BRUCH'S MEMBRANE Bruch's membrane, also called the lamina vitrea, is the inner layer
of the choroid. This thin, acellular, well-delineated zone between the
retina and choroid extends from the optic nerve to the ora serrata. Composed
of elements from both the retina and the choroid, Bruch's
membrane is an integral part of the choroid. From internal to external, the
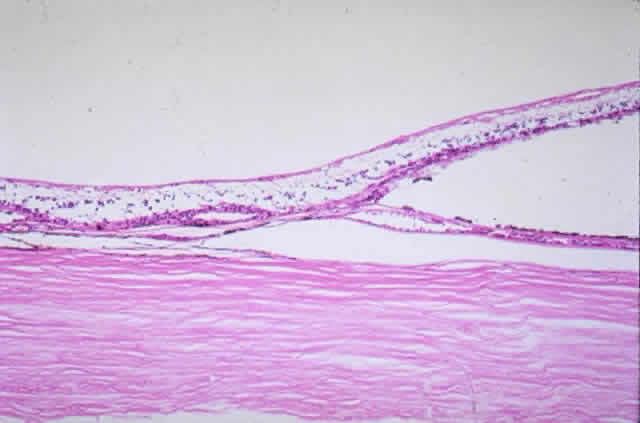
membrane is formed of five layers: the basement membrane of the RPE, the inner collagenous zone, the elastic tissue layer, the outer collagenous zone, and the basement membrane of the choriocapillaris (Fig. 8). 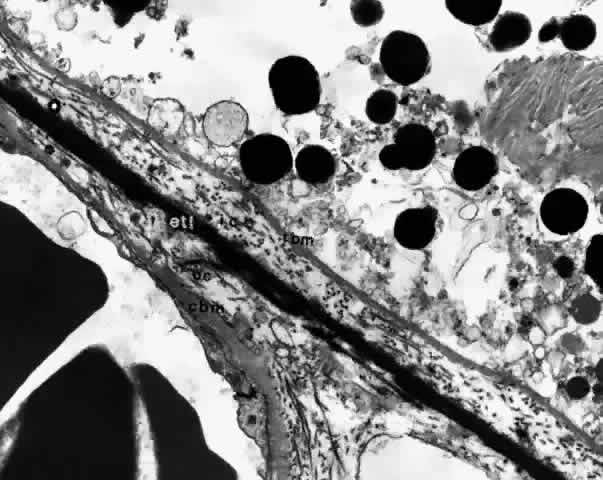 Fig. 8. Bruch's membrane. Basement membrane of retinal pigment epithelium (rbm), inner collagenous zone (ic), elastic tissue layer (etl), outer collagenous zone (oc) and basement membrane of choriocapillaris (cbm). (× 14,500) Fig. 8. Bruch's membrane. Basement membrane of retinal pigment epithelium (rbm), inner collagenous zone (ic), elastic tissue layer (etl), outer collagenous zone (oc) and basement membrane of choriocapillaris (cbm). (× 14,500)
|
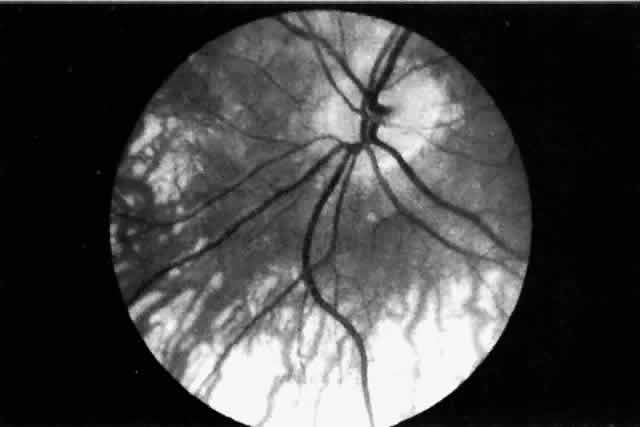
Bruch's membrane is thickest near the optic disc, measuring 2 to 4 μm, and
gradually decreases in thickness to 1 to 2 μm peripherally.16 The innermost layer, the basement membrane of the RPE, is a continuous
membrane measuring 0.3 μm thick. The outer layer, the basement membrane
of the choriocapillaris, is 0.14 μm thick and is discontinuous
at the intercapillary septa. The inner and outer collagenous layers
are continuous and measure 1.5 μm and 0.14 μm, respectively. The
middle elastic tissue layer is discontinuous. Normally, the layers of
Bruch's membrane are so closely interwoven that they cannot be separated
in a healthy globe.17–20 Drusen, ophthalmoscopically visible deposits present between the RPE basement
membrane and the inner collagenous layer of Bruch's membrane, are
more prevalent with increasing age (Fig. 9). A morphometric study of Bruch's membrane, the choriocapillaris, and
the choroid in aging was performed by Ramrattan and associates21 in eyes obtained from patients ranging from 6 to 100 years old with normal
maculae. The findings revealed that the thickness of Bruch's
membrane in the normal macula increases by 135% between the 1st and 10th
decades of life (from 2 to 4.7 μm), while the choriocapillaris
density and diameter and the choroidal thickness generally decreased in
a linear fashion in the same time interval. Statistical analysis of
the morphometric data showed that the thickness of Bruch's membrane
was directly related to age alone and that there was no relationship
between age-related atrophy of the choriocapillaris and changes in Bruch's
membrane thickness.  Fig. 9. Solitary nodular drusen (arrow) resting on inner surface of Bruch's membrane. (H & E, × 63) Fig. 9. Solitary nodular drusen (arrow) resting on inner surface of Bruch's membrane. (H & E, × 63)
|
Ultrastructurally, the basement membrane of the RPE and choriocapillaris
is made of fine filaments that blend with the collagen of the adjacent
collagenous zones. The basement membrane of the RPE is separated from
the cytoplasmic membrane of the RPE, from which it is derived, by a 100 nm
radiolucent zone. The cytoplasmic membrane of the RPE cell has
many infoldings, which its basement membrane usually does not follow, although
it may project slightly into the outer part of some folds.22,23 The basement membrane of the RPE is continuous with the basement membranes
of the pigmented epithelium of the ciliary body and anterior pigmented
epithelium of the iris and, therefore, extends from the optic disc
to the pupillary edge of the iris. The basement membrane of the choriocapillaris
is discontinuous at the intercapillary septa. The inner and outer collagenous zones are made up of randomly oriented
collagen fibers measuring 60 nm in diameter. Many are parallel to the
retina, and others pass from the inner collagenous zone through the elastic
layer into the outer collagenous zone. At the ora serrata, the inner
collagenous layer thickens, displacing the elastic layer outwardly. The elastic layer, the middle layer of Bruch's membrane, is a dense, irregularly
interrupted band composed of interwoven elastic tissue
fibers of various thickness. The elastic fibers are ultrastructurally
composed of long and straight rods with a homogeneous core and dense cortex. Variably
sized spaces are present between the elastic fibers. They
provide passageways for collagen fibers from the inner collagenous
zone to the outer collagenous zone and into the intercapillary septa
and subcapillary zone of the choriocapillaris (Fig. 10). The elastic tissue and collagenous layers of Bruch's membrane become
circularly oriented around the edge of the optic nerve. 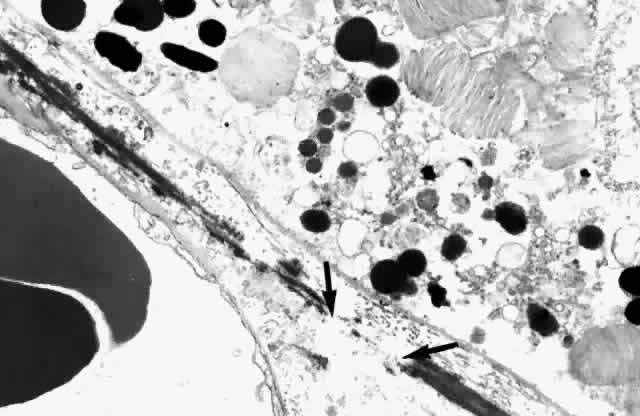 Fig. 10. Passageway between elastic fibers (between arrows). (× 12,900) Fig. 10. Passageway between elastic fibers (between arrows). (× 12,900)
|
In addition to the ultrastructurally defined components of Bruch's
membrane, vesicles, linear structures, and electron-dense bodies are
found in the collagenous and elastic zones.18,19,22,23 The ground substance, granular in appearance, may be a mucopolysaccharide-protein
complex. Nerve fibers have not been found in Bruch's
membrane.22 CHOROIDAL VASCULATURE Although the main morphologic features of the choroidal circulation are
well known, our knowledge is incomplete. The angioarchitecture of the
choroid has been studied by several in vitro methods, including intravascular
casting using neoprene latex and methyl methacrylate, scanning
electron microscopy, flat preparations of mounted choroid with and without
immunohistochemical staining, and routine histologic sections. Additionally, the
choroidal vasculature has been studied in vivo by fluorescein
angiography in normal, pathologic, and experimental eyes, with
the use of indocyanine green angiography and laser Doppler flowmetry.24–43 A major flaw of the morphologic studies that have used microvascular casting
is that they have failed to provide dynamic and physiologic information
as to the function of the choroid. The procedure of injecting
casting material into choroidal vessels under higher than normal pressures
may create vascular conduits that are otherwise absent, larger than
normal, or nonfunctional in the in vivo choroidal circulation. Arterial Blood Supply The choroid receives its arterial blood from branches of the ophthalmic artery. The nasal (medial) and temporal (lateral) short posterior ciliary arteries
and the nasal (medial) and temporal (lateral) long posterior ciliary
arteries are branches of the posterior ciliary artery (Fig. 11). The anterior ciliary arteries are all direct branches from the ophthalmic
artery, except for the one accompanying the lateral rectus muscle. This
anterior ciliary artery is derived from the ophthalmic artery
via the lacrimal artery (Fig. 12). Although the retinal vessels also arise from the ophthalmic artery, the
retinal and choroidal blood supplies are separate and distinct within
the eye and there is little, if any, connection between the two systems. 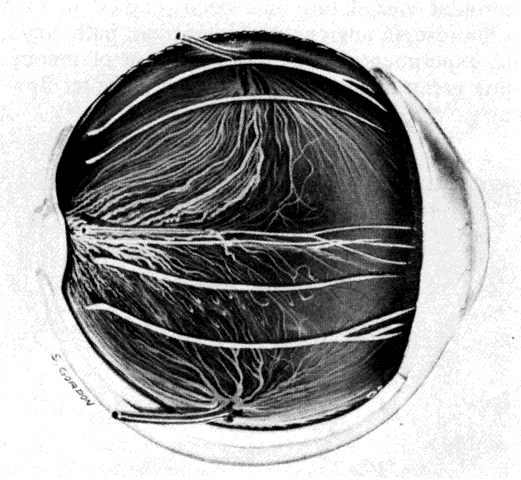 Fig. 11. Original drawing of dissected globe, showing temporal suprachoroid and
choroid. Choroidal arteries, branches of the short posterior ciliary arteries (left), appear wavy and tortuous as they radiate outward from disc. Vessels
are partially obscured by melanocytes. Long posterior ciliary artery and
nerve cross the horizontal branching near the ora. Ciliary nerves are
prominent, as they are only loosely bound by suprachoroidal lamellae. Confluence
of two vortex veins can be seen at top and bottom. (Courtesy of Stephen Gordon) Fig. 11. Original drawing of dissected globe, showing temporal suprachoroid and
choroid. Choroidal arteries, branches of the short posterior ciliary arteries (left), appear wavy and tortuous as they radiate outward from disc. Vessels
are partially obscured by melanocytes. Long posterior ciliary artery and
nerve cross the horizontal branching near the ora. Ciliary nerves are
prominent, as they are only loosely bound by suprachoroidal lamellae. Confluence
of two vortex veins can be seen at top and bottom. (Courtesy of Stephen Gordon)
|
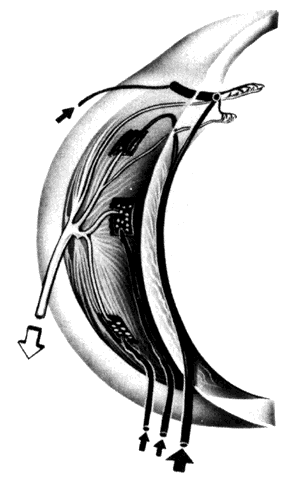 Fig. 12. Major arteries and veins of the choroid. Anterior ciliary arteries (arrow, upper left) and the long posterior ciliary artery (broad arrow, lower right) supply the major arterial circle of the iris. Arteries from the major
circle (not shown) and anterior part of the long posterior ciliary artery
send recurrent branches to the anterior and equatorial choriocapillaris. Short
posterior ciliary arteries (arrows, lower left) supply the choriocapillaris posteriorly. The two systems overlap. Vortex
veins (open arrow) drain blood from most of iris, ciliary body, and choroid. (Courtesy of Stephen Gordon) Fig. 12. Major arteries and veins of the choroid. Anterior ciliary arteries (arrow, upper left) and the long posterior ciliary artery (broad arrow, lower right) supply the major arterial circle of the iris. Arteries from the major
circle (not shown) and anterior part of the long posterior ciliary artery
send recurrent branches to the anterior and equatorial choriocapillaris. Short
posterior ciliary arteries (arrows, lower left) supply the choriocapillaris posteriorly. The two systems overlap. Vortex
veins (open arrow) drain blood from most of iris, ciliary body, and choroid. (Courtesy of Stephen Gordon)
|
POSTERIOR CILIARY ARTERIES. Great variability has been described in the vascular distribution from
the ophthalmic artery to the choroid. The ophthalmic artery most often
first gives rise to the nasal and temporal posterior ciliary arteries, which
proceed toward the globe on their respective sides of the optic
nerve. The nasal and temporal posterior ciliary arteries branch in
the orbit several times, giving rise to the short and long posterior ciliary
arteries. Occasionally a posterior ciliary artery from the ophthalmic
artery is found superior to the optic nerve. Not infrequently, the
long or short posterior ciliary arteries arise directly from the ophthalmic
artery.16,44,45 In some instances, the long posterior ciliary arteries are derived as
a branch from their respective short posterior ciliary arteries. Despite
this variability in orbital branching from the ophthalmic artery, the
pattern of the posterior ciliary vessels entering the sclera is relatively
constant (Figs. 13 and 14). 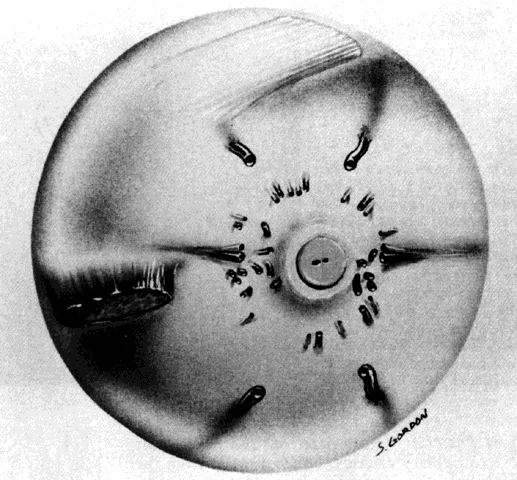 Fig. 13. Drawing of gross specimen of posterior of left globe, showing optic nerve
surrounded by short posterior ciliary arteries (SPCAs). Wreath of short
posterior ciliary nerves (lighter structures) is prominent superiorly
and inferiorly. An occasional SPCA accompanies nerves. Temporal (left of optic nerve) and nasal (right of optic nerve) canals of long posterior arteries and nerves mark the
horizontal meridian of the globe. Muscular tendon of inferior oblique
muscle (far left) partially covers canal of long temporal posterior ciliary artery and
nerve. Emissary canals of the four vortex veins lie in the oblique quadrants. Tendon
of superior oblique muscle inserts superiorly, just anterior
to superior temporal vortex vein. (Courtesy of Stephen Gordon) Fig. 13. Drawing of gross specimen of posterior of left globe, showing optic nerve
surrounded by short posterior ciliary arteries (SPCAs). Wreath of short
posterior ciliary nerves (lighter structures) is prominent superiorly
and inferiorly. An occasional SPCA accompanies nerves. Temporal (left of optic nerve) and nasal (right of optic nerve) canals of long posterior arteries and nerves mark the
horizontal meridian of the globe. Muscular tendon of inferior oblique
muscle (far left) partially covers canal of long temporal posterior ciliary artery and
nerve. Emissary canals of the four vortex veins lie in the oblique quadrants. Tendon
of superior oblique muscle inserts superiorly, just anterior
to superior temporal vortex vein. (Courtesy of Stephen Gordon)
|
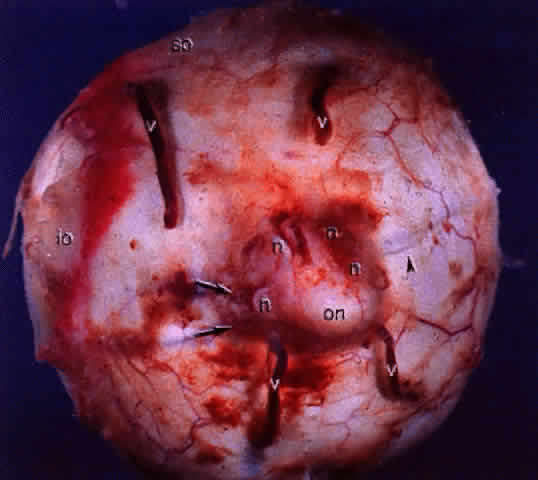 Fig. 14. Gross specimen of posterior of globe shows many of the structures shown
in Fig. 13: vortex veins (v), optic nerve (on), muscular tendon of inferior oblique (io), tendon of superior oblique (so), short posterior ciliary arteries (arrows), short posterior ciliary nerves (n), and long posterior ciliary artery and nerve (arrowhead). Fig. 14. Gross specimen of posterior of globe shows many of the structures shown
in Fig. 13: vortex veins (v), optic nerve (on), muscular tendon of inferior oblique (io), tendon of superior oblique (so), short posterior ciliary arteries (arrows), short posterior ciliary nerves (n), and long posterior ciliary artery and nerve (arrowhead).
|
There is marked interindividual variation in the areas supplied by the
nasal and temporal posterior ciliary arteries in humans as determined
by in vivo studies.46 The nasal posterior ciliary artery may supply the entire nasal choroid
and extend laterally to the level of the fovea including the optic nerve
head; its supply may stop prior to the nasal peripapillary choroid, giving
no supply to the optic nerve head; or there may be any variation
between these two extremes. The temporal posterior ciliary artery
supplies the area of the choroid not supplied by the medial posterior
ciliary artery and vice versa. When there are more than two posterior
ciliary arteries (i.e., nasal, temporal and superior) the area supplied by each of them may be
one quadrant or only a sector. Short Posterior Ciliary Arteries. Ten to 20 short posterior ciliary arteries, either directly from the ophthalmic
artery or from their respective posterior ciliary arteries, perforate
the sclera near the optic nerve. The vessels tend to cluster 2 to 2.5 mm
from the dural sheath of the disc (Fig. 15) in the horizontal meridian between the optic nerve and the wreath of
short ciliary nerves. Usually more vessels are found inferotemporal to
the scleral entrance of the temporal long posterior ciliary artery and
nerve16; however, some variability occurs. A smaller cluster of short ciliary
arteries enters nasal to the optic nerve, and a few enter the sclera above
and below the optic nerve. The short posterior ciliary vessels branch
either in the orbit, the suprachoroid, or in the outer layers of
the choroid into distal branches and smaller paraoptic branches. The distal
branches radiate toward the equator in the outermost layers of the
choroid (Fig. 16) and supply triangular areas of the choroid with the apices of the triangular
areas located posteriorly, close to their point of entry.47 The short posterior ciliary arteries terminate principally in the choroid. The
paraoptic branches of the short posterior ciliary arteries enter
straight or curve posteriorly in the choroid to supply the vertical
and peripapillary choroid either directly or via branches derived from
the circle of Haller and Zinn. Additionally, the short posterior ciliary
arteries serve the episcleral arterial plexus. Occasionally a branch
of the short posterior ciliary artery enters the retina as a cilioretinal
artery. 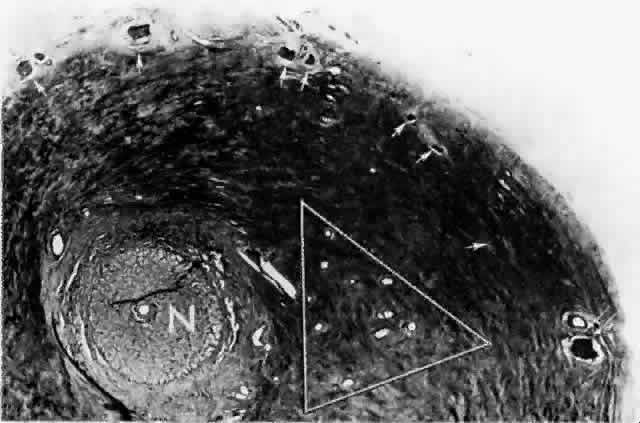 Fig. 15. Peripapillary sclera nasal to optic nerve (N). Cluster of short posterior ciliary arteries (SPCAs) (white triangle) is nasal to disc in this specimen. Other SPCAs form a ring around the
nerve. Short posterior ciliary nerves (arrows) form a wreath approximately 2 mm from the nerve. Emissary canal (thin, double arrow) for long posterior ciliary nerve (below) and artery. Cross-section at level of lamina cribrosa. (H & E, × 31) Fig. 15. Peripapillary sclera nasal to optic nerve (N). Cluster of short posterior ciliary arteries (SPCAs) (white triangle) is nasal to disc in this specimen. Other SPCAs form a ring around the
nerve. Short posterior ciliary nerves (arrows) form a wreath approximately 2 mm from the nerve. Emissary canal (thin, double arrow) for long posterior ciliary nerve (below) and artery. Cross-section at level of lamina cribrosa. (H & E, × 31)
|
 Fig. 16. Vessels of equatorial and anterior choroid are straighter and have fewer
branches than those in posterior pole. Choriocapillaris is in background. (Araki M: Observations on the corrosion casts of the choriocapillaris. Acta
Soc Ophthalmol Jpn 80:315, 1977) Fig. 16. Vessels of equatorial and anterior choroid are straighter and have fewer
branches than those in posterior pole. Choriocapillaris is in background. (Araki M: Observations on the corrosion casts of the choriocapillaris. Acta
Soc Ophthalmol Jpn 80:315, 1977)
|
The circle of Haller and Zinn is an intrascleral anastomosis between the temporal and nasal short posterior
ciliary artery branches that often forms a ring around the optic
nerve. Pial and recurrent choroidal branches originate from this anastomosis
to supply the retrolaminar optic nerve and, in a trapezoid shape, the
peripapillary and vertical meridional choroid.37 The episcleral vessels located on the posterior aspect of the globe and
the area around the optic nerve form the posterior episcleral arterial plexus. This plexus is a variable finding of rich artery-to-artery anastomoses.30 Branches to the plexus are derived from arteries on the dural sheath of
the optic nerve, the long and short posterior ciliary arteries before
scleral penetration, arteries from the inferior and superior oblique
and the deep head of the lateral rectus muscles, vessels accompanying
the short ciliary nerves, and small vessels from the surrounding loose
areolar tissue.48 The plexus also has arteriovenous connections with the vortex vein system.30 Long Posterior Ciliary Arteries. The nasal and temporal long posterior ciliary arteries arise variably—as
direct branches from the ophthalmic artery, from their respective
posterior ciliary arteries, or as branches of a short posterior ciliary
artery. Accompanied by the long posterior ciliary nerve, they pierce
the sclera 3 or 4 mm from the optic nerve and outside of the ring
of short ciliary nerves. The scleral canal of the nasal long posterior
ciliary nerve and artery, seen grossly as a blue-gray line, is a good
landmark for the horizontal meridian of the globe. On the nasal side, the
nerve lies below the artery.16 Temporally, the nerve lies superior to the artery at the external scleral
entrance. In the 3- to 7-8-mm oblique scleral canal, the temporal nerve
rotates internal to the artery and comes to lie inferior to the artery
upon entering the suprachoroidal space posterior to the equator
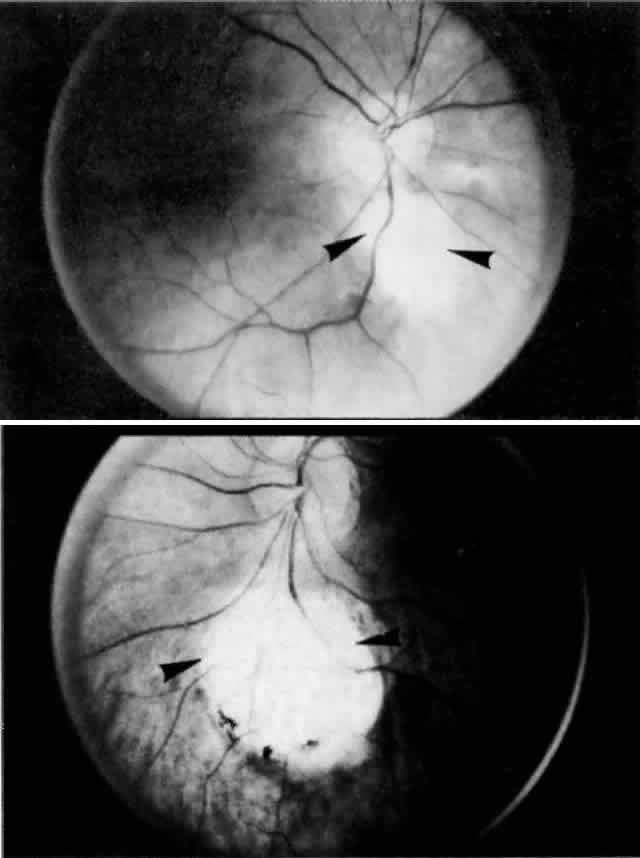
and slightly anterior to the macula (Fig. 17). Generally, the temporal long posterior ciliary artery is described as
passing through the sclera and suprachoroid without branching,16,17,29,44,45,49–52 although branching has been reported in some instances.30 A branch of the temporal long posterior ciliary artery turns posteriorly
into the choroid to supply the submacular area (Fig. 18). 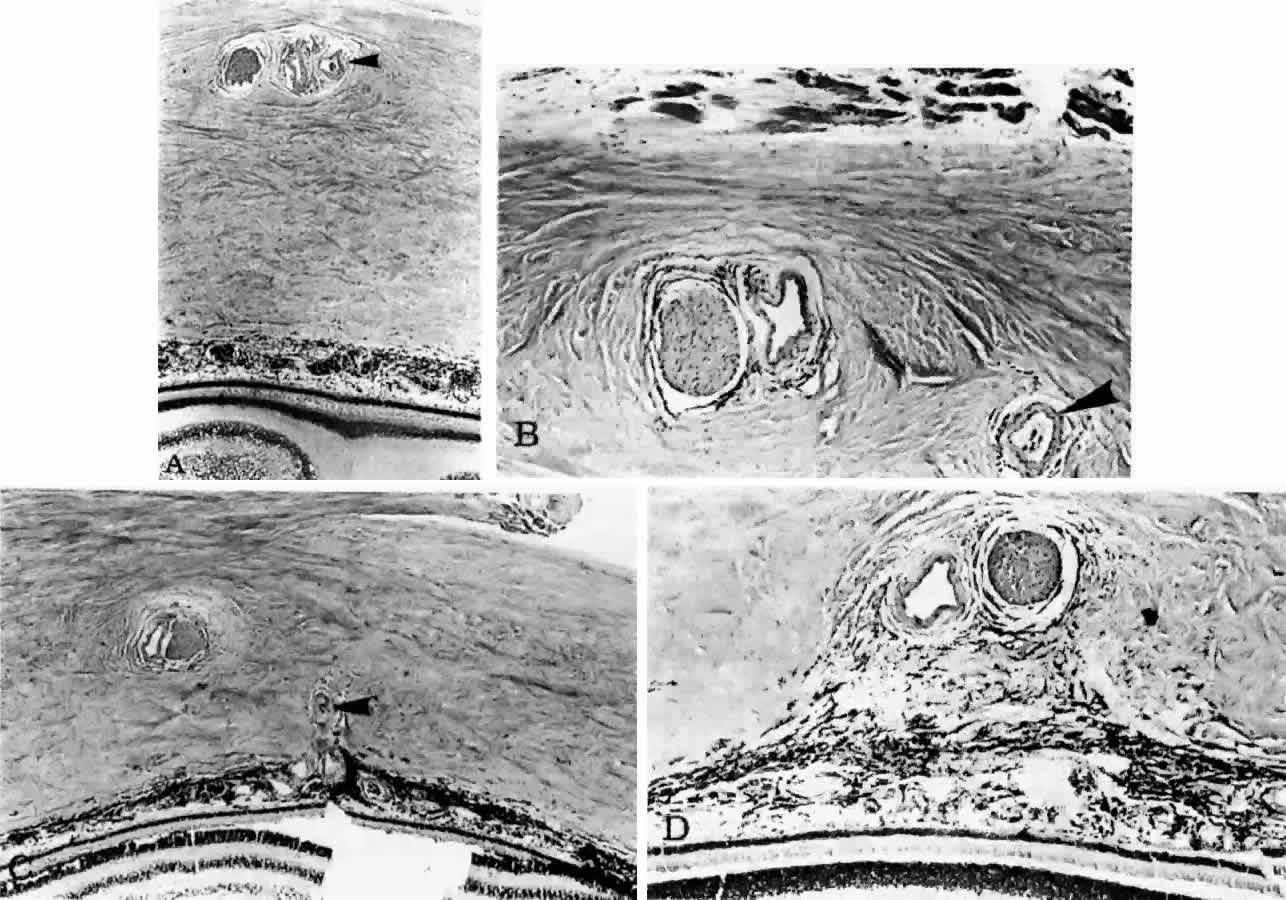 Fig. 17. Series of tangential sections showing submacular branch of long temporal
posterior ciliary artery (LTPCA). The superior pole of the globe (sectioned
perpendicular to LTPCA) is on the left; the inferior pole is on the right. A. Foveal region with long temporal posterior ciliary nerve (LTPCN) and LTPCA
with small branch (arrowhead). (H & E, × 31) B. LTPCN and LTPCA with submacular branch (arrowhead). Striated fibers of inferior oblique muscle at top. (H & E, × 125) C. Submacular branch (arrowhead) of LTPCA entering choroid. Nerve has now rotated 180° internal to
the artery and lies inferior to the artery. Inferior oblique muscle above. (H & E, × 79) D. Melanocytes and fibrocytes in suprachoroid loosely fill in area between
LTPCA and LTPCN as they enter the suprachoroid. Faint rim of perivascular-perineural
connective cells segregates nerve and artery from sclera. (H & E, × 125) Fig. 17. Series of tangential sections showing submacular branch of long temporal
posterior ciliary artery (LTPCA). The superior pole of the globe (sectioned
perpendicular to LTPCA) is on the left; the inferior pole is on the right. A. Foveal region with long temporal posterior ciliary nerve (LTPCN) and LTPCA
with small branch (arrowhead). (H & E, × 31) B. LTPCN and LTPCA with submacular branch (arrowhead). Striated fibers of inferior oblique muscle at top. (H & E, × 125) C. Submacular branch (arrowhead) of LTPCA entering choroid. Nerve has now rotated 180° internal to
the artery and lies inferior to the artery. Inferior oblique muscle above. (H & E, × 79) D. Melanocytes and fibrocytes in suprachoroid loosely fill in area between
LTPCA and LTPCN as they enter the suprachoroid. Faint rim of perivascular-perineural
connective cells segregates nerve and artery from sclera. (H & E, × 125)
|
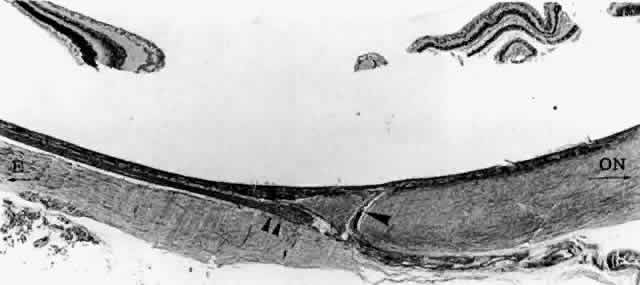 Fig. 18. Emissary canal in sclera for the long temporal posterior ciliary artery
and nerve (LTPCA and LTPCN). Submacular arterial branch (arrowhead) curves posteriorly to enter choroid abruptly. LTPCN (double arrowhead) and LTPCA slip into suprachoroidal space obliquely. Equator (E) toward left, and optic nerve (ON) toward right.(H & E, × 31) Fig. 18. Emissary canal in sclera for the long temporal posterior ciliary artery
and nerve (LTPCA and LTPCN). Submacular arterial branch (arrowhead) curves posteriorly to enter choroid abruptly. LTPCN (double arrowhead) and LTPCA slip into suprachoroidal space obliquely. Equator (E) toward left, and optic nerve (ON) toward right.(H & E, × 31)
|
Nasally, the long posterior ciliary artery does not send a branch to the
posterior pole. The main trunk of the long posterior ciliary arteries
branches at or anterior to the ora serrata, sending a few recurrent
branches to supply the anterior choroid, either directly to the choriocapillaris
or as branches from the major arterial circle of the iris. The
principal trunks of the long posterior ciliary arteries terminate
in the major arterial circle of the iris and supply the anterior uvea. ANTERIOR CILIARY ARTERIES. The muscular arteries of three rectus muscles—the medial, superior, and
inferior—originate from the ophthalmic artery and follow
their respective tendons to their scleral insertions, where they perforate
the sclera as the anterior ciliary arteries. There are two arteries
each in these rectus muscles.52 A single anterior ciliary artery courses along the lateral rectus muscle. It
originates from the lacrimal artery, which is a branch of the ophthalmic
artery. Other twigs from those muscular arteries supply the
anterior conjunctiva, episclera, and sclera near Schlemm's canal. The
anterior ciliary arteries pass through the sclera, traverse the supraciliary
space, enter the ciliary muscle, and join with the major arterial circle of the iris. The major arterial circle is not a single vessel, but rather an arterial
plexus in the root of the iris encircling the anterior uvea. Between 10 and 12 large
recurrent branches from the major arterial circle pass
posteriorly as recurrent arteries and enter the choroid to supply the
anterior choriocapillaris. Recurrent arteries anastomose with branches
of the short posterior ciliary arteries in the choroid.16,44,45,52 Choroidal arteries have the same structure as other arteries in the body. The outer adventitial layer consists of collagen fibrils oriented circumferentially around the vessels
and blending with fibers in the intervascular space. Two to three
layers of smooth muscle cells lie internal to the adventitia, forming
the muscularis. In the larger arteries, the outer smooth muscle cells lie obliquely or longitudinally. An internal elastic lamina separates the muscularis from the endothelium. A basement membrane surrounds the endothelial cells and blends with the internal elastic lamina. The
internal elastic lamina is made of elastic fibers that intermingle
on the inner side with the clusters of fine particles of the basement
membrane and on the outer side with elastic fibers (Fig. 19).16,17,22 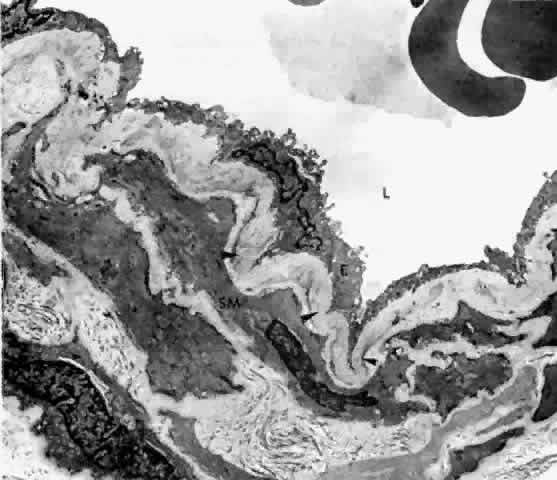 Fig. 19. Choroidal artery with folded internal elastic lamina (arrowheads) and smooth muscle cells (SM). L = Lumen; E = endothelial cell. (× 5,510) Fig. 19. Choroidal artery with folded internal elastic lamina (arrowheads) and smooth muscle cells (SM). L = Lumen; E = endothelial cell. (× 5,510)
|
The nuclei of the smooth muscle cells are cigar shaped and often contain
one or two nucleoli. The cytoplasm contains mitochondria, smooth and
rough endoplasmic reticulum, pinocytotic vesicles along the surface of
the membrane, and many myofilaments (Fig. 20). The filaments are long and parallel to each other. Dense, osmiophilic
thickenings on the myofilaments may represent Z bands where the filaments
fix for contraction.17 As the arteries diminish in caliber, they become arterioles, which have
only an intermittent layer of smooth muscle cells and no internal elastic
lamina. The adventitia is continuous with collagen fibers in the
intervascular spaces. The endothelium is continuous, and the cells are
covered by basement membrane. There is more collagen around the precapillary
arterioles than around the postcapillary venules. 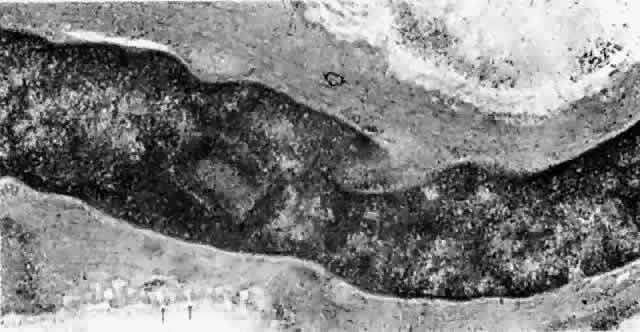 Fig. 20. Pinocytic vesicles (small arrows) and myofilaments in smooth muscle cell (open arrow). (× 43,500) Fig. 20. Pinocytic vesicles (small arrows) and myofilaments in smooth muscle cell (open arrow). (× 43,500)
|
Choriocapillaris There is disagreement in the literature regarding the appearance and organization
of the choroidal vasculature, particularly of the choriocapillaris, in
different areas of the globe (i.e., the posterior pole, equator, and periphery).27,30,36–40 Despite the controversy regarding the angioarchitecture of the choriocapillaris, one
fact is evident: There is great variation in the choroidal
pattern within different areas of the same eye and in eyes from different
individuals. This variation may explain some of the disagreement
and inconsistencies regarding the choroidal circulation. In general, the choriocapillaris appears as a single layer of broad, wide
capillaries lying in a plane internal to the arteries and veins of
the choroid and external to Bruch's membrane. The capillaries of
the choriocapillaris measure 20 to 50 μm in diameter. The density of
the capillaries is greatest in the posterior choroid.28 The capillaries of the choriocapillaris appear as a continuous meshwork
or reticulation, with intervascular spaces denoted as columns or septa. The
choriocapillaris supplies oxygen and nutrients to Bruch's
membrane and the outer third of the retina, except in the macula, where
it supplies the entire retina. It is slightly flattened under Bruch's
membrane, providing a large surface area for metabolic exchange, whereas
the external floor (scleral surface) of the capillaries is
gently undulating. Arterioles and venules join the choriocapillaris from
the external surface, either perpendicularly or obliquely, or they
come to lie in the same plane of the choriocapillaris and join the capillaries
directly or at right angles. A focus of the controversy regarding
the choroidal circulation is the organization of the choriocapillaris layer. Currently, the widely accepted concept of the choriocapillaris circulation
and anatomy is based on work first presented in the early 1970s. Based
on observations of fluorescein angiograms in monkeys, Hayreh41–43 described the choriocapillaris as a homogeneous, lobular structure with
a centrally located feeding arteriole and peripherally located collecting
venules. Noting that fluorescence did not cross lobular borders, he
concluded that the choriocapillaris had a segmental distribution and
that choroidal arteries behaved as end-arteries. Weiter and Ernest30 described the anatomy of the choroidal vasculature using vascular casts
injected with neoprene latex. By observing the vascular filling, they
were able to distinguish between precapillary arterioles and venules. They
found that the choriocapillaris was thickest and of greatest density
in the submacular region. The choriocapillaris in the peripheral
choroid was found to have a specific pattern in which a precapillary
arteriole supplied a circular capillary area 1 to 2 mm in diameter. Venule
drainage separated contiguous circular capillary beds, possibly resulting
in effective end-arteries despite the fact that adjacent capillary
beds were interconnected. This distinct choriocapillaris pattern
was not evident in the equatorial and posterior choroid, where many precapillary
arterioles were directly interconnected via the choriocapillaris
without intervening venule drainage. Torczynski and Tso27 reported on the architecture of the choriocapillaris in the posterior
pole after examining choroidal flat preparations and transverse and oblique
histologic sections (Figs. 21, 22, and 23). They described the overall appearance of the posterior choriocapillaris
as a series of adjoining lobules that was striking in some preparations
and subtle in others. The center of the lobule consisted of a single
precapillary arteriole rimmed in a thick mantle of collagen measuring 15 to 25 μm
and opening perpendicularly or curvilinearly into
a capillary bed that radiated an average distance of 300 to 400 μm
before changing from a radial to a circumferential direction. The circumferential
capillaries in the periphery of the lobule were wider and
converged from several directions, forming star-like or dendritiform configurations
in the plane of the choriocapillaris. Venular openings, outward
bulgings of the external choriocapillaris called atria, measured 30 to 37.5 μm and were present singly and in linear sequences
underlying the circumferential capillaries. The often incomplete lobules
varied in their geometric configuration, having three to six sides
and ranging in area from 420 × 605 μm to 800 × 1200 μm. The
lobular unit was thought to provide a preferred outflow route
via the perimeter of postcapillary venules so that cross-flow from
lobule to lobule would not normally occur, and thus the precapillary
arteriole would function as an end-arteriole. 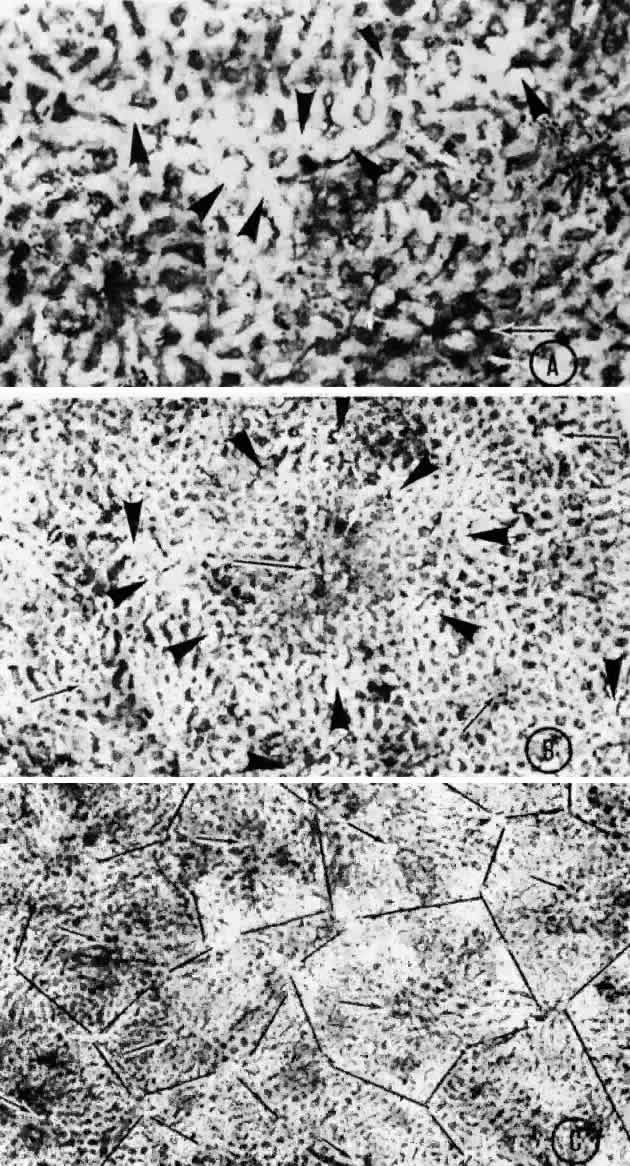 Fig. 21. Flat preparation of choriocapillaris, posterior pole. A. Arrowheads indicate oval openings to the postcapillary venules. The area
around the precapillary arteriole (white-bordered arrow) is stained more heavily because of residual subcapillary collagen. (PAS, × 180; AFIP
Neg 74-9984) B. Postcapillary venules (arrowheads) form an irregular ring bordering the capillaries that radiate from the
precapillary arteriole (white-bordered arrows), thus outlining a single lobule. The capillaries are broader and clearer
near the venules because of less subcapillary collagen. (PAS, × 100, AFIP
Neg 74-10240) C. The openings of the postcapillary venules (as shown above) are connected
with black lines; they demarcate adjoining lobules in the choriocapillaris. Capillaries
from adjoining lobules enter the intervening venules. The
lobules form a mosaic of adjoining vascular beds. Precapillary
arterioles are indicated by white-bordered arrows. (PAS × 55; AFIP
Neg 74-9985) Fig. 21. Flat preparation of choriocapillaris, posterior pole. A. Arrowheads indicate oval openings to the postcapillary venules. The area
around the precapillary arteriole (white-bordered arrow) is stained more heavily because of residual subcapillary collagen. (PAS, × 180; AFIP
Neg 74-9984) B. Postcapillary venules (arrowheads) form an irregular ring bordering the capillaries that radiate from the
precapillary arteriole (white-bordered arrows), thus outlining a single lobule. The capillaries are broader and clearer
near the venules because of less subcapillary collagen. (PAS, × 100, AFIP
Neg 74-10240) C. The openings of the postcapillary venules (as shown above) are connected
with black lines; they demarcate adjoining lobules in the choriocapillaris. Capillaries
from adjoining lobules enter the intervening venules. The
lobules form a mosaic of adjoining vascular beds. Precapillary
arterioles are indicated by white-bordered arrows. (PAS × 55; AFIP
Neg 74-9985)
|
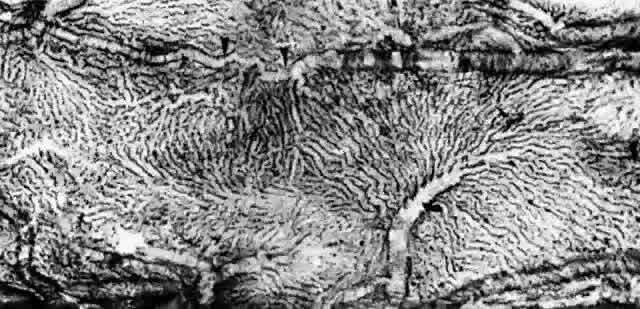 Fig. 22. Choriocapillaris in periphery has long, thin intercapillary septa. Arteriole
in capillary plane (curved arrow). Venous anastomosis (arrowheads). (AFIP Neg 73-11415 and 12110; PAS, × 50) Fig. 22. Choriocapillaris in periphery has long, thin intercapillary septa. Arteriole
in capillary plane (curved arrow). Venous anastomosis (arrowheads). (AFIP Neg 73-11415 and 12110; PAS, × 50)
|
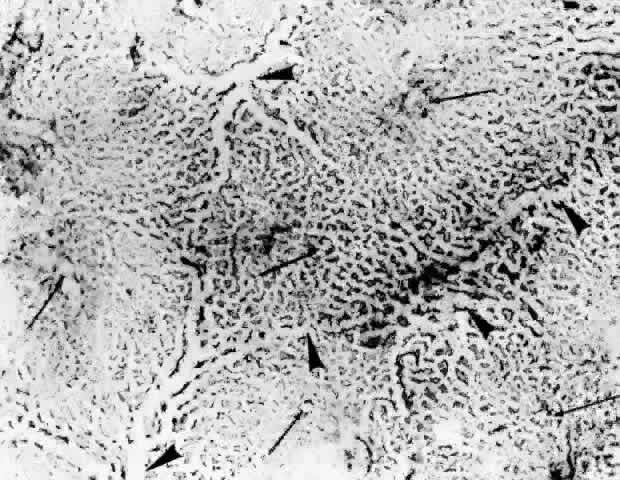 Fig. 23. Prominent lobular pattern in choriocapillaris with venules (arrowheads) surrounding arterioles (arrows). (AFIP Neg 75-3895; PAS, × 55) (Torczynski E, Tso M: The architecture of the choriocapillaris at the posterior
pole. Am J Ophthalmol 81:428, 1976) Fig. 23. Prominent lobular pattern in choriocapillaris with venules (arrowheads) surrounding arterioles (arrows). (AFIP Neg 75-3895; PAS, × 55) (Torczynski E, Tso M: The architecture of the choriocapillaris at the posterior
pole. Am J Ophthalmol 81:428, 1976)
|
Using corrosion vascular casts and scanning electron microscopy of the
human choroid, Yoneya and Tso38 studied the angioarchitecture of the entire human choroid from posterior
pole to the periphery. With painstaking dissection, they followed the
large arteries through medium-sized arteries to the precapillary arterioles
and traced the venules to the vortex veins. In the outer choroidal
layers, they found that the large choroidal arteries and veins between
the macula and optic disc were tortuous and interlacing, whereas
temporal to the macula to the equator they were arranged in an orderly
parallel manner. The medium-sized choroidal arteries and veins in the
middle choroidal layers repeatedly branched and formed interarterial
and intervenous anastomoses posteriorly, but from the equator to the
periphery they ran parallel to each other. The anastomoses were thought
to distribute blood in the choroid and to prevent the medium-sized
arteries from functioning as terminal vessels. The angioarchitecture of the choriocapillaris varied from the posterior
pole to the equator. At the posterior pole, a lobular arrangement of
the choriocapillaris was seen similar to that described by Torczynski
and Tso.27 In the equatorial region, the capillaries of the choriocapillaris ran
a more direct course, joining the precapillary arterioles to the postcapillary
venules in a spindle shape. Two or three arterioles were noted
to feed a spindled segment of the choriocapillaris, which drained into
one venule that drained adjacent segments of choriocapillaris. The
arterioles joined and the venules drained the choriocapillaris obliquely
from the scleral side. Although several adjacent spindle segments of
the choriocapillaris draining into one postcapillary venule could be
interpreted as a lobule with a central venule, the distinct lobular pattern
evident in the posterior pole was not seen in the equator. At the
periphery, the precapillary arterioles and postcapillary venules ran
parallel in the plane of the choriocapillaris. The capillaries either
joined adjacent arterioles and venules at right angles, forming a ladder
pattern, or they fanned out from the terminal arterioles to the venules. McLeod and Lutty40 published a study of the human choroid, which was prepared by flat embedding
in glycol methacrylate after immunostaining with alkaline phosphatase. The
stained choroidal sections were then examined macroscopically
and histologically. A differential immunostaining pattern for the
alkaline phosphatase permitted arteries and veins to be distinguished: arteries
generally stained less intensely than venular and capillary
endothelium. Their findings revealed a lobular organization of the choriocapillaris
in the posterior pole; however, in contrast to the findings
of other authors, a draining venule was located in the center of the
lobule, and feeding arterioles were found to be distributed around
the periphery of the lobule. A similar lobular pattern of the choriocapillaris
with a central postcapillary venule and peripheral precapillary
arterioles was demonstrated at the equator. The peripheral choriocapillaris
was shown to have a ladder-like pattern, similar to that described
by Yoneya and Tso.38 Fryczkowski39 reported on the variation in the choriocapillaris from region to region
in the choroid as determined by vascular casting and scanning electron
microscopy. In his study, arteries and veins were subjectively differentiated
by the appearance of endothelial nuclear impressions on the
surface of the intraluminal vascular casts. Accordingly, the endothelial
nuclear indentations of arteries were spindle shaped, whereas in veins
the endothelial nuclear indentations were round to oval and randomly
distributed. The choriocapillaris appeared as a regular honeycomb
pattern of freely interconnected capillaries with no evidence of a lobular
arrangement when viewed from the retinal aspect in the peripapillary
and submacular areas. A lobular appearance of the choriocapillaris
became evident in the posterior pole approximately 1 mm temporal, superior, and
inferior to the submacular area and extending to the equator. The
lobular choriocapillaris in the posterior pole and equatorial areas
were found to have a collecting venule in the center and peripheral
feeding arterioles of the anatomic lobule in 86% of cases. The arterioles
and veins opened into the choriocapillaris either at 90° angles
or tangentially. Peripherally, the lobular arrangement changed into
a palm-leaf or fan-shaped pattern, with the choriocapillaris terminating
at the ora serrata. The feeding arterioles and collecting venules
ran in the same plane as the capillaries. Fryczkowski39 introduced the concept of the choroidal functional vascular unit (Fig. 24) to explain the inconsistencies between his anatomic model of the choriocapillaris (lobules
with a central precapillary venule and peripheral
precapillary arterioles) and the functional choroidal filling pattern
described in angiographic studies. The choroidal functional vascular
unit is independent of the anatomic appearance of the choriocapillaris
and consists of two major parts. The first is a centrally located main
feeding arteriole with a centrifugal arterial capillary system. The
second part is formed by centripetal capillaries from a few surrounding
collecting venules. The mode of blood flow through the choroidal functional
vascular unit is dependent on the blood pressure gradient that
occurs on the border between the arterial and venous capillaries. Pulsatile
blood flow from the central arteriole into the centrifugal low-pressure
venous capillaries results in the appearance of lobular choriocapillaris
filling. Perturbations in the usual blood-flow gradient in
physiologic and pathologic conditions could result in blood flow from
one functional lobule to another. Stated another way, the direction
of blood flow out of the choriocapillaris lobule is determined by the peripheral resistance. Because this resistance is usually lower in the peripheral collecting
venules of the lobules than in adjacent lobular units, the blood moves
into the veins rather than into contiguous capillaries. Presumably, if
the resistance in all the collecting venules were elevated, blood could
flow from lobule to lobule in the choriocapillaris. 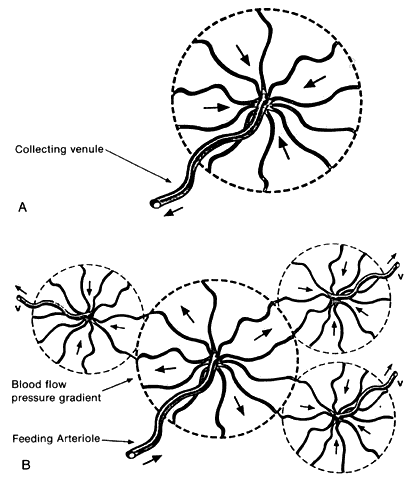 Fig. 24. Choroidal functional vascular unit as proposed by Fryczkowski. A. Anatomical choroidal lobule based on the vascular casts and SEM study. Note
centripetal arrangement of the capillaries. Collecting venule (long, thin arrow) originated from the center of the lobule forming 90° angle with choriocapillaries. B. Functional choroidal lobule outlined by the pressure gradient based on
the fluorescein and ICG angiographic study. Centrifugal arrangement of
the capillaries originated from arteriole. Feeding arteriole reaches
the choriocapillaries at 90° angle. (Courtesy of A. Fryczkowski, MD) Fig. 24. Choroidal functional vascular unit as proposed by Fryczkowski. A. Anatomical choroidal lobule based on the vascular casts and SEM study. Note
centripetal arrangement of the capillaries. Collecting venule (long, thin arrow) originated from the center of the lobule forming 90° angle with choriocapillaries. B. Functional choroidal lobule outlined by the pressure gradient based on
the fluorescein and ICG angiographic study. Centrifugal arrangement of
the capillaries originated from arteriole. Feeding arteriole reaches
the choriocapillaries at 90° angle. (Courtesy of A. Fryczkowski, MD)
|
Fryczkowski's choroidal functional vascular unit is similar to the
anatomic choriocapillary lobules described by Weiter and Ernest,30 Torczynski and Tso,27 and Yoneya and Tso.38 Its importance resides in the fact that it incorporates the role of the
blood pressure gradient in the determination of blood flow through the
choriocapillaris, a concept that could not be documented by postmortem
studies of the choroidal vasculature, although it had been suggested
by previous authors. The choriocapillaris consists of a continuous layer of fenestrated endothelial
cells surrounded by a basement membrane similar to other visceral
capillaries (i.e., capillaries in the renal glomerulus and small intestine). The circular
fenestrations, 60 to 80 nm in diameter, are covered with a thin diaphragm
consisting of a layer of attenuated cytoplasm with a central thickening
of 30 nm. The fenestrations are abundant and evenly distributed
on the inner wall of the capillaries. They seem to play an important
role in permitting the passage of glucose and vitamin A to the RPE and
retina. The nuclei of the endothelial cells are usually located on the
external side of the capillaries,22,28,53 where a decreased number of fenestrations are present.17,22,54,55 The nucleus of the endothelial cell is round, oval, or indented and contains
one or more nucleoli. The cytoplasm contains mitochondria, smooth
and rough endoplasmic reticulum, free ribonucleic particles, and some
pinocytotic vesicles, which are more prominent on the choroidal side.22 A basement membrane of finely granular material surrounds the endothelium
of the capillary. Pericytes, surrounded by the basement membrane, are
occasionally found in the external wall of the capillary, but a complete
investment of the capillaries by pericytes is not found.22,23,54,56,57 Between the endothelial cells, there are discontinuous tight junctions (i.e., zonulae occludentes); the parallel strands of the tight junctions between
endothelial cells are numerous in places and absent in others.53 Gap junctions (Fig. 25) are found near the basement membrane on the scleral side of the capillaries
and between pericytes and endothelial cells.53  Fig. 25. A. Gap junction (arrowhead) on the plasma membrane of a choroidal capillary endothelial cell. The
junction lies close to an intercellular suture (× 62,000). B. Detail of gap junction (× 160,000) (Spitznas M, Reale E: Fracture faces of fenestrations and junctions of
endothelial cells in human choroidal vessels. Invest Ophthalmol Vis Sci 14:98, 1975) Fig. 25. A. Gap junction (arrowhead) on the plasma membrane of a choroidal capillary endothelial cell. The
junction lies close to an intercellular suture (× 62,000). B. Detail of gap junction (× 160,000) (Spitznas M, Reale E: Fracture faces of fenestrations and junctions of
endothelial cells in human choroidal vessels. Invest Ophthalmol Vis Sci 14:98, 1975)
|
Collagen from Bruch's membrane extends down into the intervascular
columns or septa, and fibers in small bundles swirl outward and around
the external wall of the capillaries, intermingling with collagen in
the subcapillary layer. The capillaries are thus fixed in a rather rigid
framework with the lumina held open. The septa and subcapillary layer
are acellular, although occasional fibrocytes, but no melanocytes, are
seen in the subcapillary layer. No smooth muscle cells are found
in the capillary layer. Nerve fibers and ganglion cells are present external
to the subcapillary layer. Structural modifications that would
allow collapse or closure of the capillaries in healthy tissue are not
present.17 In histologic preparation, the capillaries are usually open, although
they may not contain any blood. Venous Drainage The arterial and venous systems in the choroid do not parallel each other
as do most arterial-venous systems in the body. Most of the vessels
of the outer choroid, except those near the disc and under the macula, are
veins. These veins carry blood from the anterior uvea, equator, and
posterior pole to drain the entire choroid via the vortex veins (Fig. 26). As smaller choroidal veins merge to form larger veins, venous anastomoses
are frequent. Before entering the vortex veins, exiting blood is
pooled in ampullae, which are dilated vascular spaces up to 5 mm long and 2 mm wide.17 Each ampulla narrows as it becomes the vortex vein at the inner opening
of the scleral canal. Two or three ampullae may drain into one vortex
vein before its descent into the scleral canal. The four vortex veins
formed by the confluence of choroidal veins lie in oblique quadrants, two
superiorly and two inferiorly. The vortex veins lie 2.5 to 3.5 mm
behind the equator, closer to the vertical meridian than to the horizontal. The
superior and inferior vortex veins drain into their respective
superior and inferior orbital veins, which in turn exit the orbit
through the superior and inferior orbital fissures, respectively. 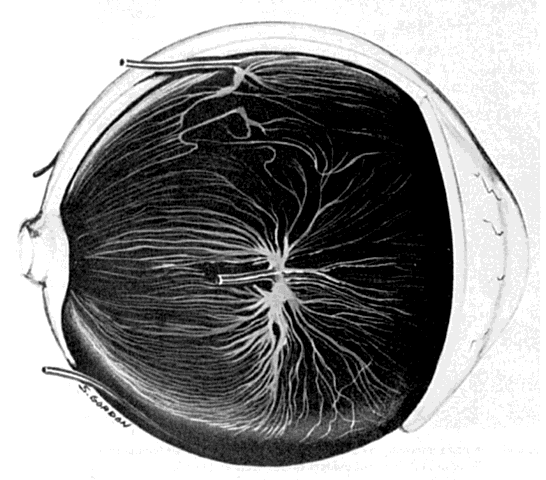 Fig. 26. Veins of anterior and posterior uvea drain into ampulla (center) and vortex vein. Short posterior ciliary nerves are removed. (Courtesy of Stephen Gordon) Fig. 26. Veins of anterior and posterior uvea drain into ampulla (center) and vortex vein. Short posterior ciliary nerves are removed. (Courtesy of Stephen Gordon)
|
The walls of choroidal veins consist of an inner lining of endothelial
cells, a media with a few irregularly and intermittently spaced smooth
muscle cells, and a thin adventitia of fibrocytes and collagen fibers (Fig. 27). The structure of all the choroidal veins, including the vortex veins, is
virtually identical. The endothelial lining is continuous. The largest
veins have a diameter as large as 300 μm; and the smallest veins, including
the postcapillary venules, are 10 to 40 μm in diameter. The
endothelial cells of the choroidal venules have discontinuous
zonulae occludentes (Fig. 28).53  Fig. 27. Thin-walled choroidal vein lined internally by endothelial cell (e) with its basal lamina (bl), and externally by a fibrocyte (f). Smooth muscle is not evident in this section. (× 4750) Fig. 27. Thin-walled choroidal vein lined internally by endothelial cell (e) with its basal lamina (bl), and externally by a fibrocyte (f). Smooth muscle is not evident in this section. (× 4750)
|
 Fig. 28. Composite of fracture faces of zonulae occludentes between endothelial
cells of a choroidal venule. Extensive zonulae occludentes (5) in center. In most places the strands are branching and interconnected; in
limited areas they are entirely missing. Higher magnification (6) of delineated area of 5 with interruption (arrow) of the zonula occludens (× 46,000). Typically angulated strands
of a zonula occludens (7), seen as imprinted grooves on cell surface (× 42,000). Zonula occludens (8) with cleavage plane jumping from one endothelial cell to another, exposing
grooves on cell surface on left and ridges on cell at right (× 54,000). Arrowheads
in all figures indicate the direction of platinum-carbon
shadowing. (Spitznas M, Reale E: Fracture faces of fenestrations and junctions of
endothelial cells in human choroidal vessels. Invest Ophthalmol Vis Sci 14:98, 1975) Fig. 28. Composite of fracture faces of zonulae occludentes between endothelial
cells of a choroidal venule. Extensive zonulae occludentes (5) in center. In most places the strands are branching and interconnected; in
limited areas they are entirely missing. Higher magnification (6) of delineated area of 5 with interruption (arrow) of the zonula occludens (× 46,000). Typically angulated strands
of a zonula occludens (7), seen as imprinted grooves on cell surface (× 42,000). Zonula occludens (8) with cleavage plane jumping from one endothelial cell to another, exposing
grooves on cell surface on left and ridges on cell at right (× 54,000). Arrowheads
in all figures indicate the direction of platinum-carbon
shadowing. (Spitznas M, Reale E: Fracture faces of fenestrations and junctions of
endothelial cells in human choroidal vessels. Invest Ophthalmol Vis Sci 14:98, 1975)
|
CHOROIDAL NERVE SUPPLY The choroid is innervated by the long and short ciliary nerves (also known
as the long and short posterior ciliary nerves). The long ciliary nerves arise from the nasociliary nerve,16 a branch of the ophthalmic division (V1) of the trigeminal nerve (cranial nerve V) and carry sensory fibers from
the cornea, iris, and ciliary muscle to the trigeminal ganglion. They
also carry sympathetic fibers from the superior cervical ganglion to
the dilator pupillae. The long posterior ciliary nerves accompanied
by the long posterior ciliary arteries enter the horizontal meridian slightly
lateral to the wreath of short ciliary nerves. The short ciliary nerves arise from the ciliary ganglion and carry sensory fibers, postganglionic
parasympathetic motor fibers, and sympathetic fibers. The sensory fibers, derived
from the cornea, iris, ciliary body, and sclera, travel
to the trigeminal ganglion. The sympathetic fibers originate in the superior
cervical ganglion and innervate the blood vessels of the eye. The
motor fibers in the short ciliary nerves are parasympathetic and originate
in the Edinger-Westphal nucleus of the cranial nerve III. They
supply the sphincter pupillae of the iris and the ciliary body and are
responsible for pupil constriction and accommodation. The short ciliary
nerves form a ring around the optic nerve 2 to 3 mm from its dural
sheath16 and are evenly distributed above and below the horizontal. The short ciliary
nerves, containing both myelinated and unmyelinated fibers, enter
the suprachoroidal space 3 to 4 mm from the optic nerve—all at
about the same meridian. They give off numerous collaterals as they
pass anteriorly and progressively diminish in size.16,55 Ruskell58 described parasympathetic nerves innervating the uvea as being derived
from the facial nerve via the greater petrosal nerve and the pterygopalatine (sphenopalatine) ganglion. The main trunks of the long and short
ciliary nerves pass into the supraciliary space, but in their passage
through the suprachoroid give off branches to the choroid and probably
the sclera.16,55 The nerves branch in the suprachoroid and choroid, anastomose extensively, and
form plexi that are widely and diffusely distributed, ramifying
in a three-dimensional manner, not strictly in layers. The terminal
axons extend into the subcapillary layer but are not found in the choriocapillaris
by electron microscopy.22,56,59 Larger branches accompany the larger muscular arteries and are found in
the outer choroid; they are free nerve endings without special modification, terminating
in the arterial walls.60 Ruskell56 estimated the density of nerve endings on arterioles to be one every 2 to 8 μm. Paravenous
nerves are present, although less frequent, with
an arterial-to-venous nerve fiber ratio of 7:1. Multipolar and bipolar
ganglion cells are seen in the layers of the suprachoroid, often forming
microganglia.55,61 The long and short ciliary nerve branches in the suprachoroid contain
both myelinated and unmyelinated nerves. Nodes of Ranvier are present
in the myelinated portions of the ciliary nerves.17 The nerve bundles in the choroid, with 50 to 100 axons, lose their myelin
sheaths and are covered by Schwann cell membranes. Axons containing
synaptic vesicles contact and indent the ganglion cells. The ganglion
cells are much larger (40 μm) than other choroidal cells, and the
central nucleus has a prominent nucleolus. The cytoplasm contains mitochondria
and ribosomes. CHOROIDAL STROMA The stroma of the choroid is relatively sparse, as most of the volume is
occupied by the blood vessels. Beneath the subcapillary layer of collagen, the
arteries and veins are surrounded by collagen fibers, oriented
in all directions and evenly distributed but with no special organization. Typically, the
collagen fibers are organized in bands at 64-nm
intervals. Flat, ribbon-like elastin fibers up to 13 μm in length
are also found throughout the intervascular spaces.17,22,52 Cell processes from melanocytic and fibrocytic cells are intermixed with
the collagen. The density of cells, especially the melanocytes, is
greatest in the outer choroid and diminishes in the middle choroidal layer. Nonmyelinated
nerve fibers and ganglion cells occur in the middle
and outer choroid. The ground substance is a watery, mucinous material
of unknown composition.62 Forming an almost continuous layer in the outer choroid, melanocytes are
most abundant near the optic nerve and less dense peripherally.52 The nuclei are oval and surrounded by a double membrane. The chromatin
is evenly dispersed, and nucleoli are present.17 The cytoplasm is heavily filled with oval, membrane-bound melanin granules, and
as much as 70% of the cytoplasm may be occupied by melanosomes (Figs. 29 and 30).22  Fig. 29. Choroidal melanocytes (mel) intermixed with fibrocytes (f). Spindle-shaped melanocytes have elongated nuclei (s), polyhedral melanocytes have oval nuclei (p), and both types of melanocytes have prominent intracytoplasmic melanin
pigment granules. (× 4750) Fig. 29. Choroidal melanocytes (mel) intermixed with fibrocytes (f). Spindle-shaped melanocytes have elongated nuclei (s), polyhedral melanocytes have oval nuclei (p), and both types of melanocytes have prominent intracytoplasmic melanin
pigment granules. (× 4750)
|
 Fig. 30. Fusiform choroidal melanocyte with tapered nucleus (f) and abundant intracytoplasmic pigment granules. (× 9000) Fig. 30. Fusiform choroidal melanocyte with tapered nucleus (f) and abundant intracytoplasmic pigment granules. (× 9000)
|
The melanocytes in the middle choroidal layers are star shaped, the cell
bodies smaller, and the processes long and thin, stretching out as tentacles
from the cell body. The processes connect with cells in adjoining
layers but do not seem to plunge deeply across several lamellae into
the more inner layers. The melanosomes, 0.3 to 0.4 μm in diameter, are
all about the same size in a given person, and are fine and evenly
distributed in the cytoplasm.52 The melanin granules are lighter brown and smaller than in the RPE. The
cytoplasm also contains free ribonucleic acid granules, a few mitochondria, Golgi
apparatus, rough endoplasmic reticulum, vesicular and lamellar
elements, and centrioles. Some melanocytes contain many mitochondria
and smaller melanin granules, less than 0.2 μm in diameter.5 Freeze-fractured melanocytes reveal melanosomes as membrane-limited organelles
with a uniform, finely divided, particulate inner structure and
no discernible internal arrangement.63 The fibrocyte is the most common nonpigmented cell found in the choroid (Fig. 31). Its long, spindle-shaped body and processes intermingle with the melanocytes
in the outer choroid, forming syncytia. They are present throughout
the vessel layers and only rarely are seen in the subcapillary
zone of collagen. The cytoplasm of the fibrocyte contains mitochondria, Golgi
apparatus, centrioles, free ribosomes, and rough endoplasmic reticulum.17,22  Fig. 31. Choroidal fibrocytes display nuclei with several nucleoli (n). Extracellular collagen is apparent. (× 9000) Fig. 31. Choroidal fibrocytes display nuclei with several nucleoli (n). Extracellular collagen is apparent. (× 9000)
|
In the healthy choroid, other nonpigmented cells are occasionally seen, including
macrophages, plasma cells, lymphocytes, and mast cells. Such
cells increase in number in response to inflammatory stimuli. Steptoe
and associates64 reported on the distribution, total number, regional density, and phenotype
of choroidal mast cells in different rat species. They found that
choroidal mast cells were of the connective tissue phenotype. They were
predominantly located in the outer choroidal layers associated with
arteries and arterioles more than 30 μm in diameter. The density
of the mast cells was greatest posteriorly and decreased anteriorly. Adhesion molecules are thought to be important in the migration of leukocytes across blood-retinal
and blood-aqueous barriers and are suspected to have an important
functional role in the pathogenesis of immunologic conditions involving
the eye. Duguid and colleagues65,66 examined the expression of adhesion molecules ICAM-1, LFA-1, LFA-3, ELAM-1, CD-44, and
VLA-2 in the human retina and choroid using immunoperoxidase
techniques. They found expression of ICAM-1, LFA-3, and ELAM-1 on
retinal endothelium and ICAM-1, ELAM-1, and CD44 in the region of
the external limiting membrane. In the choroid, ICAM-1 was noted convincingly
on the choriocapillaris endothelium, whereas there was less definitive
staining for LFA-3 and ELAM-1. SUPRACHOROID The interface between the choroid and sclera is called the suprachoroid. This
transition zone, composed of thin interconnected lamellar fibers
that bind the choroid and sclera together, is a potential space. The
suprachoroid measures 30 μm in thickness.17 Anteriorly, the suprachoroid is continuous with the supraciliary space. Posteriorly, it
extends to the optic nerve. Externally, it is limited
by the lamina fusca, the melanocytic layer lining the inner sclera. The 6 to 10 layers
of suprachoroid are crisscrossed by melanocytes and
fibrocytes. Nerve fibers and ganglion cells are abundant. No vessels, except
those passing through and destined for the choroid, are present
in the suprachoroid. The lamellae are in apposition to each other in
the healthy globe, but they may be separated by fluid or blood in the
diseased eye, thus revealing a series of communicating spaces called
suprachoroidal or perichoroidal spaces. In globes prepared routinely for
histologic examination, the sclera has a tendency to stretch slightly
more than the choroid, and the lamella separate, revealing the suprachoroidal
spaces, especially anterior to the scleral canals for the vortex
veins. The suprachoroidal space is rarely noticed beneath the macula
in histologic sections because of the many ciliary vessels and nerves
that pass from sclera to choroid in that location, preventing lamellar
separation. |