OVERVIEW
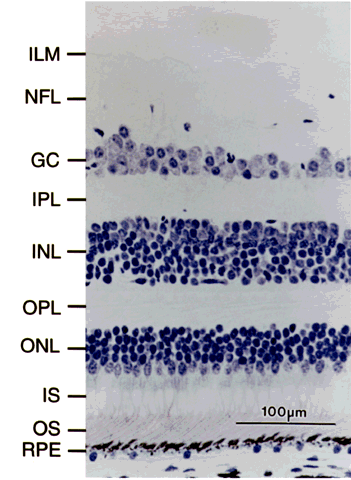
The retina is composed of millions of neurons organized into several primarily cellular layers (Fig. 1). With light microscopy, one can readily recognize three cellular layers, which contain the cell bodies of neurons: the photoreceptor-outer nuclear layer, the inner nuclear layer, and the ganglion cell layer. Connections between these neural layers are made by processes that lie in the outer and inner plexiform layers. The retinal pigment epithelium (RPE) is a single layer of contiguous cells lying beneath the photoreceptors.
Vision is initiated when the photoreceptors are activated by quanta of light. The resulting visual signals pass through the plexiform layer of neural processes and reach the bipolar cells in the inner nuclear layer. The signal is then passed through the inner plexiform layer into the ganglion cell layer and transmitted through the axons that form the optic nerve, and hence to the visual cortex.
In the primate retina, the photoreceptors of the outer nuclear layer number about six million cones and 120 million rods. In addition to containing several types of bipolar cells, the inner nuclear layer contains horizontal cells and amacrine cells, both of which mediate lateral signal interactions that are important for spatial processing of vision. The horizontal cells lie in a row closest to the outer plexiform layer, and the amacrine cells lie in a row closest to the inner plexiform layer.
The primate retina is not homogeneous from center to periphery. The central retina is specialized for cone vision, whereas the peripheral retina is organized better for rod vision. Cones are concentrated within the central-most retina and form the fovea, which is a rod-free area. The fovea is further specialized in that it contains only the photoreceptors; the second- and third-order retinal neurons are pushed to the side. This organization accomplishes two criteria that are important for fine vision:
- The image that is focused on the foveal cones is the sharpest and least
degraded because light passes through the fewest layers of the inner
retina.
- Tight packing provides minimal space between the cones in the fovea and
thus allows the sharpest reconstruction of fine image detail, corresponding
to the finest grain of a digitized photograph.
Meanwhile, the bipolar, horizontal, and amacrine cells that subserve vision processing from the densely packed foveal cones are displaced sideways into a parafoveal mound of neural tissue.
Whereas cones are packed most densely in the fovea, the rod photoreceptors reach the highest concentration 20° to 30° from the fovea. Although the retinal neurocircuitry associated with rods does not allow for acuity of better than about 20/200, the high density of rods in the midperiphery still provides the maximal sensitivity for dim-light vision at this region. The retina is thinnest in the fovea, thickest in the parafovea and macula, and of intermediate thickness in the peripheral retina. One misconception is that cones are limited to the fovea and macula. This is obviously incorrect, since we can perceive color across our entire field of vision, from edge to edge. Since color is mediated exclusively by cones, this reinforces the concept that cones exist in the peripheral retina, although at a considerably reduced number compared to the macula.
PHOTORECEPTORS
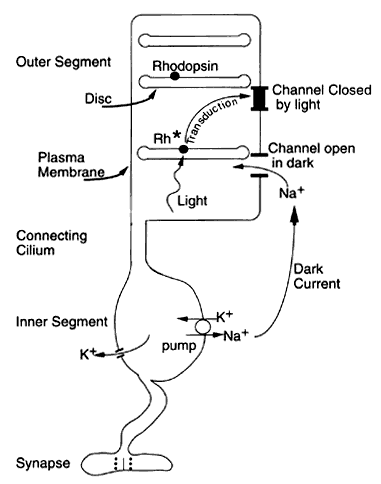
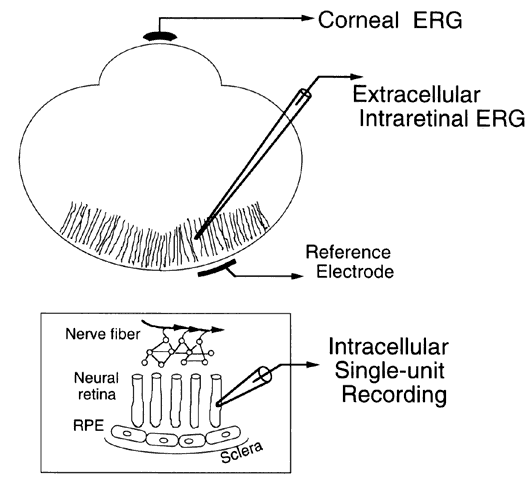
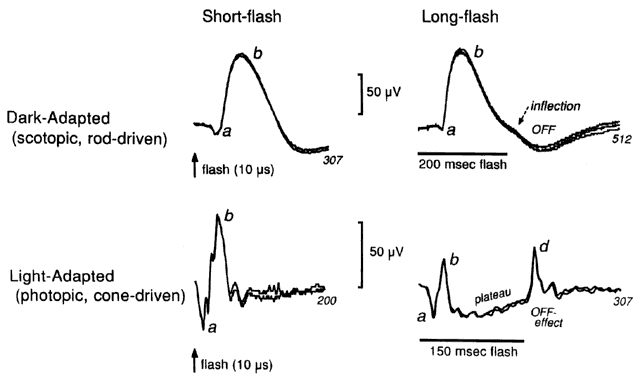
Rod and cone photoreceptors are specialized for converting light into neural signals (Fig. 2). The earliest information about electrical activity of the photoreceptors came with Granit's identification of the PIII response of the ERG as deriving from this layer of cells. In the corneal ERG, PIII is the negative-going response that forms the a-wave when elicited with stimuli of medium to bright intensity. The a-wave is the earliest and negative wave of the ERG elicited with relatively bright flashes. During the 1960s, Brown and associates inserted a microelectrode into the monkey fovea and identified the ERG a-wave signal produced by cone photoreceptors. This provided the first information about the shape of the cone response when these cells hyperpolarize to a light flash. The next advance came from impaling individual photoreceptors, and subsequently by suction electrode recordings performed by Baylor and colleagues.
Note that when a cell hyperpolarizes, the inside becomes relatively more negative compared to the outside. All this is relative, since cells normally are negative inside compared to the extracellular space. Thus the terms hyperpolarization and depolarization are with reference to the normal resting potential of the cell membrane. A depolarizing cell becomes less negative inside upon stimulation, whereas a hyperpolarizing response causes the cell membrane to become more negative.
Photoreceptor “Dark Current”
The process of translating light into electrical signals is termed transduction. The biochemical steps that are involved occur within the photoreceptor outer segments, beginning with isomerization of the vitamin A retinal from the cis to the trans form within the visual pigment. The protein portion of rhodopsin is called opsin and is arranged in the shape of seven helixes that span the membrane of the free-floating discs in the rod outer segment. Isomerization of retinal from cis to trans form opens up the space of the opsin helixes and allows rhodopsin to interact with transducin. The subsequent cascade of events leads to a decrease of cyclic guanosine monophosphate (cGMP) which closes the ionic channels on the plasma membrane of the rod outer segment. This channel normally is open, which allows entry of sodium into the rod outer segment in the dark, with a compensatory extrusion of potassium from the rod inner segment to balance the net charge within the cell. This circulating “dark current” between the inner and outer segments maintains the rod in a relatively depolarized state. Closure of the cGMP-sensitive channel by light interrupts the dark current. Interrupting the influx of sodium, which carries positive charge into the cell, causes the photoreceptor to hyperpolarize. This intracellular voltage change is conveyed to the synaptic terminal, where the visual signal is transmitted to the bipolar and horizontal cells.
To a first-time student of retinal neurophysiology, these events initiated by light may appear the reverse of what would be expected. Rather than initiating a positive response in the rod, light actually suppresses a current that exists in the dark. Furthermore, in the dark, glutamate is released from the photoreceptor synapse (its neurotransmitter), and glutamate release is stopped upon signaling by light. The outcome of signaling light, however, is the same in that light causes a signaling difference to reach the bipolar cells as the message that vision was initiated.
Rod photoreceptors are highly efficient and can signal the isomerization event from a single photon of light. Each rod contains approximately 109 rhodopsin molecules, and the probability is remarkably high that every photon will be absorbed as it passes through the length of the rod outer segment. Fortunately, rhodopsin molecules are very stable in the dark and have a 400-year half-life before they spontaneously isomerize. Occasionally, however, a rhodopsin molecule will undergo spontaneous isomerization even in the absence of light, and this thermal noise results in a spurious signal being transmitted as “vision.” Fluctuations in the photocurrent of single rods can be observed in the dark, with a frequency of approximately one every few minutes in single unit recordings of rods. These spurious events are indistinguishable from rod responses initiated by true light and are called dark-light (alternatively called dark-noise). One can experience dark-light as the infrequent, spontaneous speckles seen after being in a fully darkened room for at least 1 hour. Dark-light is a temperature-dependent event and is more frequent with higher body temperature.
When they are in constant light, rod photoreceptors become less sensitive and do not respond as readily to individual photons. The rod response saturates in bright sustained light and renders the rod incapable of further signaling. Half-saturation occurs when each rod absorbs approximately 30 photons/second. Bright room lighting, equivalent to standard office lighting with fluorescent bulbs, is sufficient to saturate human rods.
Visual Transduction
The general scheme of visual transduction in rods is as follows. Light activates rhodopsin which couples to transducin. Transducin increases the activity of phosphodiesterase which, in turn, decreases cGMP by converting it to GMP. The high concentration of cGMP in the dark normally maintains the membrane channel in an open configuration. With light activation, cGMP decreases, and the channel closes. In darkness, the open channel allows influx of sodium. When the channel closes, the sodium influx decreases and interrupts the dark current flow that is maintained between the rod inner segment and outer segment. This, in turn, causes electrical hyperpolarization of the rod and produces the a-wave.
Closure of membrane channels by light also interrupts the normal influx of calcium into the outer segment. Since calcium is important for maintaining phosphodiesterase levels, the calcium change interrupts the degradation of cGMP to GMP. This, in turn, partially nullifies the light-activated decrease of cGMP and allows channels to reopen, terminating the light response and causing light adaptation.
The transduction process requires a number of constituents. It has recently been appreciated that genetic mutations that alter the molecular structure of the proteins and enzymes involved in transduction frequently lead to retinal degeneration. Rhodopsin mutations are found in 20% to 40% of persons with autosomal dominant retinitis pigmentosa. Retinal dystrophies are also associated with mutations in phosphodiesterase, in the outer segment channel protein, and in transducin. A detailed understanding of how these genetic mutations result in death of rods and widespread retinal degeneration remains an unsolved problem. One particular mutation in the rhodopsin molecule at codon 90 (glycine-90-aspartate) was found to alter the stability of rhodopsin and to increase the level of dark-light in human subjects with a special form of congenital night blindness.
PARALLEL PATHWAYS: VISION IN STARLIGHT AND DAYLIGHT
Separate Rod and Cone Signals
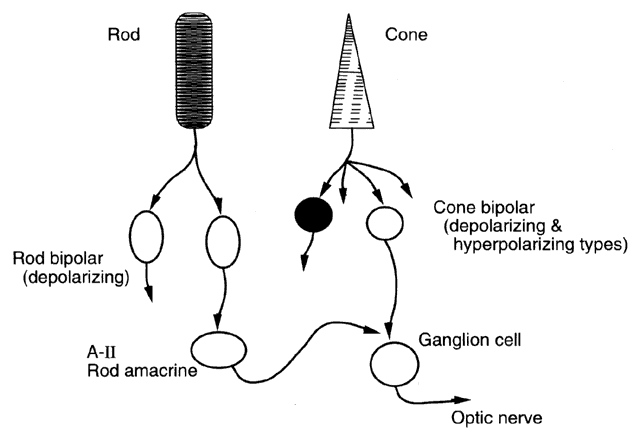
For the most part, the tasks of the rods and cones are quite different, and their signals remain separate within the retinal circuitry (Fig. 3). Rods respond to extraordinarily dim light equivalent to starlight, and a single photon is sufficient to activate a rod photoreceptor. The sensitivity of the rod pathway is further increased by pooling of signals, with signals from as many as 1000 rods all converging onto a single ganglion cell. This effectively enhances the sensitivity of rod vision in very dim light.
By comparison, each cone acts relatively independently of other cones: they do not pool signals substantially. This autonomy is necessary to ensure the precise spatial discrimination required for fine visual acuity. Pooling of cone signals would blur vision.
Each cone photoreceptor is activated only when struck by several photons simultaneously. This leads to an intrinsic difference in sensitivity from rods, which are activated by single photons. The dimmest light range of sensitivity is called scotopic, and implies that vision is mediated exclusively by rods. For bright stimuli, rods saturate and no longer respond significantly to further light, which leaves the cone circuit to mediate vision in the photopic range. The mesopic range overlaps the range of dim and bright light, and is therefore a transition zone between scotopic rod vision and photopic cone vision. Both rods and cones can respond under mesopic adaptation conditions, which correspond to the dim light at dusk and dawn. Patients with retinal disease frequently experience their worst vision impairment under mesopic conditions.
Rod Circuit in Starlight
The rod microcircuitry employs two clever tricks to increase the sensitivity of vision in starlight. A single rod connects to two depolarizing bipolar cells (DBCs). This divergence effectively amplifies the rod signal by a factor of two. Further amplification occurs because the rod synapse onto these DBCs has an intrinsic gain of about fivefold. These two rod DBCs make synaptic contact onto five amacrine cells, which further spreads the signals from each individual rod cell.
Rod signal sensitivity also benefits from convergence, in that signals from as many as 1000 rod photoreceptors are pooled onto a single ganglion cell. The convergent pooling of rod signals causes a loss of spatial discrimination, since a single photon captured by any of the 1000 rod photoreceptors in the pool will signal the same set of ganglion cells for transmission to the brain. Although this design may appear to be sloppy, it provides a tremendous increase in visual sensitivity in very dim light, with the tradeoff of reduced spatial discrimination.
Cone Circuit in Daylight
The cone circuit operates inherently differently from rods. Whereas individual rod photoreceptors are activated by absorption of a single photon, activation of an individual cone requires four to six simultaneous quantal hits. An immediate advantage to this scheme is that cones will be insensitive to vision in dimmest light and will not interfere with rod vision, since they will not send overlapping signals to the brain. The cone microcircuitry is also more complicated even at the first stage of vision, since individual cones synapse onto as many as 30 postsynaptic cells, including horizontal cells and several classes of bipolar cells. Some cone bipolar cells function by depolarizing when stimulated (depolarizing bipolar cells [DBCs]) whereas others hyperpolarize when stimulated (hyperpolarizing bipolar cells [HBCs]). This design is useful for daylight vision, in which both increments and decrements of light are important. Cone circuitry exhibits convergence to a lesser extent than rod circuitry.
Output Pathway from Retina to Brain
Despite the seemingly independent rod and cone circuitry of the retina, only a single set of ganglion cells actually transmits retinal signals toward the brain. Thus, signals from both rod and cone photoreceptors ultimately must feed into the same ganglion cells. In the cone circuit, transmission is from cones to cone bipolar cells to ganglion cells. The rod pathway is more circuitous. The rod bipolar cells do not synapse onto ganglion cells directly but only indirectly through the amacrine cells. Rods synapse onto bipolar cells and then onto amacrine cells that pass the signal to ganglion cells. The retina is rich in amacrine cells; the cat and rabbit are known to have 30 to 40 types. The connection from the rod bipolar cell to the ganglion cell is primarily through the AII amacrine cell, which has multiple feedback and feedforward control mechanisms through the other types of amacrine cells. This amacrine network ultimately controls signal transmission from the rod pathway to the ganglion cell by controlling the AII amacrine cell. From an engineering perspective, the AII amacrine cell serves as a switch that can disconnect the rod pathway from the cone pathway in bright light photopic vision, where the cone pathway needs to have exclusive access to the vision outflow to the brain, without interference from rod signals.
INTERACTION BETWEEN ROD AND CONE SIGNALS
Rod and cone signals are maintained relatively separate under purely scotopic and purely photopic conditions. For the intermediate realm of the mesopic vision in twilight conditions, however, there is evidence that the rod and cone signals can interact. One example involves detection of flickering-light stimuli by the cones, which becomes less sensitive when the rods begin to adapt to the dark and regain their sensitivity in dimmer light. Evidently, rods can have a considerable impact on the cone circuit under this particular condition. Another example involves color and spatial discrimination. Both are mediated by cone systems but are partially suppressed under mesopic conditions in which rods are active.
To explain rod and cone interactions, it must be demonstrated that there is some anatomic connection between these two pathways. One site of interaction would be through the AII amacrine cell, where rod signals can be fed onto the cone bipolar cells and thereby impinge on the cone signals. Another site is through the horizontal cells that lie immediately adjacent to both rods and cones, in the microcircuitry of the outer retina. Furthermore, it has been demonstrated that rod signals in the primate retina are found in cone photoreceptors, which indicates the presence of electrical coupling between rods and cones.
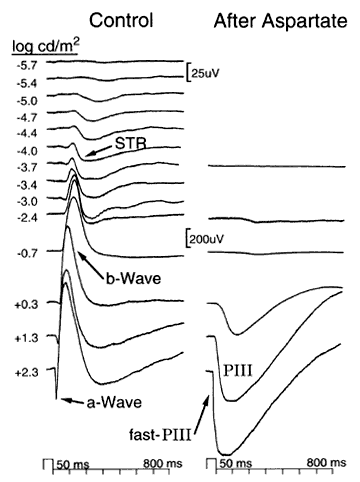
Signals that are conducted through the rod AII amacrine cell can be studied by recording the scotopic threshold response (STR) of the ERG. This is a tiny, negative-going ERG wave, best recorded under extremely dim light conditions and after complete dark adaptation of 1 hour or more. The STR is present in the ERG of a variety of species, including mouse, rat, cat, dog, sheep, monkey, and human. Although the full diagnostic value of the STR remains to be determined, the STR is absent in some types of congenital stationary night blindness (CSNB) patients and is diminished in glaucoma.
RETINAL NEUROTRANSMITTERS
As befits the neural complexity of the retina, all of the major neurotransmitters have been identified in this tissue. Glutamate is the principal transmitter from photoreceptors onto bipolar and horizontal cells. Glutamate is also used by bipolar cells to communicate with amacrine cells. At both rod and cone photoreceptors, glutamate is continuously released in the synaptic cleft in darkness, and its release is interrupted by the light response of the photoreceptor. The postsynaptic machinery is of two principal types:
OFF-bipolar cells (HBCs) receive direct chemical synaptic input from glutamate. The presence
of glutamate in the dark depolarizes the OFF-bipolar cells, whereas
the interruption of glutamate in the light hyperpolarizes these cells.
ON-bipolar cells (DBCs) work in reverse. These cells are hyperpolarized in their unexcited
state in the dark during glutamate release, whereas cessation of glutamate
release in the light causes these bipolar cells to depolarize. This
involves inverting the signaling process and indicates that a second
messenger system is involved at the synapse, which can reverse the
normal chemical signaling and cause bipolar cells to depolarize only
upon withdrawal of synaptic glutamate during the light-evoked photoreceptor
response.
The sign-inverting synapse between the rods and the depolarizing rod bipolar cell uses a metabotropic glutamate receptor that can be blocked by 2-amino-4-phosphonobutyric acid (APB). This particular APB-sensitive mGluR6 glutamate receptor was molecularly cloned in 1993. Although multiple subtypes of the glutamate receptor have been identified, the APB-sensitive form appears to be unique to the retina. Experiments show that applying APB to the retina of many species, including primates, will block synaptic transmission and cause a loss of the dark-adapted b-wave (i.e., it blocks bipolar depolarization) without altering the a-wave (from rod photoreceptors). This particular ERG change mimics quite closely the ERG found in the type of CSNB patients who have rod photoreceptors and rod visual pigment, but who still cannot see at night, presumably because of faulty synaptic transmission. It may be that genetic mutations that alter the function of this metabotropic receptor could result in CSNB abnormality.
Inner retinal neurons utilize a variety of neurotransmitters, including gamma-aminobutyric acid (GABA), glycine, and dopamine. Specialized NMDA receptors have been identified in the proximal retina of many species. Dopamine is involved in setting the dark- or light-adaptation state of the retina. Dopamine can modulate the coupling or uncoupling of photoreceptors as well as produce retinomotor movement of the pigment epithelial sheaths that surround the rod and cone photoreceptors. Dopamine is released from dopaminergic amacrine cells in the proximal retina and diffuses to the outer retina, where it works in exocrine fashion on the photoreceptors and RPE. Work with experimental myopia in the chick model has implicated dopamine in development of myopia.