Of course, a real objective difference does exist between a red and a green apple because color is determined in large part by the composition of light that enters the eye after being transmitted through or reflected off objects. However, many properties of the human eye and brain influence the color name that we give the object. Some of the physiologic processes of color perception are now understood in terms of the way light energy is transformed and coded into color signals and the way those signals are interpreted in the visual system.
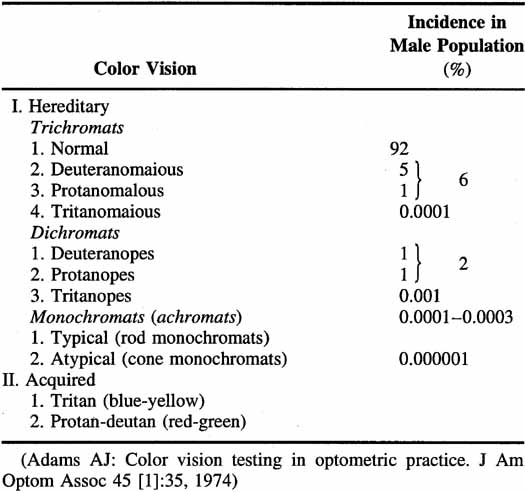
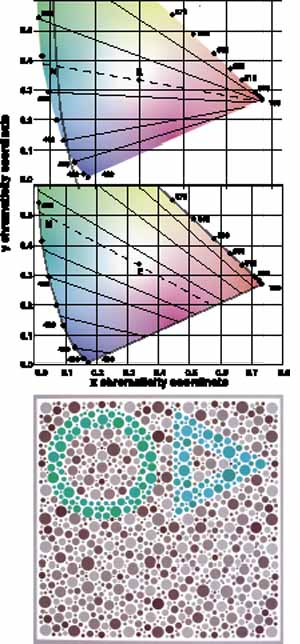
Not all humans see colors the same way. Most of us are resigned to occasional arguments about whether a carpet is beige or cream-colored. However, individual differences in color perception can be more profound and more important. Almost 10% of the male population and approximately 0.5% of the female population have defective color vision that makes it difficult for them to discriminate some colors—or at least more difficult than normal. This so-called color blindness is one of the most common of all genetic defects and has fascinated geneticists, vision scientists, and medical practitioners. Great advances have been made in understanding the genetics of normal and congenitally defective color vision. We now know the precise gene coding sequence for the rod and cone photopigments, and we understand more about variations in normal color vision and the underlying pigment changes that account for color vision defects. Very few people, perhaps 1 in 10,000, are truly color blind (that is, they see everything in shades of gray), but even the previously mentioned 10% of color-defective males and the 0.5% of color-defective females who do see color yet fail on tests of “color blindness” are likely to be precluded from engaging in certain occupations that require normal color vision (e.g., becoming policemen, firemen, pilots, or drivers).
In this chapter, we present some of the results of physiologic and psychophysical studies made on encoding color vision signals in the visual system. Further, we relate these findings to the color discrimination of normals and color defectives in an attempt to understand why there are these differences between the two groups and discuss these differences in the context of clinical testing of color vision.
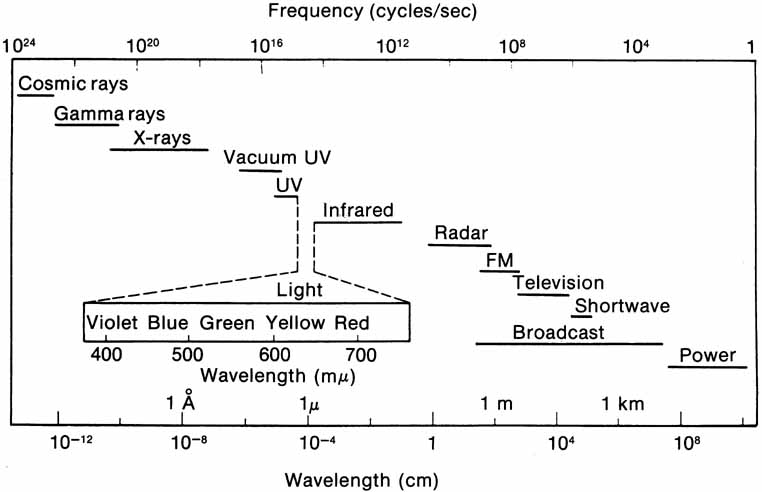
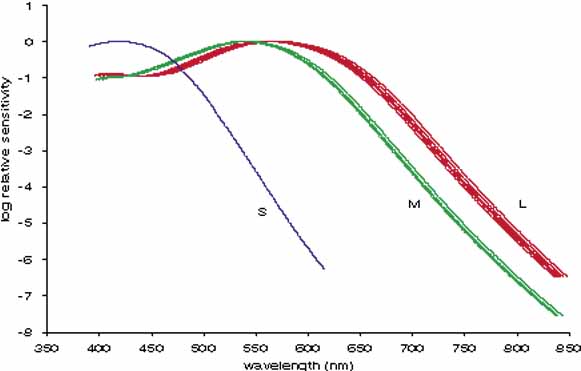
The radiant energy that gives rise to the sensation of vision is a very narrow section of the electromagnetic spectrum (Fig. 1), with the wavelength of light being less than one ten-thousandth of a millimeter. The wavelength limits of visible light extend from the extreme violet end (wavelength approximately 400 nm or four ten-thousandths of a millimeter) to the extreme red part of the spectrum (wavelength 700 nm). Yet within this range, the human eye is able to distinguish many different color sensations.
|
Light also has particle properties and wave characteristics; the radiant energy can be considered as extremely small discontinuous particles of energy called quanta. The energy in a quantum is directly proportional to the frequency of the electromagnetic energy. Consequently, high-frequency violet light (400 nm) contains twice the energy per quantum as radiation at 800 nm.
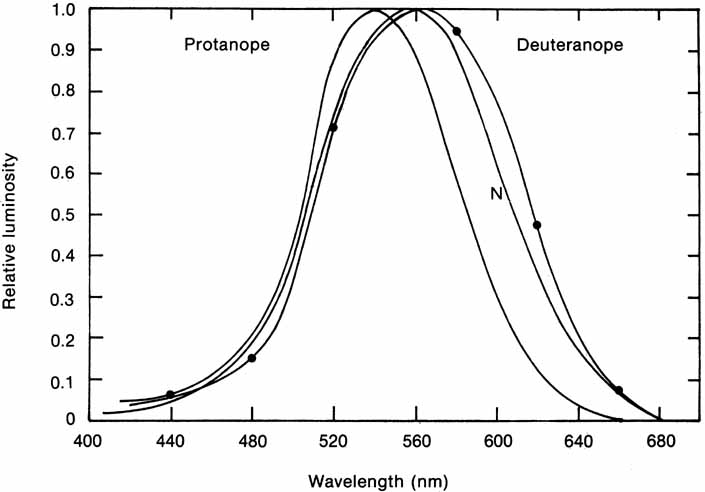
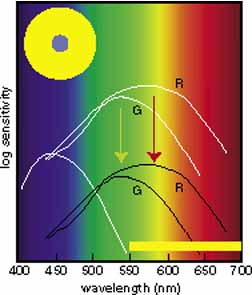
In 1666 Isaac Newton spread the spectral components of normal white sunlight across the wall of his room by allowing a shaft of white light to pass through a small glass prism. A repetition of this simple experiment will illustrate a number of important aspects of color vision to be considered in some detail later in this chapter (Fig. 2, in color). If your vision is normal, you will note first that the ends of such a spectrum (violet and red) are quite dim compared with the middle of the spectrum (yellow and green). The fact that the middle appears to be the brightest suggests that the eye is not equally sensitive to all wavelengths. Second, as you move your eyes across the color spectrum, you will find two areas in which, for a given space (or number of nanometers), you seem to perceive more than the usual number of different colors. One of these areas is in the blue and green region; the other in the yellow region. This phenomenon suggests that the eye is able to make finer discrimination between adjacent wavelengths in certain sections of the spectrum. Third, you will probably find that the region of the spectrum that looks the least colored or most like white lies in the yellow portion of the spectrum (approximately 570 nm).
|
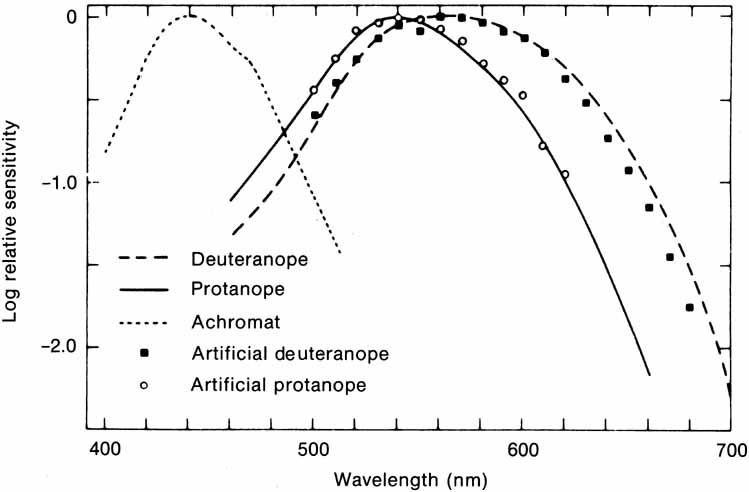
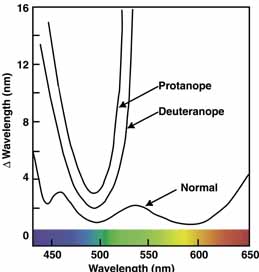
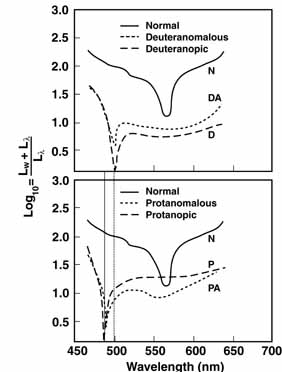
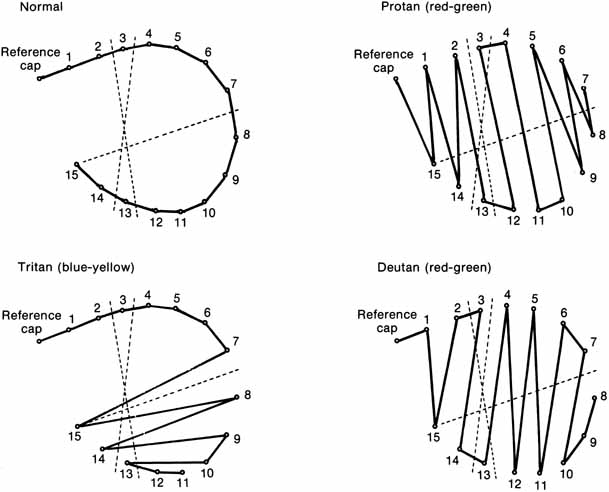
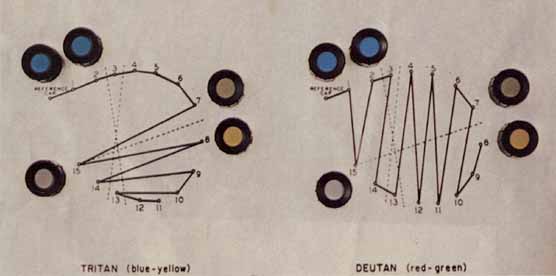
The foregoing are the observations of people with normal color vision. The same spectrum when viewed by many color–-vision-defective subjects will result in different observations. For example, in contrast to normal subjects, color-defective subjects may see the range of light waves that would normally be green as the brightest part of the spectrum. They may see the area that normally would be blue–green as the most like white, and they may be unable to make color distinctions in what would ordinarily be the green through red region. All of these variations in observations may be understood in terms of differences in color-vision mechanisms between normal and hereditary color defectives.
The various theories about how humans perceive color and light developed slowly. Newton's interest in the subject did not stop at prisms. He speculated that light sets up vibrations in the optic nerves that are tuned to respond to each color. Approximately 100 years later, Thomas Young decided that to postulate the existence in the retina of an almost infinite number of receptors was to ignore some basic facts.1
If an individual had to have a very large number of color receptors—one for each color sensation—then at a particular point in the retina, where he or she is able to distinguish one colored object from another, he or she would have to have a complete set. This arrangement would call for an area so large that objects would have to be very far apart to distinguish them. Because we know that this is not the case—the eye has good resolution and can distinguish between things that are very close together—it becomes difficult to conceive of a way in which that many receptors could be piled into as small a location as is indicated by our excellent spatial resolution.
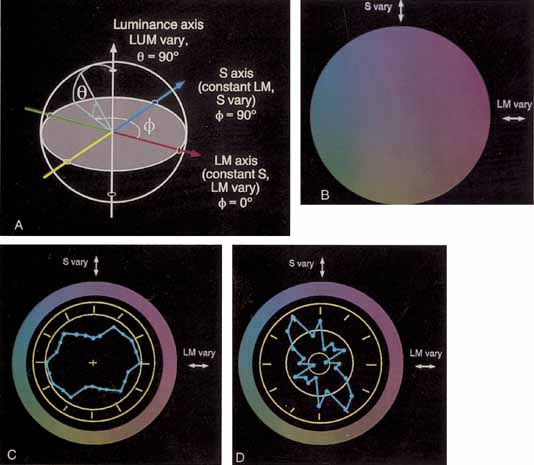
Instead, Young proposed three resonator groups responding maximally to one of three principal colors. Helmholtz, in the mid-19th century, elaborated on this theory and pointed out that although each receptor responded maximally to a particular region of the spectrum, they all responded over practically the entire spectrum.2 In such a scheme, the various color sensations would be the result of the relative strengths with which the three groups of receptors were stimulated. Color vision would thus require a limited number of receptors of different but overlapping spectral sensitivity and a neural system that compares the output of different receptor types.
Even though Helmholtz and, later Hering, proposed the elements of these requirements more than a century ago, until very recently their theoretic contributions were seen as mutually exclusive.3 Hering and Helmholtz seemed to see it this way also.
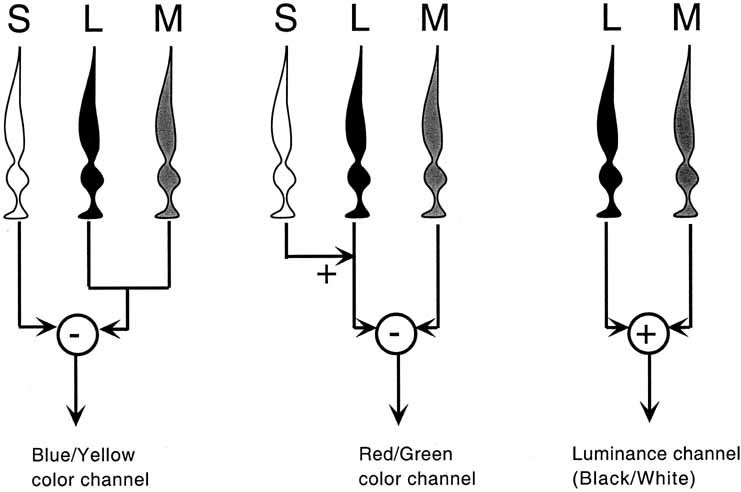
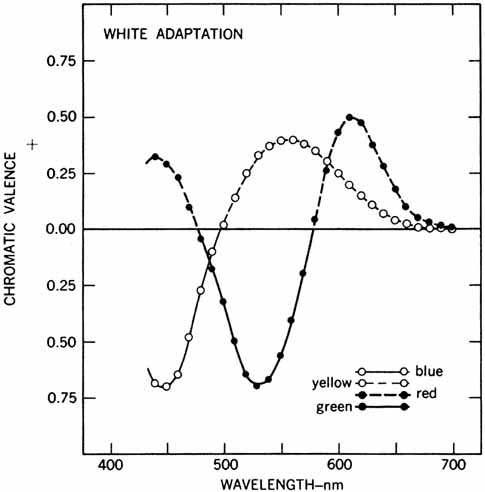
Hering proposed that there were three kinds of “catching material,” two substances providing signals about color and one signaling blackness or whiteness. The three substances were responsible for producing warm (white, yellow, red) and cold (black, blue, green) color sensations. Hering explained this in terms of a warm color being paired with cold color in each substance (yellow–blue, red–green, white–black). Light had two opposing actions: catabolism produced the warm colors, anabolism the cold colors. The theory was primarily aimed at accounting for the six distinct sensations that appeared to be paired in everyday sensations of color (e.g., a red afterimage follows exposure to green light).
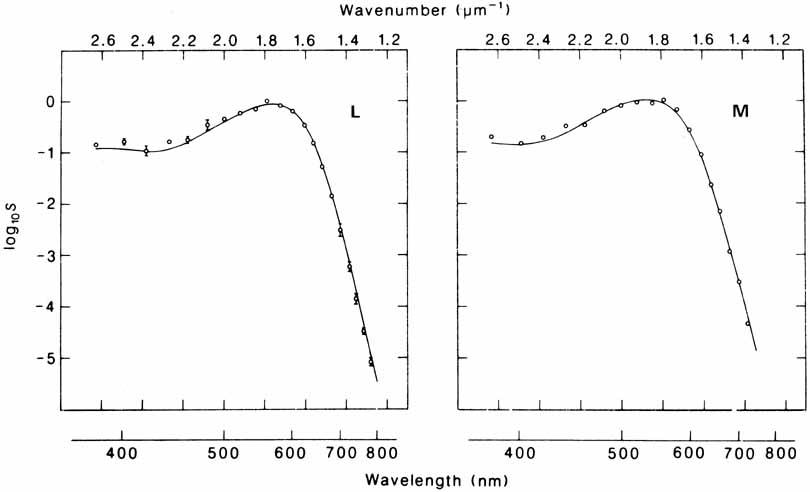
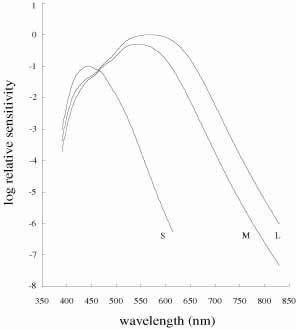
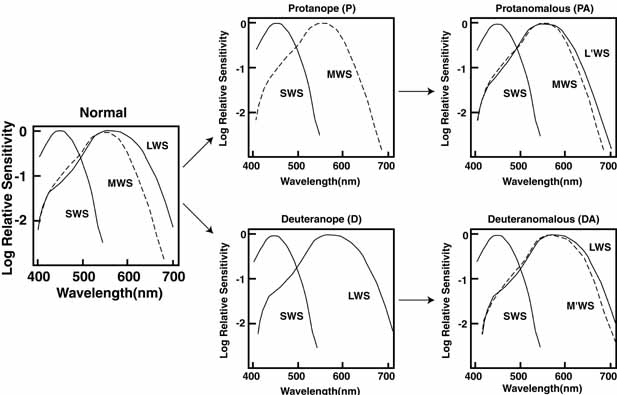
De Valois and De Valois have pointed out that the long arguments and confusion about the Helmholtz and Hering theories were aided by the unfortunate fact that Hering proposed that the opponent process occurred at the receptor level.4 Today, there is considerable evidence that in the normal eye there are three separate receptor types, as Young and Helmholtz proposed, and subsequent neural pathways that compare the outputs of the different receptor types (spectrally opponent interactions), much like the organization that formed the basis of Hering's proposal.
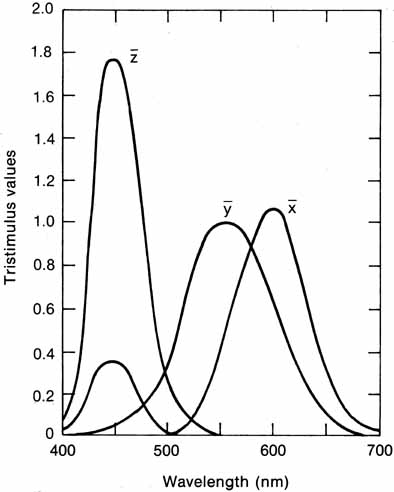
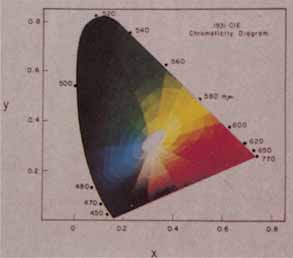
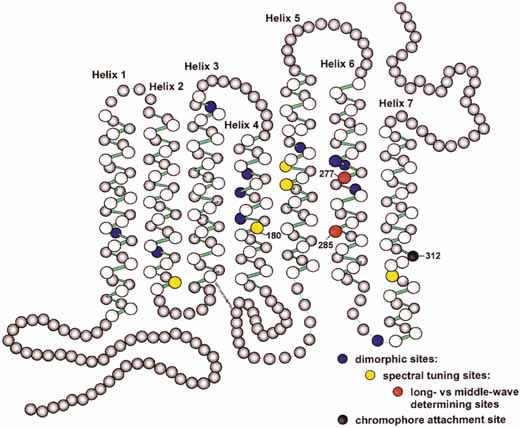
The presence of three cone receptors in the normal human retina is entirely consistent with the so-called trichromacy of vision that was known even before Newton's time and quantitatively established by Maxwell.5 Maxwell's carefully performed color-matching experiments showed that all spectral colors could be matched with some mixture of three primary colors chosen from the red, green, and blue regions of the spectrum. These and subsequent studies supported the description of the normal color vision as requiring three independent variables. With the recent advances in molecular genetics and refinement of psychophysical techniques to assess color vision, the presence of only three cone pigments is coming under scrutiny. Whether all color-normals have the same three cone pigments and whether an individual can possess more than three cone pigments are at the forefront of color science.