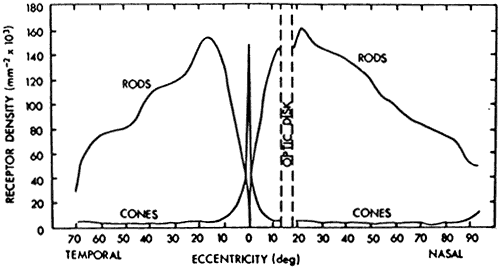
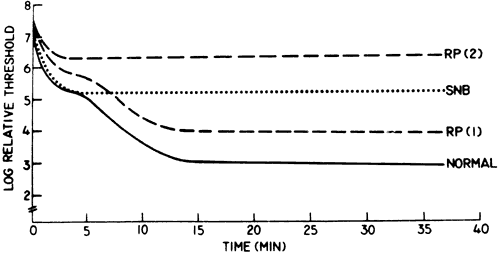
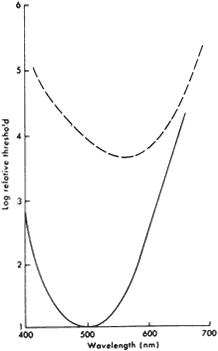
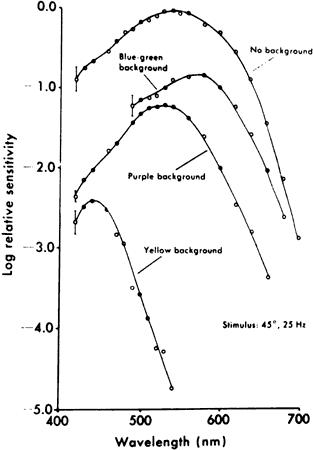
1. Osterberg G: Topography of the layer of rods and cones in the human retina. Acta Ophthalmol 6(Suppl):1, 1935 2. Hecht S: Rods, cones and the chemical basis of vision. Physiol Rev 17:239, 1937 3. Mandelbaum J: Dark adaptation: Some physiological and clinical considerations. Arch Ophthalmol 26:203, 1941 4. Berson EL: Night blindness: Some aspects of management. In Faye E, ed: Clinical
Low Vision, p 301. Boston, Little, Brown, 1976 5. Berson EL: Electrical phenomena in the retina. In Hart WM, ed: Adler's
Physiology of the Eye. Clinical Application, 9th ed, p 641. St. Louis, CV
Mosby, 1992 6. Mehaffey L III, Berson EL: Cone mechanisms in the electroretinogram of the cynomolgus monkey. Invest Ophthalmol 13:266, 1974 7. Brown PK, Wald G: Visual pigments in single rods and cones of the human retina. Science 144:45, 1964 8. Marks WB, Dobelle WH, MacNichol EF Jr: Visual pigments of single primate cones. Science 143:1181, 1964 9. Pokorny J, Smith V: Color vision and night vision. In Ryan SJ, ed: Retina, Vol. 1: Basic
Science and Inherited Retinal Disease, p 109. St. Louis, CV
Mosby, 1989 10. Reichel E: Hereditary cone dysfunction syndromes. In Jakobiec FA, Albert
DM: Principles and Practice of Ophthalmology, 2nd ed, vol 3, p 2290. Philadelphia, WB
Saunders, 2000 11. Pokorny J, Smith VC: Eye disease and color defects. Vision Res 26:1573, 1986 12. Berson EL, Sandberg MA, Rosner B, Sullivan PL: Color plates to help identify patients with blue cone monochromatism. Am J Ophthalmol 95:741, 1983 13. Wyszechi G, Stiles WS: Color Science, p 206. New York, John Wiley and Sons, 1967 14. Gouras P: Electroretinography: Some basic principles. Invest Ophthalmol 9:557, 1970 15. Berson EL, Gouras P, Hoff M: Temporal aspects of the electroretinogram. Arch Ophthalmol 81:207, 1969 16. Berson EL: Retinitis pigmentosa and allied retinal diseases: Electrophysiologic findings. Trans Am Acad Ophthalmol Otolryngol 81:659, 1976 17. Berson EL, Sandberg MA, Rosner B et al: Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol 99:240, 1985 18. Andreasson SOL, Sandberg MA, Berson EL: Narrowband filtering for monitoring low-amplitude cone electroretinograms
in retinitis pigmentosa. Am J Ophthalmol 105:500, 1988 19. Berson EL, Rosner B, Sandberg MA et al: A randomized trial of vitamin A and vitamin E supplementation for retinitis
pigmentosa. Arch Ophthalmol 111:761, 1993 20. Berson EL: Treatment of retinitis pigmentosa with vitamin A. Proceedings
of the Fernström Symposium on Tapetoretinal Degenerations, Lund, Sweden. Digital
J Ophthalmol 1998; http://www.djo.harvard.edu/meei/OA/RPB/RPB.html 21. Sandberg MA, Berson EL, Ariel M: Visually evoked response testing with a stimulator-ophthalmoscope: Macular
scars, hereditary macular degeneration, and retinitis pigmentosa. Arch Ophthalmol 95:1805, 1977 22. Sandberg MA, Jacobson SG, Berson EL: Foveal cone electroretinograms in retinitis pigmentosa and juvenile macular
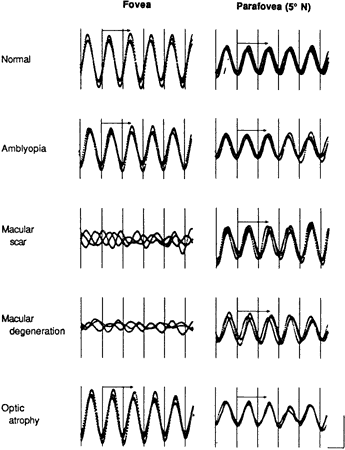
degenerations. Am J Ophthalmol 88:702, 1979 23. Jacobson SG, Sandberg MA, Effron MH, Berson EL: Foveal cone electroretinograms in strabismic amblyopia: Comparison with
juvenile macular degeneration, macular scars and optic atrophy. Trans Ophthalmol Soc UK 99:353, 1980 24. Sandberg MA, Hanson AH, Berson EL: Focal and parafoveal cone electroretinograms in juvenile macular degeneration. Ophthalmol Pediatr Gen 3:83, 1983 25. Maffei L, Fiorentini A: Electroretinographic responses to alternating gratings before and after
section of the optic nerve. Science 211:953, 1981 26. Harrison JM, O'Connor PS, Young RSL et al: The pattern ERG in man following surgical resection of the optic nerve. Invest Ophthalmol Vis Sci 28:492, 1987 27. Lawwill T: The bar-pattern electroretinogram for clinical evaluation of the central
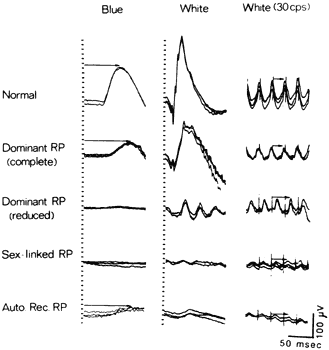
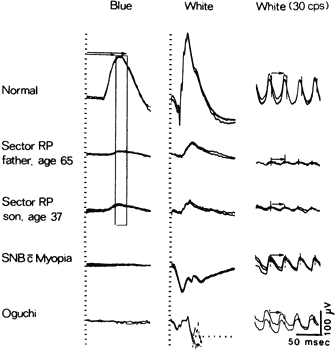
retina. Am J Opthalmol 78:1231, 1979 28. Sutter EE, Tran D: The field topography of ERG components in man, I. The photopic luminance
rsponse. Vis Res 32:433, 1992 29. Hood DC, Holopigian K, Seiple W et al: Assessment of local retinal function in patients with retinitis pigmentosa
using the multi-focal ERG technique. Vis Res 38:163, 1998 30. Sutter EE, Bearse MA: The optic nerve head component of the human ERG. Vis Res 39:419, 1999 31. Hood DC: Assessing retinal function with the multifocal technique. In Osborne
NN, Chader GJ, eds: Progress in Retinal and Eye Research, vol 19, p 607. Oxford, Pergamon
Press, 2000 32. Heynen H, Wachtmeister L, van Norren D: Origin of the oscillatory potentials in the primate retina. Vision Res 25:1365, 1985 33. Bresnick GH, Korth K, Groo A, Palta M: Electroretinographic oscillatory potentials predict progression of diabetic
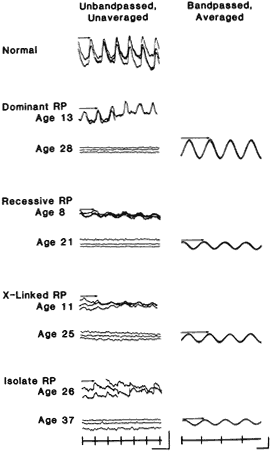
retinopathy: Preliminary report. Arch Ophthalmol 102:1307, 1984 34. Sandberg MA, Lee H, Matthews GP, Gaudio AR: Relationship of oscillatory potential amplitude to a-wave slope over a
range of flash luminances in normal subjects. Invest Ophthalmol Vis Sci 32:1508, 1991 35. Kurtenbach A, Langrova H, Zrenner E: Multifocal oscillatory potentials in type 1 diabetes without retinopathy. Invest Ophthalmol Vis Sci 41:3234, 2000 36. Hood DC, Zhang X, Greenstein VC et al: An interocular comparison of the multifocal VEP: A possible technique for
detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 41:1580, 2000 37. Weiner A, Sandberg MA, Gaudio AR et al: Hydroxycholorquine retinopathy. Am J Ophthalmol 112:528, 1991 38. Berson EL: Hereditary retinal diseases: Classification with the full-field
electroretinogram. In Lawwill T, ed: ERG, VER and Psychophysics, Documenta
Ophthalmologica Proceedings Series, 13-XIV ISCERG Symposium, May 10–14, 1976, Louisville, KY, p 149. The Hague, Dr. W. Junk, 1977 39. Berson EL: Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34:1659, 1993 40. RetNet: Summary of Genes Causing Retinal Diseases.http://www.sph.uth.tmc.edu/Retnet/summary.htm 41. Berson EL, Rosner B, Sandberg MA, Dryja T: Ocular findings in patients with autosomal dominant retinitis pigmentosa
and a rhodopsin gene defect (Pro23His). Arch Ophthalmol 109:92, 1991 42. Berson EL, Rosner B, Sandberg MA, Dryja TP: Ocular findings in patients with autosomal dominant retinitis pigmentosa
and rhodopsin, proline-347-leucine. Am J Ophthalmol 111:614, 1991 |