system model with latent/manifest latent
nystagmus (LMLN) and dual-mode fast phases. In SharpeJA (ed): Neuro-ophthalmology at the beginning of the new millennium. Englewood: Medimond Medical Publications, 2000:113 72. Dell'Osso LF, Jacobs JB: A normal ocular motor system model that simulates the dual-mode
fast phases of latent/manifest latent nystagmus. Biological Cybernetics 85:459, 2001 73. Jacobs JB: An ocular motor system model that simulates congenital nystagmus, including
braking and foveating saccades (Ph.D. Dissertation). In: Biomedical Engineering. Case Western Reserve University, Cleveland, 2001:1 74. Dell'Osso LF: A hypothetical fixation system capable of extending foveation in congenital
nystagmus. J Neuro-Ophthalmol 22:185, 2002 75. Jacobs JB, Dell'Osso LF: A robust, normal ocular motor system model with latent/manifest latent
nystagmus (LMLN) and dual-mode fast phases. In Kaminski HJ, Leigh RJ (eds): Neurobiology of eye movements. From molecules to behavior—Ann NY
Acad Sci 956. New York: NYAS, 2002:604 76. Jacobs JB, Dell'Osso LF: A hypothetical fixation system capable of extending foveation in congenital
nystagmus. In Kaminski HJ, Leigh RJ (eds): Neurobiology of eye movements. From molecules to behavior—Ann NY
Acad Sci 956. New York: NYAS, 2002:608 77. Garbutt S, Dell'Osso LF, Jacobs JB: “Staircase” saccadic intrusions and transient yoking and neural
integrator failures associated with cerebellar hypoplasia. p. ARVO
Abstr 1968. Annual Meeting Abstract and Program Planner [on CD-ROM
or accessed at www.arvo.org], 2003 78. Robinson DA: A method of measuring eye movement using a scleral search coil in a magnetic
field. IEEE Trans Bio Med Electron BME 10:137, 1963 79. Frens MA, van der Geest JN: Scleral search coils influence saccade dynamics. J Neurophysiology 88:676, 2002 80. DiScenna AO, Das V, Zivotofsky AZ, et al: Evaluation of a video tracking device for measurement of horizontal and
vertical eye rotations during locomotion. J Neurosci Methods 58:89, 1995 |