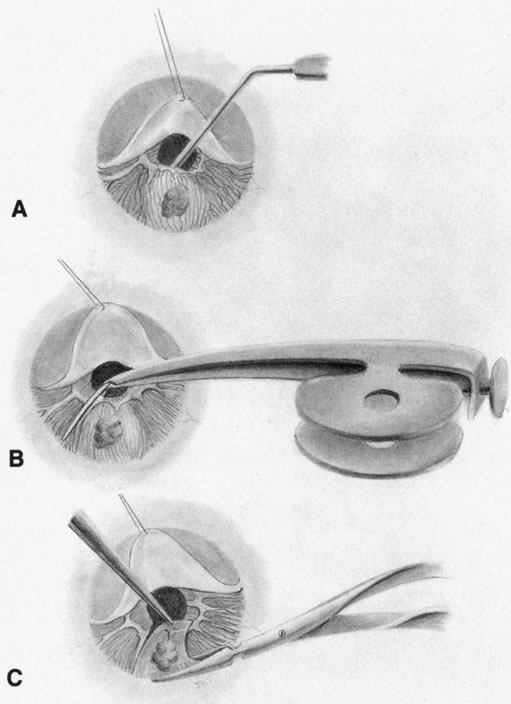
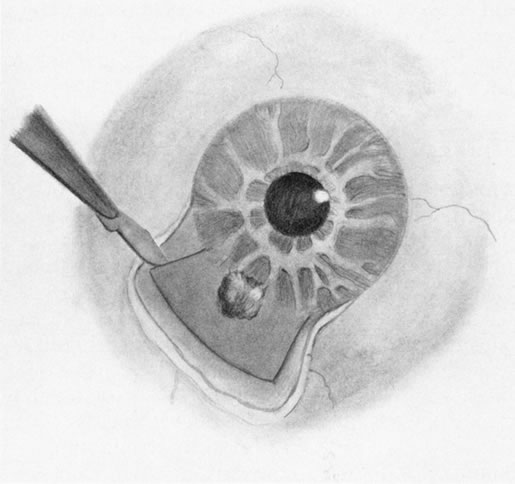
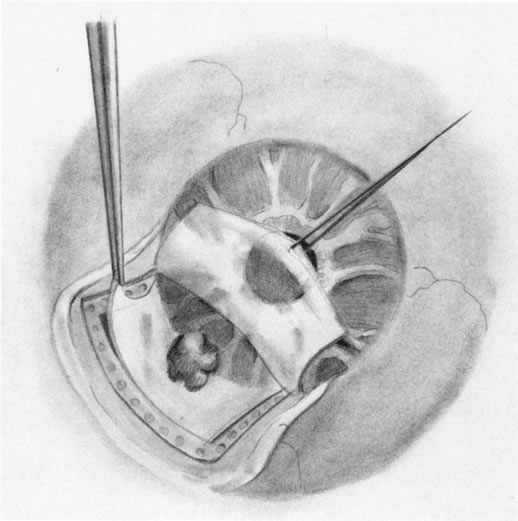
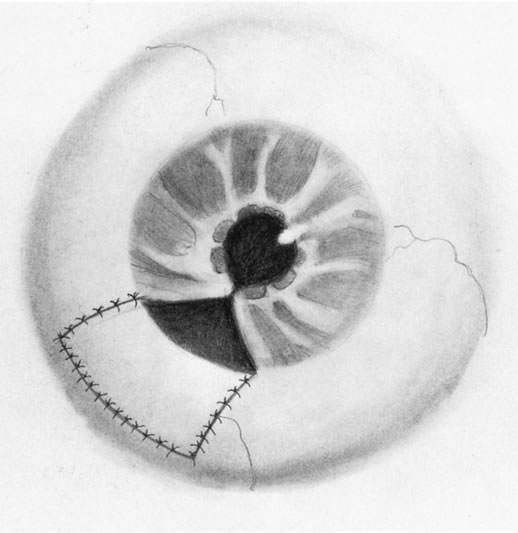
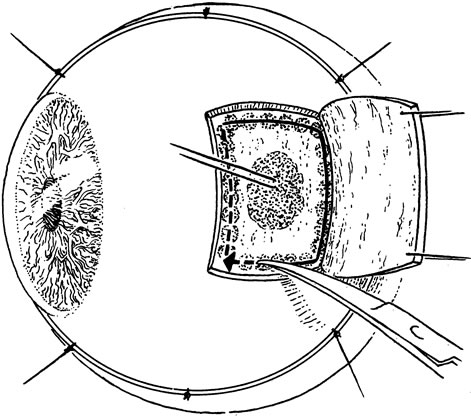
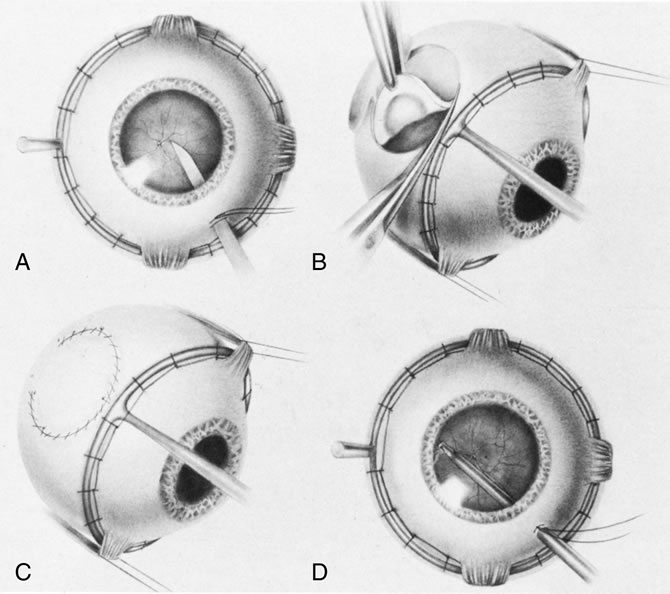
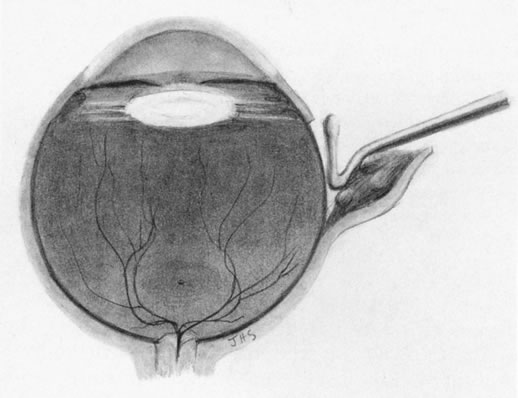
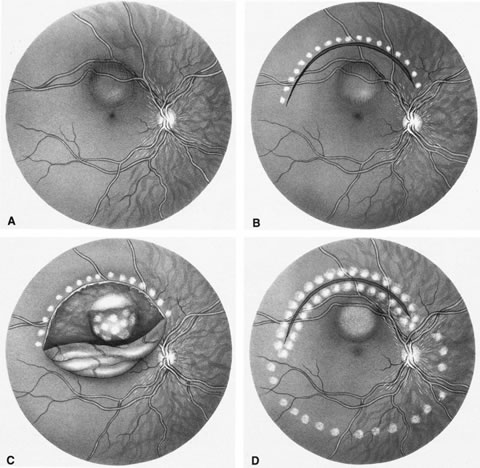
1. Foulds WS, Lee WR, Roxburgh STD, et al: Can chorioretinal biopsy be justified? Trans Ophthalmol Soc UK 104:864, 1985 2. Peyman GA, Nelson NC Jr, Paris CL, et al: Internal choroidectomy of posterior uveal melanomas under a retinal flap. Int Ophthalmol 16:439, 1992 3. Shields JA, Augsburger JJ, Brown GC, et al: The differential diagnosis of posterior uveal melanoma. Ophthalmology 87:518, 1980 4. Jakobiec FA, Coleman DJ, Chattock A, et al: Ultrasonically guided needle biopsy and cytologic diagnosis of solid intraocular
tumors. Ophthalmology 86:1662, 1979 5. Char DH, Ljung B, Miller T, et al: Primary intraocular lymphoma (ocular reticulum cell sarcoma): Diagnosis
and management. Ophthalmology 95:625, 1988 6. Gregor RJ, Chang CA, Augsburger JJ, et al: Endogenous Nocardia asteroides subretinal abscess diagnosed by transvitreal fine-needle aspiration
biopsy. Retina 9:118, 1989 7. Carroll DM, Franklin RM: Vitreous biopsy in uveitis of unknown cause. Retina 1:245, 1981 8. Taylor D, Day S, Tiedemann K, et al: Chorioretinal biopsy in a patient with leukaemia. Br J Ophthalmol 65:489, 1981 9. Augsburger JJ: Fine needle aspiration biopsy of suspected metastatic cancers to the posterior
uvea. Trans Am Ophthalmol Soc 86:499, 1988 10. Michels RG, Knox DL, Erozan YS, et al: Intraocular reticulum cell sarcoma: Diagnosis by pars plana vitrectomy. Arch Ophthalmol 93:1331, 1975 11. Kirmani MH, Thomas EL, Rao NA, et al: Intraocular reticulum cell sarcoma: Diagnosis by choroidal biopsy. Br J Ophthalmol 71:748, 1987 12. Peyman GA, Fishman GA, Sanders DR, et al: Histopathology of Goldmann-Favre syndrome obtained by full-thickness
eye-wall biopsy. Ann Ophthalmol 9:479, 1977 13. Peyman GA, Fishman GA, Sanders DR, et al: Biopsy of human scleral-chorioretinal tissue. Invest Ophthalmol 14:707, 1975 14. Shields JA, Shields CL, Shah P, et al: Partial lamellar sclerouvectomy for ciliary body and choroidal tumors. Ophthalmology 98:971, 1991 15. Shields JA, Shields CL, Donoso LA: Management of posterior uveal melanoma. Surv Ophthalmol 36: 161, 1991 16. Foulds WS: Experience with local excision of uveal melanomas. Trans Ophthalmol Soc UK 97:412, 1977 17. Lee KJ, Peyman GA, Raichand S: Internal eye wall resection for posterior uveal melanoma. Jpn J Ophthalmol 37:287, 1993 18. Rones B, Zimmerman LE: The prognosis of primary tumors of the iris treated by iridectomy. Arch Ophthalmol 60:193, 1958 19. Kersten RC, Tse DT, Anderson R: Iris melanoma: Nevus or malignancy? Surv Ophthalmol 29:423, 1985 20. Reese AB, Cleasby GW: The treatment of iris melanoma. Am J Ophthalmol 47:118, 1959 21. Shields CL, Shields JA, Materin M, et al: Iris melanoma: risk factors for metastasis in 169 consecutive patients. Ophthalmology 108:172, 2001 22. Demirci H, Shields CL, Shields JA, et al: Diffuse iris melanoma: A report of 25 cases. Ophthalmology 109:1553, 2002 23. Brown D, Boniuk M, Font RL: Diffuse malignant melanoma of iris with metastases. Surv Ophthalmol 34:357, 1990 24. Dick AD, Jagger J, McCartney AC: Refsum's disease: electron microscopy of an iris biopsy. Br J Ophthalmol 74:370, 1990 25. Preac-Mursic V, Pfister HW, Spiegel H, et al: First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmol 13:155, 1993 26. Moorthy RS, Rao NA, Sidikaro Y, et al: Coccidioidomycosis iridocyclitis. Ophthalmology 101:1923, 1994 27. MacLean H, Clarke MP, Strong NP, et al: Primary ocular relapse in acute lymphoblastic leukemia. Eye 10:719, 1996 28. Karcioglu ZA, Mullaney PB: Diagnosis and management of iris juvenile xanthogranuloma. J Pediatr Ophthalmol Strabismus 34:44, 1997 29. Chan SM, Hutnik CM, Heathcote JG, et al: Iris lymphoma in a pediatric cardiac transplant recipient: Clinicopathologic
findings. Ophthalmology 107:1479, 2000 30. Char DH, Crawford JB, Kroll S: Iris melanomas. Diagnostic problems. Ophthalmology 103:251, 1996 31. Shields JA, Shields CL: Surgical approach to lamellar sclerouvectomy for posterior uveal melanomas: The 1986 Schoenberg
lecture. Ophthalmic Surg 19:774, 1988 32. Arentsen JJ, Green WR: Melanoma of the iris: Report of 72 cases treated surgically. Ophthalmic Surg 6:23, 1975 33. Vail DT: Iridocyclectomy: a review. Am J Ophthalmol 71:161, 1971 34. LoRusso FJ, Boniuk M, Font RL: Melanocytoma (magnocellular nevus) of the ciliary body: Report
of 10 cases and review of the literature. Ophthalmology 107:795, 2000 35. Shields JA, Eagle RCJr , Shields CL: Adenoma of nonpigmented ciliary epithelium with smooth muscle differentiation. Arch Ophthalmol 117:117, 1999 36. Shields JA, Eagle RCJr , Shields CL, et al: Natural course and histopathologic findings of lacrimal gland choristoma
of the iris and ciliary body. Am J Ophthalmol 119:219, 1995 37. Broughton WL, Zimmerman LE: A clinicopathologic study of 56 cases of intraocular medulloepitheliomas. Am J Ophthalmol 85:407, 1978 38. Pavlin CJ, McWhae JA, McGowan HD, et al: Ultrasound biomicroscopy of anterior segment tumors. Ophthalmology 99:1220, 1992 39. Augsburger JJ, Affel LL, Benarosh DA: Ultrasound biomicroscopy of cystic lesions of the iris and ciliary body. Trans Am Ophthalmol Soc 94:259, 1996 40. Reminick LR, Finger PT, Ritch R, et al: Ultrasound biomicroscopy in the diagnosis and management of anterior segment
tumors. J Am Optom Assoc 69:575, 1998 41. Shields JA, Shields CL, Mercado G, et al: Adenoma of the iris pigment epithelium: A report of 20 cases: the 1998 Pan-American
Lecture. Arch Ophthalmol 117:736, 1999 42. Mannino G, Malagola R, Abdolrahimzadeh S, et al: Ultrasound biomicroscopy of the peripheral retina and the ciliary body
in degenerative retinoschisis associated with pars plana cysts. Br J Ophthalmol 85:976, 2001 43. Reese AB: Tumors of the Eye, p 286. New York, Paul B. Hoeber, 1951 44. Shields JA, Shields CL: Intraocular Tumors: A Text and Atlas. Philadelphia, WB Saunders, 1992 45. Müller HK, Lund OE, Söllner F et al: Die operative behandlung von tumoren des kammerwinkels und des ciliarkörpers. Doc Ophthalmol 20:500, 1966 46. Barrada A, Peyman GA, Palacio MN: Limitations of iris and ciliary body resection in primates. Retina 4:119, 1984 47. Gupta M, Puri P, Rennie IG: Iris seeding following iridocyclectomy for localised iris melanoma. Eye 15:808, 2001 48. Damato BE, Paul J, Foulds WS: Risk factors for residual and recurrent uveal melanoma after trans-scleral
local resection. Br J Ophthalmol 80:102, 1996 49. Stallard HB: Partial cyclectomy: Some further modifications in technique. Br J Ophthalmol 48:1, 1964 50. Flieringa HJ: A method of surgery in the angle of the anterior chamber of the eye. Trans Ophthalmol Soc UK 81:421, 1961 51. Robertson DM, Campbell RJ: Errors in the diagnosis of malignant melanoma of the choroid. Am J Ophthalmol 87:269, 1979 52. Ferry AP: Lesions mistaken for malignant melanoma of the posterior uvea: A clinicopathologic
analysis of 100 cases with ophthalmoscopically visible lesions. Arch Ophthalmol 72:463, 1964 53. Histopathologic characteristics of uveal melanomas in eyes enucleated from
the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol 125:745, 1998 54. Augsburger JJ, Shields JA, Folberg RT, et al: Fine needle aspiration biopsy in the diagnosis of intraocular cancer: Cytologic-histologic
correlations. Ophthalmology 92:39, 1985 55. Char DH, Kroll SM: Cytomorphometry of uveal melanoma: Fine needle aspiration biopsy versus
standard histology. Trans Am Ophthalmol Soc 87:197, 1989 56. Midena E, Segato T, Piermarocchi S, et al: Fine needle aspiration biopsy in ophthalmology. Surv Ophthalmol 29:410, 1985 57. Augsburger JJ, Shields JA: Fine needle aspiration biopsy of solid intraocular tumors: Indications, instrumentation
and techniques. Ophthalmic Surg 15:34, 1984 58. Karcioglu ZA, Gordon RA, Karcioglu GL: Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology 92:1763, 1985 59. Char DH, Miller T: Accuracy of presumed uveal melanoma diagnosis before alternative therapy. Br J Ophthalmol 79:692, 1995 60. Cohen VM, Dinakaran S, Parsons MA, et al: Transvitreal fine needle aspiration biopsy: The influence of intraocular
lesion size on diagnostic biopsy result. Eye 15:143, 2001 61. Peyman GA, Ericson ES, Axelrod AJ, et al: Full-thickness eye wall resection in primates: An experimental approach
for treatment of choroidal melanoma. Arch Ophthalmol 89:410, 1973 62. Peyman GA, Koziol JE: Limitation of eye wall resection. Can J Ophthalmol 9:328, 1974 63. Peyman GA, Conway MD, Karaĉorlu M, et al: Evaluation of silicone gel as a long-term or permanent vitreous
substitute in non-human primates [abstract]. Invest Ophthalmol Vis Sci 33(suppl):899, 1992 64. Foulds WS, Damato BE: Alternatives to enucleation in the management of choroidal melanoma. Aust N Z J Ophthalmol 14:19,1986 65. Peyman GA, Cohen SB: Ab interno resection of uveal melanoma. Int Ophthalmol 9:29, 1986 66. Peyman GA: Internal retinal biopsy: Surgical technique and results. Int Ophthalmol 14:101, 1990 67. Damato B, Groenewald CP, McGalliard JN, et al: Rhegmatogenous retinal detachment after transscleral local resection of
choroidal melanoma. Ophthalmology 109:2137, 2002 68. Jonas JB, Groh MJ, Rummelt V, et al: Rhegmatogenous retinal detachment after block excision of epithelial implantation
cysts and tumors of the anterior uvea. Ophthalmology 106:1942, 1999 69. Peyman GA, Charles H: Internal eye wall resection in the management of uveal melanoma. Can J Ophthalmol 23:219,1988 70. Peyman GA, Juarez CP, Raichand M: Full-thickness eye-wall biopsy: Long-term results
in 9 patients. Br J Ophthalmol 65:722, 1981 71. Peyman GA, Axelrod AJ, Graham RO: Full-thickness eye wall resection: An experimental approach for
treatment of choroidal melanoma: Evaluation of cryotherapy, diathermy
and photocoagulation. Arch Ophthalmol 91:219, 1974 72. Constable IJ, Chester GH, Horne R, et al: Human chorioretinal biopsy under controlled systemic hypotensive anaesthesia. Br J Ophthalmol 64:559, 1980 73. Peyman GA, Diamond JG, Axelrod AJ: Sclero-chorio-retinal (SCR) resection in humans. Ann Ophthalmol 6:1347, 1974 74. Peyman GA, Nelson PT, Axelrod AJ, et al: Full thickness eye wall resection: Evaluation of preoperative photocoagulation. Invest Ophthalmol 12:262, 1973 75. Peyman GA, May DR, Ericson ES, et al: Full thickness eyewall resection: An experimental approach for treatment
of choroidal melanoma. II. Homo- and heterograft. Invest Ophthalmol 11:668, 1972 76. Shields JA, Shields CL: Surgical approach to lamellar sclerouvectomy for posterior uveal melanomas: The 1986 Shoenberg
Lecture. Ophthalmic Surg 19:774, 1988 77. Peyman GA, Juarez CP, Diamond JG, et al: Ten years experience with eye wall resection of uveal malignant melanomas. Ophthalmology 91:1720, 1984 78. Zimmerman LE, McLean IW, Foster WD: Does enucleation of the eye containing a malignant melanoma prevent or
accelerate the dissemination of tumour cells? Br J Ophthalmol 62:420, 1978 79. Kertes PJ, Johnson JC, Peyman GA: Internal resection of posterior uveal melanomas. Br J Ophthalmol 82:1147, 1998 80. Freeman WR, Gross JG, Labelle J, et al: Pneumocystis carinii choroidopathy: A new clinical entity. Arch Ophthalmol 107:863, 1989 81. Durant WJ, Flood T, Goldberg MF, et al: Vitrectomy and Whipple's disease. Arch Ophthalmol 102:848, 1984 |