ANATOMIC FACTORS
Several anatomic features predispose an eye to primary angle-closure glaucoma. These relate to the size and shape of the eye, its cornea, anterior chamber, angle, lens, iris, and ciliary body.
The Eye
Ultrasonographic measurements have shown that eyes with primary angle-closure glaucoma have an average axial length 1 mm shorter than normals.16,17 Hyperopes are therefore more predisposed than emmetropes or myopes to angle closure. However, myopia does not preclude the development of angle closure.18
The Cornea
The corneal radius of curvature and diameter are usually smaller than average in primary angle-closure glaucoma eyes. There is a tendency for corneae with smaller diameters to have steeper curvatures, which partially compensate for the change in refractive power.19
The Anterior Chamber
The central anterior chamber in eyes with angle closure appears to be 1 mm shallower, on average, than that in normal eyes. It seems that the 1 mm shallowing of the anterior chamber is caused by increased volume and forward positioning of the lens.20 Eyes with anterior chamber depth greater than 2.5 mm seldom develop angle closure. According to Barkan,21 the central anterior chamber depth was less than 1.5 mm in 75% of angle-closure glaucoma cases.
It should be emphasized that the depth of the anterior chamber can vary in a given individual and in a given eye. Generally, the anterior chamber becomes more narrow with advancing age. A patient seen with a moderately narrow angle at age 50 is likely to have a much more narrow angle at age 60, and perhaps a dangerously narrow angle at age 70 or 80. This appears to be due to increasing size of the crystalline lens with age and perhaps to increasing laxity of the zonules, which allows the lens-zonular diaphragm to move anteriorly.
In addition, variations in angle depth can occur as aqueous production alters. Chandler and Grant frequently observed that an angle that is anatomically extremely narrow or closed could be opened and appear wide and nonoccludable when aqueous production was markedly reduced, such as when carbonic anhydrase therapy was instituted. Thus, a patient treated for angle-closure glaucoma in one eye might be misdiagnosed as having a nonoccludable angle in the fellow eye when, in fact, the fellow eye was extremely narrow before treatment with carbonic anhydrase inhibitors. There may be variation in the angle depth with any therapeutic agent that alters aqueous production. Similar effects could be produced as a result of other variations in aqueous production, such as the reduction in aqueous humor production after contusion to the eye, choroidal separation, or chronic uveitis.
The Lens
The average axial lens thickness is 0.6 mm greater in angle-closure glaucoma eyes compared with normals. Axial thickening of the lens and its forward displacement due to laxity of the zonules increases with age. The lens size relative to the anterior chamber volume may bring about shallowing of the anterior chamber. An eye with a normal size anterior segment may develop anterior chamber shallowing with an unusually large lens. This same effect may be seen with a normal size lens in a small anterior segment. In addition, ultrasonographic measurements of the radius of curvature of the anterior lens surface is on average 2.3 mm steeper in angle-closure glaucoma eyes than normals.22
The Angle
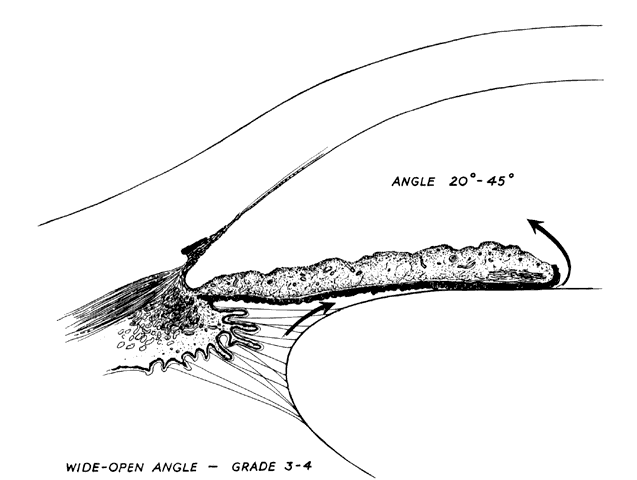
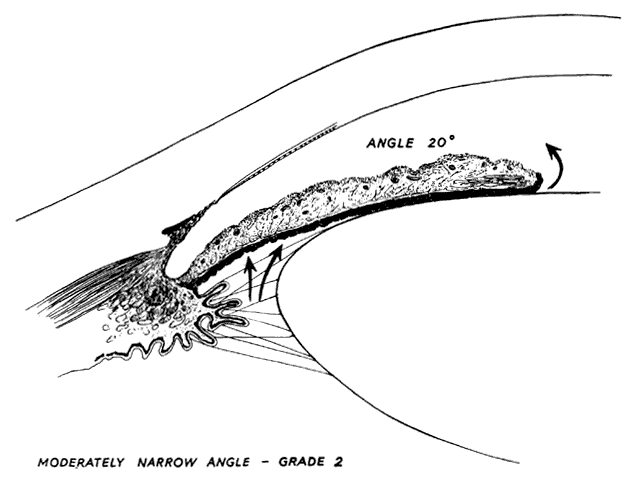
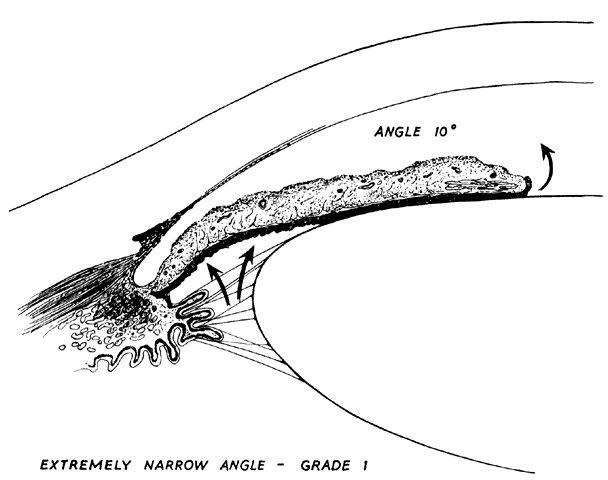
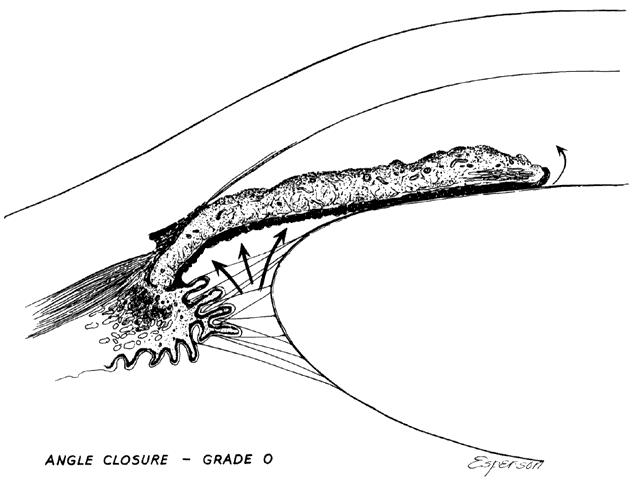
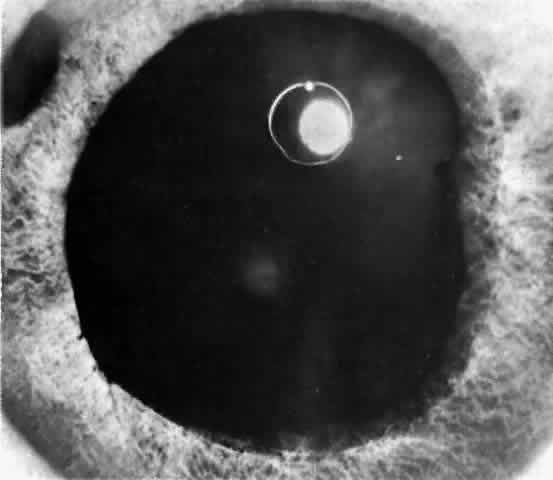
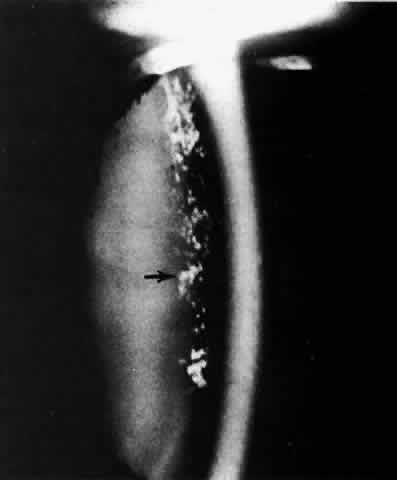
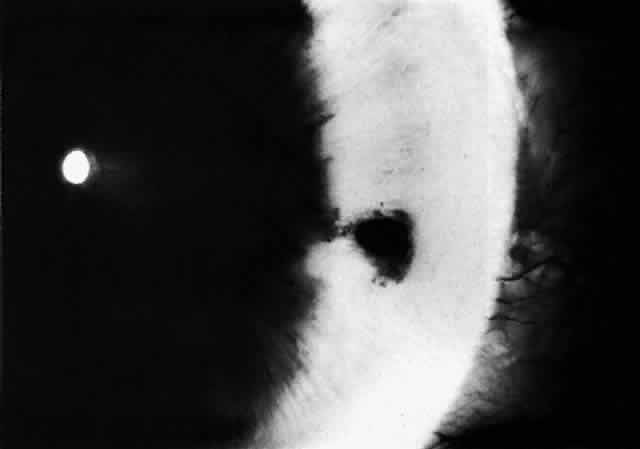
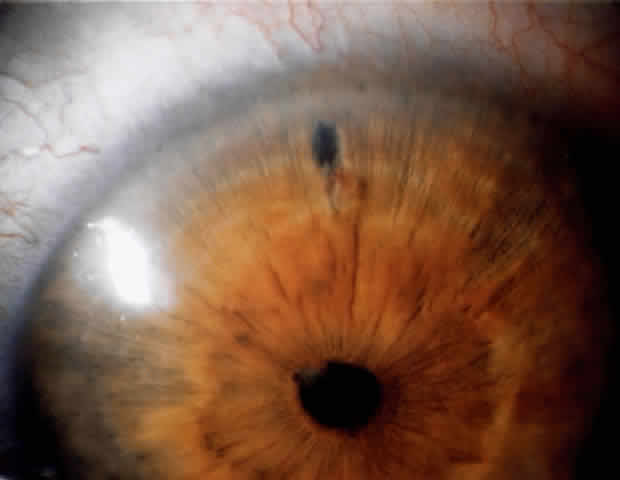
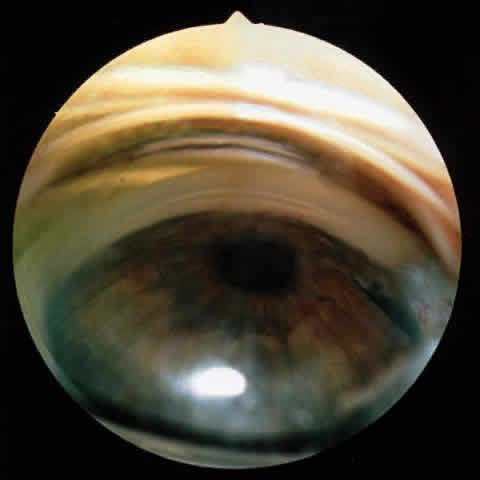
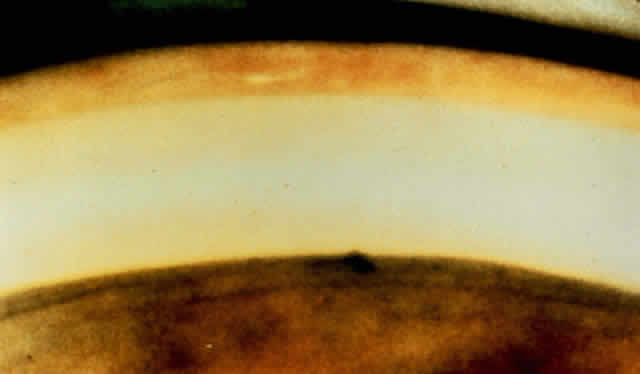
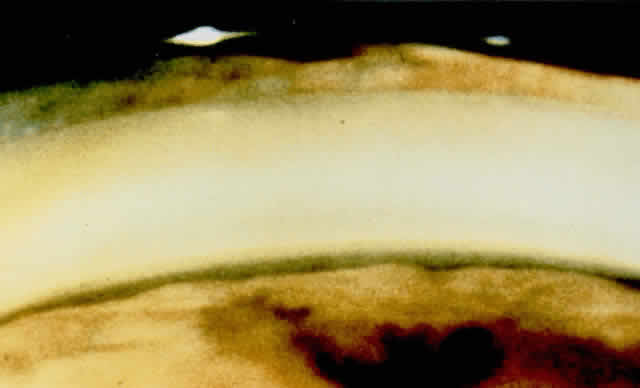
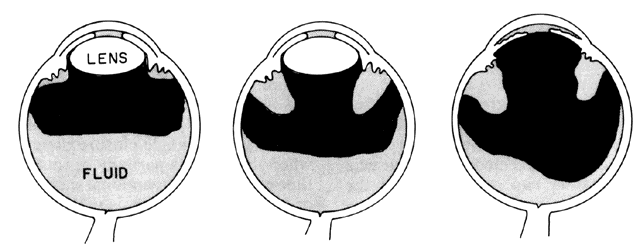
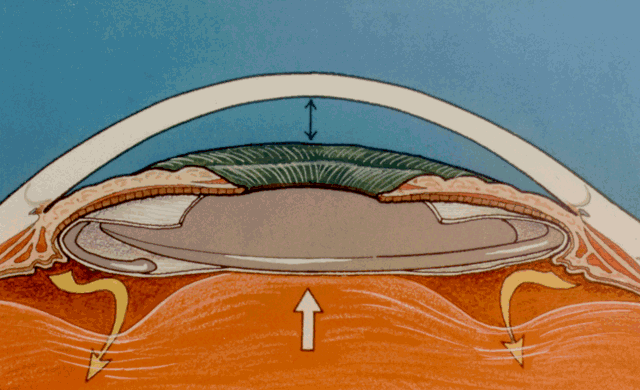
There are several classifications that have been developed in an attempt to identify those patients at risk for angle closure. Van Herick15 developed a slit lamp grading system based on the depth of the peripheral anterior chamber as measured by the adjacent corneal thickness seen in the slit lamp beam directed at the limbus. In van Herick's system, a grade 1 depth is the narrowest and a grade 4 depth is a deep anterior chamber. When a grade 1 or 2 depth is present, that is, peripheral anterior chamber depth less than one half corneal thickness, gonioscopy should be performed to rule out occludable angles. Scheie's23 grading system is based on the depth of angle structures visible on gonioscopy with a grade IV angle being narrow and visible only to Schwalbe's line. Shaffer developed the following classification for describing angle width and the likelihood of angle closure. If an angle recess width is 20 degrees or more, angle closure is improbable (Fig. 1). Angles between 10 and 20 degrees have an increased risk of developing angle closure (Fig. 2). Below 10 degrees, angle closure is probable or actually present (Figs. 3 and 4).24 When evaluating an angle, however, it is also necessary to consider the peripheral iris configuration and iris root insertion.25 Spaeth developed a gonioscopic classification system based on three elements: (1) the angle width in degrees; (2) the configuration of the peripheral iris; and (3) the insertion of the iris root.26
|
This large number of classification systems can generate confusion in communication between observers. By one grading system, a dangerously narrow angle may be described as grade IV. By another system, the same angle might be labeled grade I. The diversity of the grading systems requires both the retranslation from grade to anatomic interpretation and the identification of whose grade I or IV is being used. It seems that with frequent modern international communication of medical information, it is simpler and safer to use straightforward scientific English prose to describe angle anatomy. Chandler and Grant8 prefer to describe each angle verbally and to state whether it is judged closable or not.
The Iris and Ciliary Body
In some emmetropic and hyperopic, but rarely myopic eyes, the anterior chamber is shallow in structure with a narrow angle. This configuration can be related to heredity, a large lens, a structurally crowded anterior segment, or other anatomic characteristics. In some eyes, especially hyperopic eyes, the iris root inserts more anteriorly on the anteromedial surface of the ciliary body. The iris plane is typically convex. In addition, the ciliary body is more developed in hyperopic eyes, perhaps due to increased accommodative effort.27 These factors lead to increased narrowing of the angle recess.
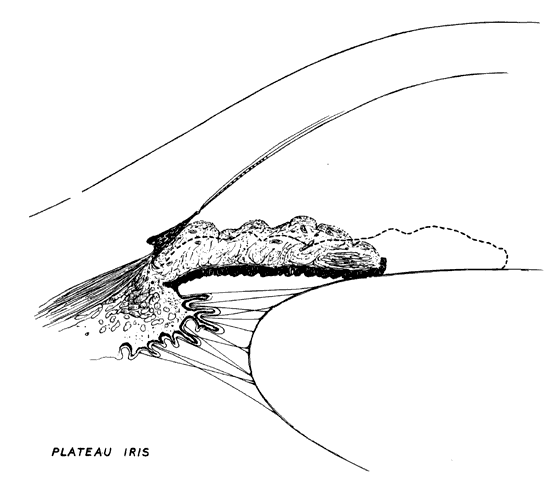
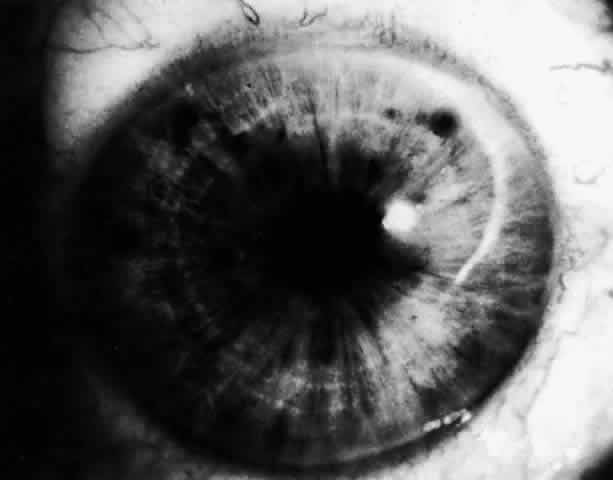
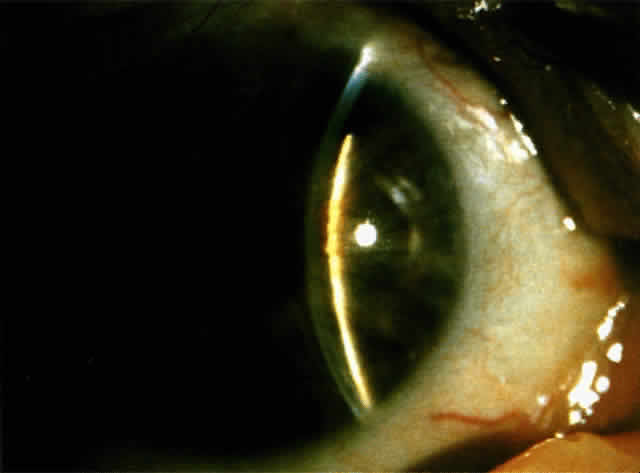
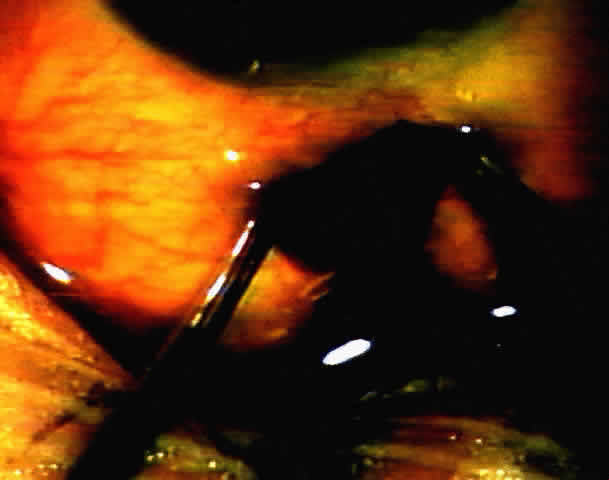
Another anatomic configuration, the so-called plateau iris configuration, may predispose to closure of the angle. This is a relatively rare anatomic configuration of the peripheral iris and was first described by Tornquist.28 In plateau iris configuration eyes, the anterior chamber appears to have normal axial depth and the iris plane remains flat. The chamber angle may be narrow, however, due to the location of the iris plane adjacent to anterior chamber structures. In the far periphery, the iris drops abruptly to a more posterior plane to create a very narrow recess over the filtration meshwork. In a somewhat different anatomic description, Barkan stated that the peripheral iris appears to be redundant with marked folds crowding the angle.29 This particular arrangement of the anterior iris plane gives the angle an appearance resembling a deep valley surrounding a flat-topped hill, thus the designation “plateau” (Fig. 5). Such eyes are susceptible to closure of the angle when the pupil dilates physiologically or pharmacologically.
These anatomic factors may be said to predispose to closure of the angle but other factors, either physiologic or pharmacologic, must be superimposed to bring about actual closure of the angle.
PHYSIOLOGIC FACTORS
In an anatomically predisposed eye, several physiologic mechanisms can trigger angle-closure glaucoma. Of particular interest are the iris-lens interaction, pupil size, accommodation, and uveal vascular status.
Relative Pupillary Block
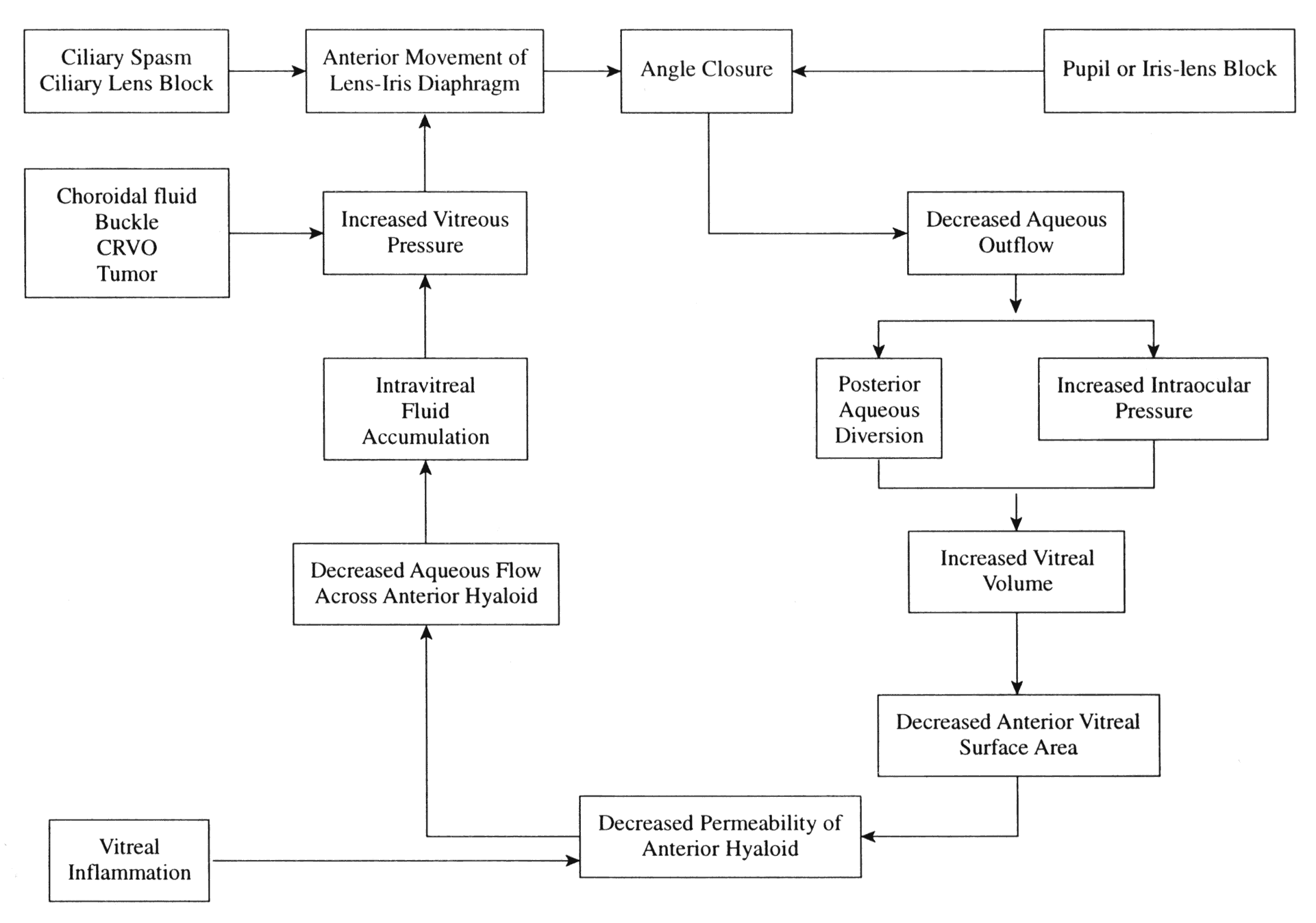
IRIS-LENS INTERACTION.Aqueous humor flows from the posterior chamber, between the posterior iris surface and anterior lens capsule, around the pupil edge and into the anterior chamber. In a normal eye, the iris is in contact with the lens only in the immediate vicinity of the pupillary margin. In eyes with shallow anterior chambers, the forward position of the lens brings the lens-iris diaphragm forward relative to the iris insertion plane. In addition, the anterior lens surface's greater curvature increases the area of apposition of the posterior iris surface to the lens. The increased surface apposition of the iris to lens causes an increased resistance to forward aqueous flow causing a pressure differential between the anterior and posterior chamber. The slightly greater pressure in the posterior chamber leads to forward bowing of the peripheral iris. This mechanism is known as relative pupillary block and it depends greatly on pupillary size and rigidity of the peripheral iris. Under normal circumstances, the iris tends to hang lax in the fluid medium and is sensitive to any pressure differential between the two chambers. Some degree of relative pupillary block is constantly present under normal circumstances, but eyes with a greater extent of lens-iris apposition have more relative pupillary block and are the ones most vulnerable to angle closure.
PUPIL SIZE. One of the most important factors in closing the angle in an anatomically predisposed eye is dilation of the pupil. Dilation leading to closure of the angle may occur as a result of a variety of causes including darkness, emotion, and both topical and systemic dilating drugs. Iris-lens contact is maximal with pupil sizes between 3 and 4.5 mm, enhancing the relative pupillary block mechanism.30 In mid-dilation (3 to 4.5 mm), peripheral iris laxness combined with persistent relative pupillary block leads to closure of a narrow angle by the peripheral iris in predisposed eyes. Wide and rapid dilation minimizes the relative pupillary block phenomenon since iris-lenticular contact is reduced, and the aqueous passes forward through the widely dilated pupil easily. Dilation with sympathomimetic agents, alone or with cycloplegics, tends to decrease peripheral iris laxity by contracting the dilator fibers and thus decreases the likelihood of angle closure while the pupil is rapidly dilating. However, when the iris and ciliary body are slowly recovering from the drug effects and the pupil is gradually becoming smaller in size, the iris at mid-dilation reaches its worst relationship with the lens; relative pupillary block is maximal and the peripheral iris is lax. In this position of mid-dilation following wide dilation, an attack of acute angle closure commonly occurs.
Topical anticholinergics are safe in the majority of eyes but they also increase the risk of angle closure in predisposed eyes. Cycloplegia results in a posterior displacement of the ciliary body, tightening of the zonules, flattening of the lens, and theoretical deepening of the anterior chamber. Mydriasis increases the lens-iris contact. When the pupil is in mid-dilation, the lens-iris contact is maximal thereby increasing relative pupillary block. In addition, cycloplegic mydriasis increases peripheral laxity of the iris since the dilator fibers are not contracted by cycloplegics alone and this further increases the likelihood of closure of the angle. Cycloplegia deepens the anterior chamber in a normal eye, but in a small anterior segment with a relatively large lens bearing a steeper than normal anterior curvature, the slight benefit of a retroplacement of the lens is overcome by the increased relative pupillary block related to midmydriasis. Pure miosis, as seen after instillation of the alpha adrenergic blocking agent thymoxamine, can break angle closure by relieving iris crowding of the angle.31 Thymoxamine hydrochloride acts by inhibition of the iris dilator muscle and has no effect on the ciliary muscle and therefore on accommodation.
ACCOMMODATION. In situations where miosis, whether physiologic or pharmacologic, is seen concurrently with an angle-closure attack, the mechanism appears to be more related to accommodation than to the pupil size. Contraction of the circular muscle of the ciliary body relaxes the zonules and allows forward displacement of the lens. During accommodation, the central portion of the lens becomes more curved while the peripheral zone of the lens surface flattens. In young people, the radius of curvature of the central anterior surface of the lens changes from 10 mm at rest to 5.3 mm in full accommodation.32 There is a slight increase in the axial length of the lens due to the forward bulge of the anterior surface. The coexistence of the aforementioned physiologic mechanisms increases the lens-iris surface contact and enhances the pupillary block phenomenon in predisposed eyes.
UVEAL VASCULAR STATUS. Some authors postulate that the iris-lens diaphragm may suddenly move anteriorly under the influence of increased choroidal blood volume. This mechanism is uncertain and any relationship is unproven. While this may explain an acute angle-closure attack following severe emotional stress, a more plausible explanation relates it to mydriasis due to increased sympathetic tone.
PATHOPHYSIOLOGIC FACTORS
According to Goldmann's equation, assuming constant aqueous production, the pressure driving fluid through the trabecular meshwork out of the eye (perfusion pressure) is directly proportional to the resistance to flow in the eye. The perfusion pressure is the result of intraocular pressure minus episcleral venous pressure. Chandler and Grant concluded that in angle-closure glaucoma, the rate of the rise of intraocular pressure is directly proportional to the degree of obstruction to outflow. A normal eye has excess capacity for outflow through the trabecular meshwork to withstand partial angle closure without significant rise in intraocular pressure. The unaffected trabecular meshwork increases its filtration and keeps the intraocular pressure from rising significantly.
In eyes with anatomically narrow but open angles without glaucoma, the filtration apparatus is normal and therefore the intraocular pressure remains at a normal level. As in normal eyes, there is asymmetry in the width of the angle in different quadrants. The angle appears more narrow in the upper than in the lower quadrants. This observation is constant despite the type of gonio lens used to visualize the angle. It presents even with Koeppe lens direct gonioscopy with the patient in the supine position. The closure of the angle probably evolves gradually from above downward. As closure develops throughout the angle, more and more of the trabecular meshwork is obstructed and an increase in intraocular pressure occurs.
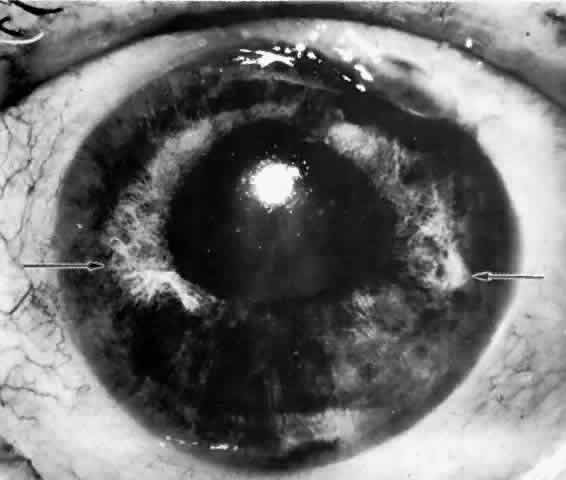
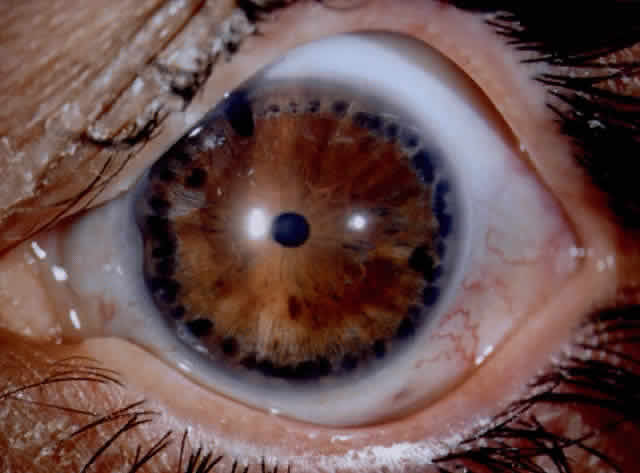
In primary angle-closure glaucoma, obstruction of outflow starts by apposition of the peripheral iris to the trabecular meshwork. There may be temporary apposition of the iris to trabecular meshwork, or the closure may become permanent due to formation of peripheral anterior synechiae. Goniosynechiae tend to occur earlier and more frequently superiorly than inferiorly. The extent of closure at any given time determines the reduction of outflow facility and the level of elevation of the intraocular pressure. Furthermore, in partially closed angles, the efficiency of the remaining functional angle will determine the facility of outflow and pressure elevation.
How the closure of the angle evolves over time and in extent will determine the actual clinical presentation of each particular eye.