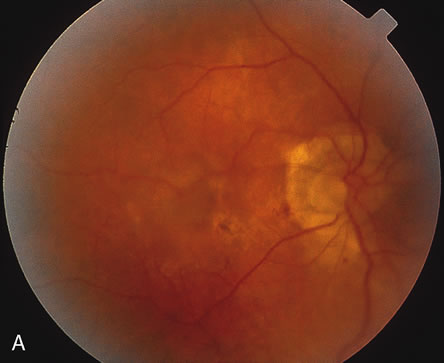
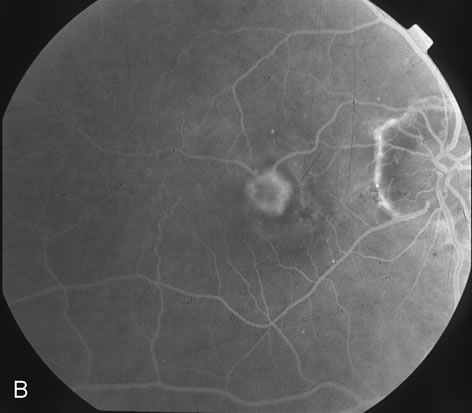
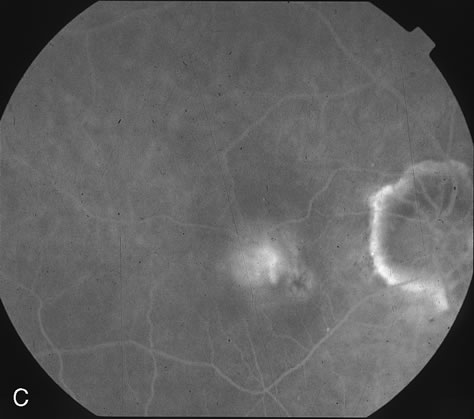
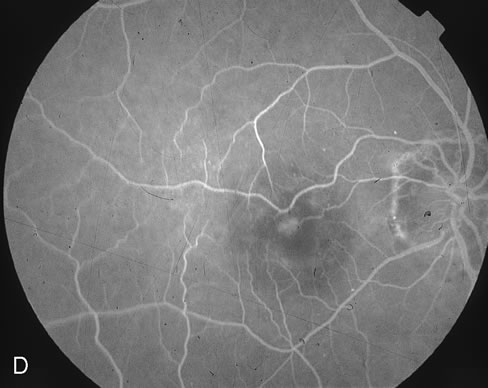
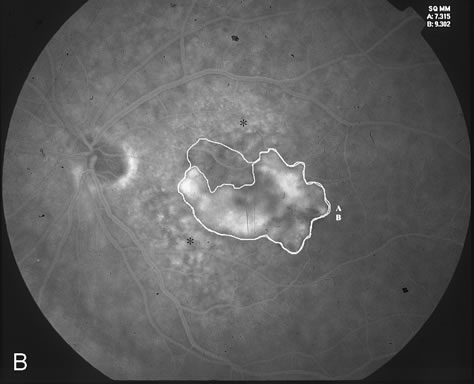
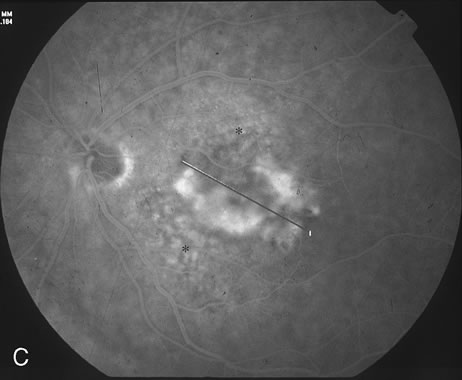
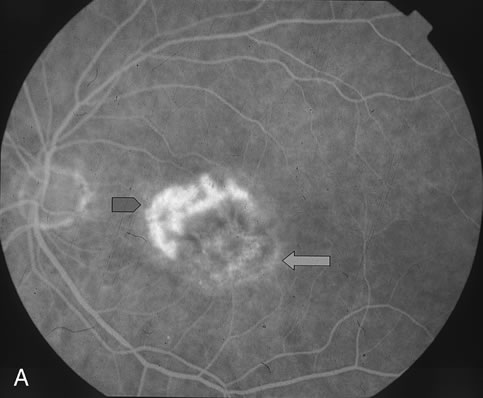
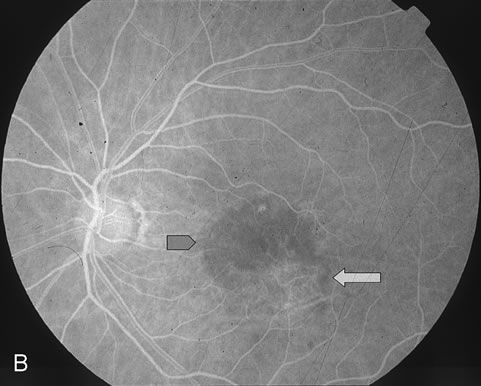
1. Dougherty TJ, Grindey GB, Fiel R et al: Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin
and light. J Natl Cancer Inst 55:115, 1975 2. Henderson BW, Dougherty TJ: How does photodynamic therapy work? Photochem Photobiol; 55:145, 1992 3. Gomer CJ, Doiron DR, White L et al: Hematoporphyrin derivative photoradiation induced damage to normal and
tumor tissue of the pigmented rabbit eye. Curr Eye Res; 3:229, 1984 4. Nanda SK, Hatchell DL, Tiedeman JS et al: A new method for vascular occlusion. Photochemical initiation of thrombosis. Arch Ophthalmol; 105:1121, 1987 5. Stender IM, Na R, Fogh H, et al: Photodynamic therapy with 5-aminolaevulinic acid or placebo for
recalcitrant foot and hand warts: Randomised double-blind trial. Lancet 355:963, 2000 6. Wang I, Bendsoe N, Klinteberg CA et al: Photodynamic therapy vs. cryosurgery of basal cell carcinomas: Results
of a phase III clinical trial. Br J Dermatol 144:832, 2001 7. Roberts WG, Hasan T: Role of neovasculature and vascular permeability on the tumor retention
of photodynamic agents. Cancer Res 52:924, 1992 8. Miller JW, Walsh AW, Kramer M et al: Photodynamic therapy of experimental choroidal neovascularization using
lipoprotein-delivered benzoporphyrin. Arch Ophthalmol 113:810, 1995 9. Buettner GR, Oberley LW: The apparent production of superoxide and hydroxyl radicals by hematoporphyrin
and light as seen by spin-trapping. FEBS Lett 121:161, 1980 10. Schmidt-Erfurth U, Hasan T, Gragoudas E, Birngruber R: [Selective occlusion of subretinal neovascularization with photodynamic
therapy]. Ophthalmologe 91:789, 1994 11. Schmidt-Erfurth U, Bauman W, Gragoudas E et al: Photodynamic therapy of experimental choroidal melanoma using lipoprotein-delivered
benzoporphyrin. Ophthalmology; 101:89, 1994 12. Kim RY, Hu LK, Foster BS et al: Photodynamic therapy of pigmented choroidal melanomas of greater than 3-mm
thickness. Ophthalmology 103:2029, 1996 13. Young LH, Howard MA, Hu LK et al: Photodynamic therapy of pigmented choroidal melanomas using a liposomal
preparation of benzoporphyrin derivative. Arch Ophthalmol 114:186, 1996 14. Husain D, Miller JW, Michaud N et al: Intravenous infusion of liposomal benzoporphyrin derivative for photodynamic
therapy of experimental choroidal neovascularization. Arch Ophthalmol 114:978, 1996 15. Hager A, Schmidt-Erfurth U, Barbazetto I et al: [Photodynamic therapy: ICG angiography findings]. Ophthalmologe 96:291, 1999 16. Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H: Photodynamic effects on choroidal neovascularization and physiological
choroid. Invest Ophthalmol Vis Sci 43:830, 2002 17. Flower RW: Expanded hypothesis on the mechanism of photodynamic therapy action on
choroidal neovascularization. Retina 19:365, 1999 18. Schmidt-Erfurth U: Indocyanine green angiography and retinal sensitivity after photodynamic
therapy of subfoveal choroidal neovascularization. Semin Ophthalmol 14:35, 1999 19. Michels S, Barbazetto I, Schmidt-Erfurth U: [Choroidal changes after photodynamic therapy (PDT). A two-year
follow-up study of 38 patients]. Klin Monatsbl Augenheilkd; 217:94, 2000 20. Sickenberg M, Schmidt-Erfurth U, Miller JW et al: A preliminary study of photodynamic therapy using verteporfin for choroidal
neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid
streaks, and idiopathic causes. Arch Ophthalmol 118:327, 2000 21. Miller JW, Schmidt-Erfurth U, Sickenberg M et al: Photodynamic therapy with verteporfin for choroidal neovascularization
caused by age-related macular degeneration: Results of a single
treatment in a phase 1 and 2 study. Arch Ophthalmol 117:1161,1999 22. Schmidt-Erfurth U, Miller JW, Sickenberg M et al: Photodynamic therapy with verteporfin for choroidal neovascularization
caused by age-related macular degeneration: Results of retreatments
in a phase 1 and 2 study. Arch Ophthalmol; 117:1177, 1999 23. Photodynamic therapy of subfoveal choroidal neovascularization in age-related
macular degeneration with verteporfin: one-year results
of 2 randomized clinical trials—TAP report. Treatment of
age-related macular degeneration with photodynamic therapy (TAP) Study
Group. Arch Ophthalmol 117:1329, 1999 24. Lam S, Kostashuk EC, Coy EP et al: A randomized comparative study of the safety and efficacy of photodynamic
therapy using Photofrin II combined with palliative radiotherapy versus
palliative radiotherapy alone in patients with inoperable obstructive
non-small cell bronchogenic carcinoma. Photochem Photobiol 46:893, 1987 25. Keefe KA, Chahine EB, DiSaia PJ et al: Fluorescence detection of cervical intraepithelial neoplasia for photodynamic
therapy with the topical agents 5-aminolevulinic acid and
benzoporphyrin-derivative monoacid ring. Am J Obstet Gynecol 184:1164, 2001 26. Kreimer-Birnbaum M: Modified porphyrins, chlorins, phthalocyanines, and purpurins: second-generation
photosensitizers for photodynamic therapy. Semin Hematol 26:157, 1989 27. Richter AM, Kelly B, Chow J et al: Preliminary studies on a more effective phototoxic agent than hematoporphyrin. J Natl Cancer Inst 79:1327, 1987 28. Rivellese MJ, Baumal CR: Photodynamic therapy of eye diseases. Ophthalmic Surg Lasers 30:653, 1999 29. Husain D, Kramer M, Kenny AG et al: Effects of photodynamic therapy using verteporfin on experimental choroidal
neovascularization and normal retina and choroid up to 7 weeks after
treatment. Invest Ophthalmol Vis Sci 40:2322, 1999 30. Verteporfin therapy of subfoveal choroidal neovascularization in age-related
macular degeneration: Two-year results of a randomized
clinical trial including lesions with occult with no classic choroidal
neovascularization—verteporfin in photodynamic therapy report 2. Am J Ophthalmol 131:541, 2001 31. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic
myopia with verteporfin. 1-year results of a randomized clinical
trial—VIP report no. 1. Ophthalmology 108:841, 2001. 32. Verteporfin in Photodynamic Therapy (VIP) Study Group: Verteporfin
therapy of subfoveal choroidal neovascularization in pathologic
myopia. Two-year results of a randomized clinical trial—VIP
report no. 3. Ophthalmology 110:667, 2003 33. Baumal C, Puliafito CA, Pieroth L et al: Photodynamic therapy (PDT) of experimental choroidal neovascularization
with tin ethyl etiopurpurin, The Association of Research in
Vision and Ophthalmology, Fort Lauderdale, FL, April 1996. 34. Renno RZ, Miller JW: Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv Drug Deliv Rev 52:63, 2001 35. Blumenkranz MS WK, Verdooner S: Lutetium texaphyrin angiography: A new method for the evaluation and treatment
of retinal and choroidal vascular disorders [abstract]. Invest Ophthalmol Vis Sci 39:S486, 1998 36. Macular Photocoagulation Study Group: Laser photocoagulation of subfoveal
neovascular lesions in age-related macular degeneration. Results
of a randomized clinical trial. Arch Ophthalmol 109:1220, 1991 37. Macular Photocoagulation Study Group: Laser photocoagulation of subfoveal
recurrent neovascular lesions in age-related macular degeneration. Results
of a randomized clinical trial. Arch Ophthalmol 109:1232, 1991 38. Macular Photocoagulation Study Group: Subfoveal neovascular lesions in
age-related macular degeneration. Guidelines for evaluation and
treatment in the macular photocoagulation study. Arch Ophthalmol; 109:1242, 1991 39. Macular Photocoagulation Study Group: Laser photocoagulation of subfoveal
neovascular lesions of age-related macular degeneration. Updated
findings from two clinical trials. Arch Ophthalmol 111:1200, 1993 40. Bressler NM: Photodynamic therapy of subfoveal choroidal neovascularization in age-related
macular degeneration with verteporfin: Two-year results
of 2 randomized clinical trials-tap report 2. Arch Ophthalmol 119:198, 2001 41. Bressler NM, Arnold J, Benchaboune M et al: Verteporfin therapy of subfoveal choroidal neovascularization in patients
with age-related macular degeneration: Additional information
regarding baseline lesion composition's impact on vision outcomes—TAP
report No. 3. Arch Ophthalmol 120:1443,2002 42. Blumenkranz MS, Bressler NM, Bressler SB et al: Verteporfin therapy for subfoveal choroidal neovascularization in age-related
macular degeneration: Three-year results of an open-label
extension of 2 randomized clinical trials—TAP Report
no. 5. Arch Ophthalmol; 120:1307, 2002 43. Rubin GS, Bressler NM: Effects of verteporfin therapy on contrast on sensitivity: Results from
the treatment of age-related macular degeneration with photodynamic
therapy (TAP) investigation—TAP report No 4. Retina; 22:536, 2002 44. Bressler NM: Verteporfin therapy of subfoveal choroidal neovascularization in age-related
macular degeneration: Two-year results of a randomized
clinical trial including lesions with occult with no classic choroidal
neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol 133:168, 2002 45. Macular Photocoagulation Study Group: Five-year follow-up
of fellow eyes of patients with age-related macular degeneration
and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol 111:1189, 1993 46. Macular Photocoagulation Study Group: Laser photocoagulation for juxtafoveal
choroidal neovascularization. Five-year results from randomized
clinical trials. Arch Ophthalmol 112:500, 1994 47. Jampol LM, Scott L: Treatment of juxtafoveal and extrafoveal choroidal neovascularization in
the era of photodynamic therapy with verteporfin. Am J Ophthalmol 134:99, 2002 48. Sharma S, Hollands H, Brown GC et al: Improvement in quality of life from photodynamic therapy: a Canadian perspective. Can J Ophthalmol 36:332, 200 49. Sharma S, Brown GC, Brown MM et al: The cost-effectiveness of photodynamic therapy for fellow eyes with
subfoveal choroidal neovascularization secondary to age-related
macular degeneration. Ophthalmology 108:2051,2001 50. Piermarocchi S, Lo Giudice G, Sartore M et al: Photodynamic therapy increases the eligibility for feeder vessel treatment
of choroidal neovascularization caused by age-related macular
degeneration. Am J Ophthalmol 133:572, 2002 51. Borodoker N SR, Maranan L, Murray J et al: Verteporfin infusion-associated pain. Am J Ophthalmol; 133:211,2002 52. Spaide RF, Maranan L: Neutrophil margination as a possible mechanism for verteporfin infusion-associated
pain. Am J Ophthalmol 135:549, 2003 53. Tornambe P: Using intravenous diphenhydramine to minimize back pain associated with
photodynamic therapy with verteporfin. Arch Ophthalmol 120:872, 2002. 54. Saperstein DA, Rosenfeld PJ, Bressler NM et al: Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin
in the ocular histoplasmosis syndrome: One-year results
of an uncontrolled, prospective case series. Ophthalmology; 109:1499, 2002 55. Spaide RF, Martin ML, Slakter J et al: Treatment of idiopathic subfoveal choroidal neovascular lesions using photodynamic
therapy with verteporfin. Am J Ophthalmol; 134:62,2002 56. Karacorlu M, Karacorlu S, Ozdemir H, Mat C: Photodynamic therapy with verteporfin for choroidal neovascularization
in patients with angioid streaks. Am J Ophthalmol 134:360, 2002. 57. Battaglia Parodi M, Da Pozzo S, Toto L et al: Photodynamic therapy for choroidal neovascularization associated with choroidal
osteoma. Retina 21:660,2001. 58. Valmaggia C, Niederberger H, Helbig H: Photodynamic therapy for choroidal neovascularization in fundus flavimaculatus. Retina 22:111,2002 59. Coquelet P, Postelmans L, Snyers B, Verougstraete C: Successful photodynamic therapy combined with laser photocoagulation in
three eyes with classic subfoveal choroidal neovascularisation affecting
two patients with multifocal choroiditis: Case reports. Bull Soc Belge Ophtalmol:69, 2002 60. Quaranta M, Mauget-Faysse M, Coscas G: Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic
therapy with verteporfin. Am J Ophthalmol; 134:277, 2002 61. Potter MJ, Szabo SM, Chan EY, Morris AH: Photodynamic therapy of a subretinal neovascular membrane in type 2A idiopathic
juxtafoveolar retinal telangiectasis. Am J Ophthalmol 133:149,2002 62. Ladd BS, Solomon SD, Bressler NM, Bressler SB: Photodynamic therapy with verteporfin for choroidal neovascularization
in patients with diabetic retinopathy. Am J Ophthalmol; 132:659, 2001 63. Schmidt-Erfurth UM, Kusserow C, Barbazetto IA, Laqua H: Benefits and complications of photodynamic therapy of papillary capillary
hemangiomas. Ophthalmology 109:1256,2002 64. Madreperla SA: Choroidal hemangioma treated with photodynamic therapy using verteporfin. Arch Ophthalmol 119:1606,2001 65. Barbazetto I, Schmidt-Erfurth U: Photodynamic therapy of choroidal hemangioma: Two case reports. Graefes Arch Clin Exp Ophthalmol 238:214, 2000. 66. Diestelhorst M, Grisanti S: Photodynamic therapy to control fibrosis in human glaucomatous eyes after
trabeculectomy: A clinical pilot study. Arch Ophthalmol 120:130, 2002 67. Davidorf J, Davidorf F: Treatment of iris melanoma with photodynamic therapy. Ophthalmic Surg 23:522,1992 68. Stevens TS, Bressler NM, Maguire MG et al: Occult choroidal neovascularization in age-related macular degeneration. A
natural history study. Arch Ophthalmol 115:345, 1997 |