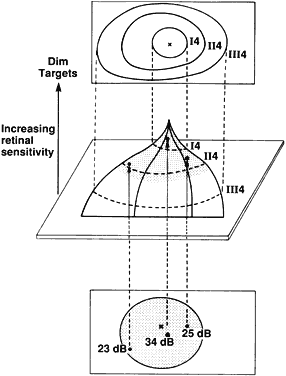
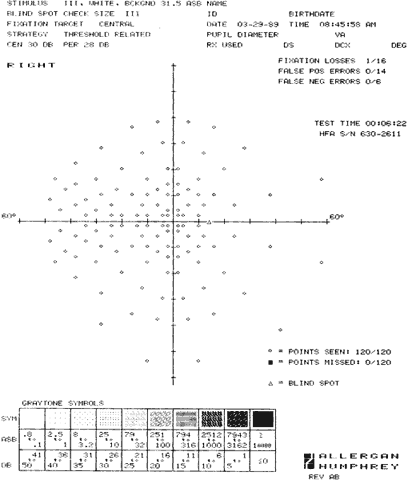
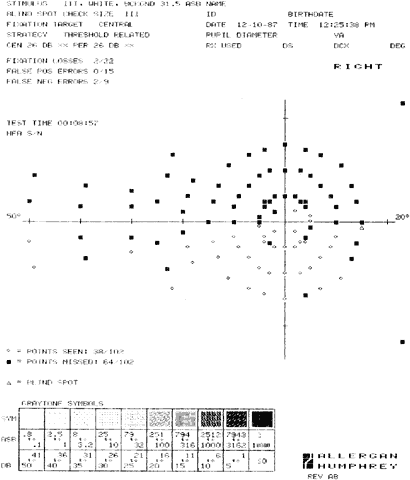
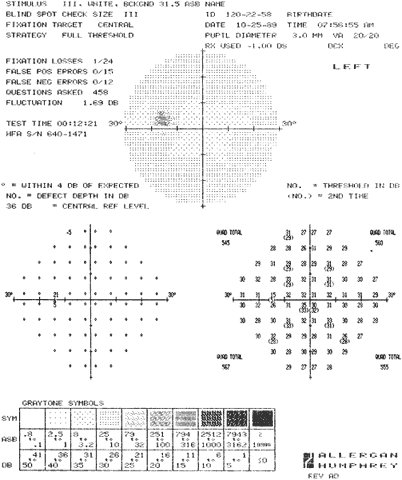
1. Young T: The Bakerian lecture on the mechanism of the eye. Philos Trans R Soc Lond (Biol) 91:23, 1801 2. Graefe A von: Uber die Untersuchung des gesichtsfeldes bei amblyopischen Affectionen. Graefes Arch Klin Exp Ophthalmol 2:258, 1856 3. Förster R: Vorzeigung des Perimeter. Klin Monatsbl Augenheilk 7:411, 1869 4. Bjerrum JP: Omen tilfojelse til saedvanlige synsfeltundersogelse saint om synfeltet
ved glaukom. Nord Ophth Tidsskr Kjobenh 2:144, 1889 5. Sloan LL: Instruments and techniques for the clinical testing of light sense—an
apparatus for studying regional differences in light sense. Arch Ophthalmol 22:233, 1939 6. Goldmann H: Grundlagen exakter Perimetrie. Ophthalmologica 109:57, 1945 7. Harms H, Aulhorn E: Vergleichende Untersuchungen über den Wert der Quantitativen Perimetrie, Skiaskotometrie
und Verschmelzungsfrequenz fur die Erkennung beginnender
Gesichtsfeldstorungen beim Glaukom. Doc Ophthalmol 13:303, 1959 8. Dubois-Poulsen A, Magis C: Premieres applications a l'ophthalmologie des techniques modernes d'automation
et d'analyse de l'information. Bull Mere Soc Fr Ophthalmol 79: 576, 1966 9. Lynn JR, Tate GW: Computer-controlled apparatus for automatic visual field
examination. US patent 3,883,234, issued May 1975 10. Traquair HM: Perimetry in the study of glaucoma. Trans Ophthalmol Soc UK 51:585, 1931 11. Armaly, MF: Ocular pressure and visual fields: A ten-year follow-up study. Arch Ophthalmol 81:25, 1969 12. Drance SM: The early field defects in glaucoma. Invest Ophthalmol Vis Sci 8:84, 1969 13. Armaly MF: Selective perimetry for glaucomatous defects in ocular hypertension. Arch Ophthalmol 87:518, 1972 14. Drance SM, Brais P, Fairclough M, Bryett J: A screening method for temporal visual defects in chronic simple glaucoma. Can J Ophthalmol 7:428, 1972 15. Rock WJ, Drance SM, Morgan RW: A modification of the Armaly visual field screening technique for glaucoma. Can J Ophthalmol 6:283, 1971 16. Rock WJ, Drance SM, Morgan RW: Visual field screening in glaucoma: An evaluation of the Armaly technique
for screening glaucomatous visual field defects. Arch Ophthalmol 89:287, 1973 17. Harms H: Entwicklungsmoglichkeiten der Perimetrie. Graefes Arch Ophthalmol 150:28, 1950 18. Le Blanc RP: The Octopus system. In Drance SM, Anderson DA (eds): Automatic
Perimetry in Glaucoma. A Practical Guide, pp 69–87. Orlando, FL, Grune & Stratton, 1985 19. Heijl A: Humphrey field analyzer. In Drance SM, Anderson DA (eds): Automatic
Perimetry in Glaucoma. A Practical Guide, pp 129–140. Orlando, FL, Grune & Stratton, 1985 20. Heijl A: Automatic perimetry in glaucoma visual field screening: A clinical study. Graefes Arch Clin Exp Ophthalmol 200:21, 1976 21. Li SG, Spaeth GL, Scimeca HA et al: Clinical experience with the uses of an automated perimeter (Octopus) in
the diagnosis and management of patients with glaucoma and neurologic
diseases. Ophthalmology 86:1302, 1979 22. Johnson CA, Keltner JL, Balestrery FG: Suprathreshold static perimetry in glaucoma and other optic nerve disease. Ophthalmology 86:1278, 1979 23. Johnson CA, Keltner JA: Automated suprathreshold static perimetry. Am J Ophthalmol 89:731, 1980 24. Heijl A, Drance SM: A clinical comparison of three computerized automatic perimeters in the
detection of glaucoma defects. Arch Ophthalmol 99:832, 1981 25. Beck RW, Bergstrom TJ, Lichter PR: A clinical comparison of visual field testing with a new automated perimeter, the
Humphrey field analyzer, and the Goldmann perimeter. Ophthalmology 92:77, 1985 26. Trope GE, Britton R: A comparison of Goldmann and Humphrey automated perimetry in patients with
glaucoma. Br J Ophthalmol 71:489, 1987 27. Tate GW Jr: The physiological basis for perimetry. In Drance SM, Anderson
DA (eds): Automatic Perimetry in Glaucoma: A Practical Guide, pp 1–28. Orlando, FL, Grune & Stratton, 1985 28. Anderson DR: Perimetry, With and Without Automation, 2d ed, p 228. St. Louis, CV
Mosby, 1987 29. Klewin K, Radius R: Background illumination and automated perimetry. Arch Ophthalmol 104:395, 1986 30. Britt JM, Mills RP: The black hole effect in perimetry. Invest Ophthalmol Vis Sci 29:795, 1988 31. Dejardins D, Anderson DR: Threshold variability with an automated LED perimeter. Invest Ophthalmol Vis Sci 29:915, 1988 32. Wilensky JT, Mermelstein JR, Siegel HG: The use of different-sized stimuli in automated perimetry. Am J Ophthalmol 101:710, 1986 33. Heijl A, Krakau CET: An automatic perimeter for glaucoma visual field screening and control: Construction
and clinical cases. Graefes Arch Klin Exp Ophthalmol 197:13, 1975 34. Anderson DR: Principles of static perimetry. In Drance SM, Anderson DA (eds): Automatic
Perimetry in Glaucoma. A Practical Guide, pp 69–87. Orlando, FL, Grune & Stratton, 1985 35. O'Brien C, Poinoosawmy D, Wu J, Hitchings R: Evaluation of the Humphrey FASTPAC threshold program in glaucoma. Br J Ophthalmol 78:516, 1994 36. Flanagan JG, Wild JW, Trope GE: Evaluation of FASTPAC, a new strategy for threshold estimation with the
Humphrey Field Analyzer, in a glaucomatous population. Ophthalmology 100:949, 1993 37. Schaumberger M, Schafer B, Lachenmayr BJ: Glaucomatous visual fields: FASTPAC versus full threshold strategy of the
Humphrey Field Analyzer. Invest Ophthalmol Vis Sci 36:1390, 1995 38. Bengtsson B, Heijl A, Olsson J: Evaluation of a new threshold visual field strategy, SITA, in normal subjects. Acta Ophthalmol Scand 76:165, 1998 39. Bengtsson B, Heijl A: Evaluation of a new perimetric threshold strategy, SITA, in patients with
manifest and suspect glaucoma. Acta Ophthalmol Scand 76:268, 1998 40. Bengtsson B, Heijl A: SITA Fast, a new rapid perimetric threshold test. Description of methods
and evaluation in patients with manifest and suspect glaucoma. Acta Ophthalmol Scand 76:431, 1998 41. Liebermann MF, Drake MV: A Simplified Guide to Automated Perimetry, pp. 34–42, 103–128. Thorofare, NJ, Slack, 1987 42. Abramson DH: Computerized visual field of choroidal melanoma. Glaucoma 10:39, 1988 43. Palazzi M, Abramson DH, Myers C et al: Computerized visual fields in uveal melanomas after treatment. Glaucoma 11:114, 1989 44. McCrary JA, Feigon J: Computerized perimetry in neuro-ophthalmology. Ophthalmology 86:1287, 1979 45. Keltner JL, Johnson CA: Automated and manual perimetry: A six-year overview: Special emphasis on
neuro-ophthalmic problems. Ophthalmology 91:68, 1983 46. Younge BR, Trautmann JC: Computer-assisted perimetry in neuro-ophthalmic disease. Mayo Clin Proc 55:207, 1980 47. Hard-Boberg A, Wirtschafter JD: Evaluating the usefulness in neuro-ophthalmology of visual field examination
peripheral to 30 degrees. Doc Ophthal Proc Series 42:197, 1985 48. Mills RP: Usefulness of peripheral testing in automated screening perimetry. Doc Ophthal Proc Series 42:207, 1985 49. Beck RW: Optic Neuritis Treatment Trial. Operations Manual. 1988 50. Smith TJ, Baker RS: Visual field findings in pseudotumor cerebri. Ophthalmology 92 (suppl 2):69, 1985 51. Aulhorn E, Harms H: Early visual field defects in glaucoma. In Leydhecker
W (ed): Glaucoma: Tutzing Symposium, 1966, pp 151–186. Basel, Karger, 1967 52. Drance SM, Berry V, Hughes A: Studies on the effects of age on the central and peripheral isopters of
the visual fields in normal subjects. Am J Ophthalmol 63:1667, 1967 53. Werner EB, Drance SM, Schulzer M: Early visual field disturbances in glaucoma. Arch Ophthalmol 95:1173, 1977 54. Hart WM Jr, Becker B: The onset and evolution of glaucomatous visual field defects. Ophthalmology 89:268, 1982 55. Werner EB, Saheb N, Thomas D: Variability of static visual field threshold responses in patients with
elevated IOPs. Arch Ophthalmol 100:1627, 1982 56. Flammer J, Drance SM, Zulauf M: Differential light threshold: Short- and long-term fluctuation in patients
with glaucoma, normal controls and patients with suspected glaucoma. Arch Ophthalmol 102:704, 1984 57. Flammer J, Drance SM, Fankhauser F, Augustiny L: Differential light threshold in automated static perimetry: Factors influencing
short-term fluctuation. Arch Ophthalmol 102:876, 1984 58. Mikelberg FS, Drance SM: The mode of progression of visual field defects in glaucoma. Am J Ophthalmol 98:443, 1984 59. Werner EB, Beraskow J: Peripheral nasal field defects in glaucoma. Ophthalmology 86:1875, 1979 60. LeBlanc RP, Becker B: Peripheral nasal field defects. Am J Ophthalmol 72:415, 1971 61. Seamone C, LeBlanc R, Rubillowicz M et al: The value of indices in the central and peripheral visual fields for the
detection of glaucoma. Am J Ophthalmol 106:180, 1988 62. Schulzer M, Mikelberg FS, Drance SM: A study of the value of the central and peripheral isopters in assessing
visual field progression in the presence of paracentral scotoma measurements. Br J Ophthalmol 71:422, 1987 63. Flammer J, Jenni F, Bebie JH, Keller B: The Octopus glaucoma G-1 program. Glaucoma 9:67, 1987 64. Johnson CA, Brandt JD, Khong AM, Adams AJ: Short-wavelength automated perimetry in low-, medium-, and high-risk ocular
hypertensive eyes: Initial baseline results. Arch Ophthalmol 113:70, 1995 65. Wild JM, Cubbidge RP, Pacey IE, Robinson R: Statistical aspects of the normal visual field in short-wavelength automated
perimetry. Invest Ophthalmol Vis Sci 39:54, 1998 66. Sample PA, Bosworth CF, Weinreb RN: Short-wavelength automated perimetry and motion automated perimetry in
patients with glaucoma. Arch Ophthalmol 115:1129, 1997 67. Wall M, Jennisch CS, Munden PM: Motion perimetry identifies nerve fiber bundlelike defects in ocular hypertension. Arch Ophthalmol 115:26, 1997 68. Sponsel WE, Arango S, Trigo Y, Mensah J: Clinical classification of glaucomatous visual field loss by frequency-doubling
perimetry. Am J Ophthalmol 125:830, 1998 69. Johnson CA, Samuels SJ: Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci 38:413, 1997 70. Hass A, Flammer J, Schneider U: Influence of age on the visual field of normal subjects. Am J Ophthalmol 101:199, 1986 71. Jaffe GJ, Alvarado JA, Juster RP. Age-related changes in the normal visual
field. Arch Ophthalmol 104:1021, 1986 72. Katz J, Sommer A: Asymmetry and variation in the normal hill of vision. Arch Ophthalmol 104:65, 1986 73. Heijl J, Lindgren G, Olsson J: Normal variability of static perimetric threshold value across the central
field. Arch Ophthalmol 105:1544, 1987 74. Katz J, Sommer A: A longitudinal study of the age-adjusted variability of automated visual
fields. Arch Ophthalmol 105:1083, 1987 75. Heijl J, Lindgren G, Olsson J: The effect of perimetric experience in normal subjects. Arch Ophthalmol 107:81, 1989 76. Werner EB, Adelson A, Krupin T: Effect of patient experience on the results of automated perimetry in clinically
stable glaucoma patients. Ophthalmology 95:764, 1988 77. Weinreb RN, Perlman JP: The effect of refractive correction on automated perimetric thresholds. Am J Ophthalmol 101:706, 1986 78. Goldstick B, Weinreb RN: The effect of refractive error on automated global analysis program G1. Am J Ophthalmol 104:229, 1987 79. McCluskey DJ, Douglas JP, O'Connor PS et al: The effect of pilocarpine on the visual field in normals. Ophthalmology 93:843, 1986 80. Lindenmuth KA, Skuta GL, Rabbani R et al: Effects of pupillary constriction on automated perimetry in normal eyes. Ophthalmology 96:1298, 1989 81. Guthauser U, Flammer J: Quantifying visual field damage caused by cataract. Am J Ophthalmol 106:480, 1988 82. Zalta AH: Lens rim artifact in automated threshold perimetry. Ophthalmology 96:1302, 1989 83. Heijl A, Lindgren A, Lindgren G: Test-retest variability in glaucomatous visual fields. Am J Ophthalmol 108:130, 1989 84. Parrish RK, Schiffman J, Anderson SR: Static and kinetic visual field testing: Reproducibility in normal volunteers. Arch Ophthalmol 102:1497, 1984 85. Lewis RA, Johnson CA, Keltner JL, Labermeier PK: Variability of quantitative automated perimetry in normal observers. Ophthalmology 93:878, 1986 86. Brenton RS, Argus WA: Fluctuations on the Humphrey and Octopus perimeters. Invest Ophthalmol Vis Sci 28:767, 1987 87. Katz J, Sommer A: Reliability indexes of automated perimetric tests. Arch Ophthalmol 106:1252, 1988 88. Flammer J, Drance SM, Augustiny L, Funkhouser A: Quantification of glaucomatous visual field defects with automated perimetry. Invest Ophthalmol Vis Sci 26:176, 1985 89. Wilensky JT, Joondeph BC: Variation in visual field measurements with an automated perimeter. Arch Ophthalmol 97:328, 1984 90. deJong DGMM, Greve EL, Bakker D, van den Berg TJTP: Psychological factors in computer assisted perimetry; automatic and semiautomatic
perimetry. Doc Ophthalmol Proc Series 42:137, 1985 91. Bickler-Bluth M, Trick GL, Kolker AE, Cooper DG: Assessing the utility of reliability indices for automated visual fields: Testing
ocular hypertensives. Ophthalmology 96:616, 1989 92. Heijl A, Lindgren G, Olsson, Jet al: STATPAC User's Guide. San Leandro, CA, Allergan
Humphrey, 1986 93. Sommer A, Enger C, Witt K: Screening for glaucomatous visual field loss with automated threshold perimetry. Am J Ophthalmol 103:681, 1987 94. Liao PM, Gollamudi SR, Hirsch J: Evaluation of corrected loss variance as a visual field index (Part I). Ophthalmologica 197:136, 1988 95. Hoskins HD, Magee SD, Drake MV, Kidd MN: Confidence intervals for change in automated visual fields. Br J Ophthalmol 72:591, 1988 96. Werner EB, Bishop K, Koelle J et al: A comparison of experienced clinical observers and statistical tests in
detection of progressive visual field loss in glaucoma using automated
perimetry. Arch Ophthalmol 106:619, 1988 97. Brenton RS, Phelps CD, Rojas P, Woolson RF: Interocular differences of the visual field in normal subjects. Invest Ophthalmol Vis Sci 27:799, 1986 98. Feuer WJ, Anderson DA: Static threshold asymmetry in early glaucomatous visual field loss. Ophthalmology 96: 1285, 1989 |