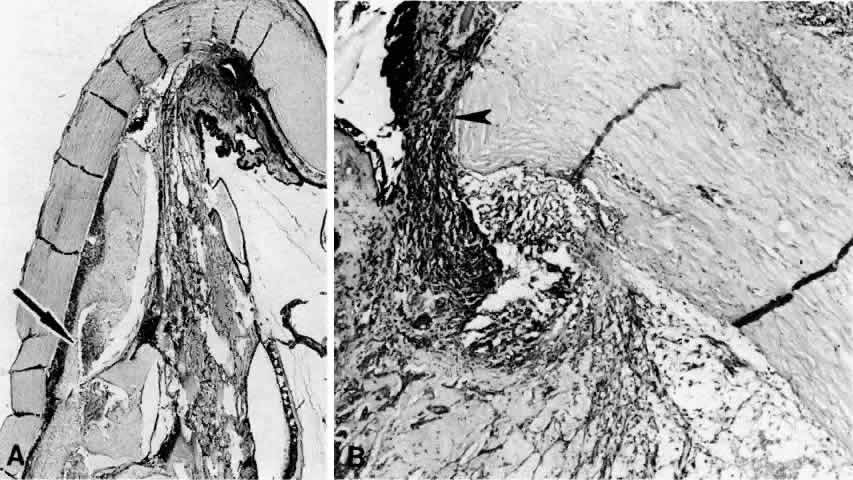
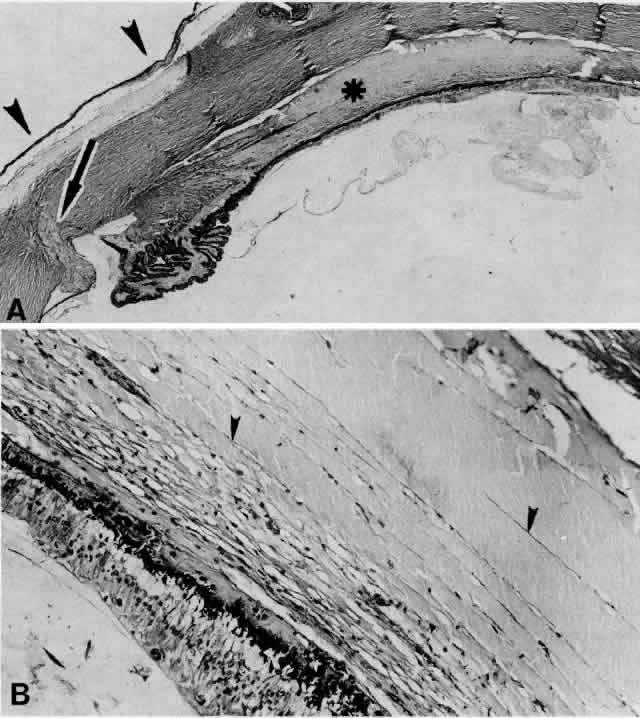
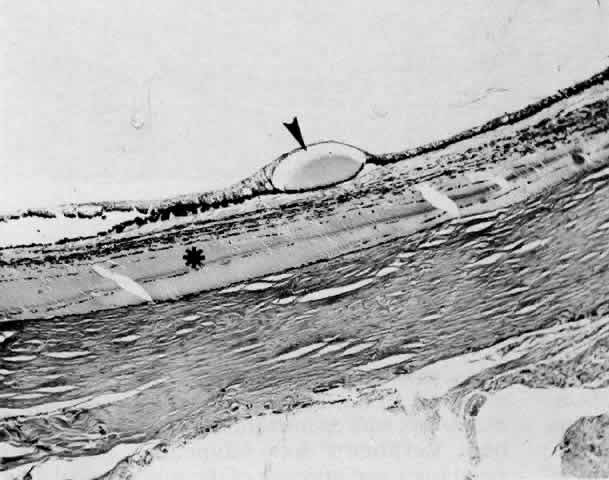
1. Spaeth EP, de Long P: Detachment of the choroid: A clinical and histopathologic analysis. Arch Ophthalmol 32:217, 1944 2. Brav SS: Serous choroidal detachment. Surv Ophthalmol 6:395, 1961 3. Von Graefe A: Zur diagnose des beginnenden intraocularen krebses. Graefes Arch Ophthalmol 4:218, 1858 4. Weiter JJ, Ernest JT: Anatomy of the choroidal vasculature. Am J Ophthalmol 78:583, 1974 5. Bill A: Intraocular pressure and blood flow through the uvea. Arch Ophthalmol 67:336, 1962 6. Inomata H, Bill A, Smesler GK: Unconventional routes of aqueous humor outflow in cynomolgus monkey (Macaca
irus). Am J Ophthalmol 73:893, 1972 7. Moses RA: Accommodation. In Moses RA (ed): Alder's Physiology of the
Eye: Clinical Application, p 313. St. Louis, CV Mosby, 1981 8. Moses RA: Detachment of the ciliary body: Anatomic and physical considerations. Invest Ophthalmol 4:935, 1965 9. Hertz V: Choroidal detachment with notes on scleral depression and pigmented streaks
in the retina. Acta Ophthalmol 41(suppl):3, 1954 10. Hyman BN, Hagler WS: Bilateral annular detachment and myopia. Am J Ophthalmol 70:853, 1970 11. Soylev MF, Green RL, Feldon SE: Choroidal effusion as a mechanism for transient myopia induced by hydrochlorothiazide
and triamterene. Am J Ophthalmol 120: 395, 1995 12. Kimbrough RL, Trempe CS, Brockhurst RJ, Simmons RJ: Angle-closure glaucoma in nanophthalmos. Am J Ophthalmol 88:572, 1979 13. Schepens CL, Brockhurst RJ: Uveal effusion: I. Clinical picture. Arch Ophthalmol 70:189, 1963 14. Fourman S: Angle-closure glaucoma complicating ciliochoroidal detachment. Ophthalmology 96:646, 1989 15. Jin JC, Anderson DR: Laser and unsutured sclerotomy in nanophthalmos. Am J Ophthalmol 109:575, 1990 16. Koster HR, Liebmann JM, Ritch R et al: Acute angle-closure glaucoma in a patient with acquired immunodeficiency
syndrome successfully treated with argon laser peripheral iridoplasty. Ophthalmic Surg 21:501, 1990 17. Nash RW, Lindquist TD: Bilateral angle-closure glaucoma associated with uveal effusion: Presenting
sign of HIV infection. Surv Ophthalmol 36:255, 1992 18. Ullman S, Wilson RP, Schwartz L: Bilateral angle-closure glaucoma in association with the acquired immune
deficiency syndrome. Am J Ophthalmol 101:419, 1986 19. Dodds EM, Lowder CY, Barnhorst DA et al: Posterior scleritis with annular ciliochoroidal detachment. Am J Ophthalmol 120:677, 1995 20. Quinlan MP, Hitchings RA: Angle-closure glaucoma secondary to posterior scleritis. Br J Ophthalmol 62:330, 1978 21. Brockhurst RJ, Lam KW: Uveal effusion: II. Report of a case with analysis of subretinal fluid. Arch Ophthalmol 90:399, 1973 22. Verhoeff FH: The nature and origin of the pigmented streaks caused by separation of
the choroid. JAMA 92:1873, 1931 23. Gottlieb F: Combined choroidal and retinal detachment. Arch Ophthalmol 88:481, 1972 24. Graham PA: Unusual evolution of retinal detachments. Trans Ophthalmol Soc UK 78:359, 1958 25. Seelenfreund MH, Kraushar MF, Schepens CL et al: Choroidal detachment associated with primary retinal detachment. Arch Ophthalmol 91:254, 1974 26. Ferry AP: Lesions mistaken for malignant melanoma of the posterior uvea. Arch Ophthalmol 92:463, 1964 27. Hagen S: Die serose postoperative Choroideallablosung und ihre pathogenese. Klin Monatsbl Augenheilkd 66: 121, 1921 28. Shields JA, Leonard BC, Sarin LK: Multilobed uveal melanoma masquerading as postoperative choroidal detachment. Br J Ophthalmol 60:386, 1976 29. Coleman DJ: Ocular diagnosis. In Coleman DJ, Lizzi FL, Jack RL (eds): Ultrasonography
of the Eye and Orbit. Philadelphia, Lea & Febiger, 1977 30. Wing GL, Schepens CL, Trempe CL: Serous choroidal detachment and the thickened-choroid sign detected by
ultrasonography. Am J Ophthalmol 94:499, 1982 31. Peyman GA, Mafee M, Schulman J: Computerized tomography in choroidal detachment. Ophthalmology 91:156, 1984 32. Rosen E, Lyne A: Uveal effusions: I. A clinical picture. Am J Ophthalmol 65:509, 1968 33. Villaseca A: Late emptying of anterior chamber and choroidal detachment in cataract
operations. Arch Ophthalmol 52:250, 1954 34. Ruiz RS, Salmonsen PC: Expulsive choroidal effusion. Arch Ophthalmol 94:69, 1975 35. Campbell JK: Expulsive choroidal hemorrhage and effusion: A reappraisal. Ann Ophthalmol 12:332, 1980 36. Maumenee AE, Schwartz MF: Acute intraoperative choroidal effusion. Am J Ophthalmol 100:147, 1985 37. Bellows AR, Chylack LT Jr, Hutchinson BT: Choroidal detachment. Ophthalmology 88:1107, 1981 38. Fuchs E: Ablosung der Aderhaut nach Staaroperation. Arch Ophthalmol 51:199, 1900 39. Bard LA: Eyes with choroidal detachments removed for suspected melanoma. Arch Ophthalmol 73:320, 1965 40. Cotlier E: Aphakic flat anterior chamber: III. Effect of inflation of the anterior
chamber and drainage of choroidal detachments. Arch Ophthalmol 88:16, 1972 41. McClure HL: Massive bilateral choroidal detachment occurring in an aphakic patient
six years and nine months postoperatively. Am J Ophthalmol 63:295, 1967 42. Shah RR: Flat anterior chamber and choroidal detachment in aphakia: Study of 500 cataract
extractions. Br J Ophthalmol 55:48, 1971 43. Savir H: Expulsive choroidal effusion during cataract surgery. Ann Ophthalmol 11:113, 1979 44. Swyers EM: Choroidal detachment immediately following cataract extraction. Arch Ophthalmol 88:632, 1972 45. Rosengren B: Aqueous leakage through the uveal vessels: A factor in choroidal detachment? Acta Ophthalmol 48:901, 1970 46. Janotka H, Huczynska B: Choroidal detachment following cataract extraction. Klin Oczna 42:743, 1972 47. Pederson JE, Gaasterland DE, McClean HM: Experimental ciliochoroidal detachment: Effect on intraocular pressure
and aqueous humor flow. Arch Ophthalmol 97:536, 1979 48. Sakai T, Tamashita S: Choroidal detachment after glaucoma surgery. Folia Ophthalmol Japan 19:174, 1968 49. Ursin KY: On spontaneous choroidal detachment after acute glaucoma in the light of
an exceptional case. Acta Ophthalmol 43:751, 1965 50. Hawkins WR, Schepens CL: Choroidal detachment and retinal surgery. Am J Ophthalmol 62:813, 1966 51. Packer AJ, Maggiano JM, Aaberg TM et al: Serous choroidal detachment after retinal detachment surgery. Arch Ophthalmol 101:1221, 1983 52. Kishimoto M: Choroidal detachment after retinal detachment surgery. Folia Ophthalmol Japan 17:569, 1966 53. Chignell AH: Choroidal detachment following retinal detachment surgery without drainage
of subretinal fluid. Am J Ophthalmol 78:860, 1972 54. Topilow HW, Ackerman AL: Massive exudative retinal and choroidal detachments following scleral buckle
surgery. Ophthalmology 90:143, 1983 55. Huamonte FU, Peyman GA, Goldberg MF: Immediate fundus complications after retinal scatter photocoagulation. Ophthalmic Surg 7:88, 1976 56. Weiter JJ, Brockhurst RJ, Tolentino FI: Uveal effusion following panretinal photocoagulation. Ann Ophthalmol 11:1723, 1979 56a. Gentile RC, Stegman Z, Liebmann JM et al: Risk factors for ciliochoroidal effusion after panretinal photocoagulation. Ophthalmology 103:827, 1996 57. Vela MA, Campbell DG: Hypotony and ciliochoroidal detachment following pharmacologic aqueous
suppressant therapy in previously filtered patients. Ophthalmology 92:50, 1985 58. Hayreh SS, Baines JAB: Occlusion of the vortex veins: An experimental study. Br J Ophthalmol 57:217, 1973 59. Dotan S, Oliver M: Shallow anterior chamber and uveal effusion after nonperforating trauma
to the eye. Am J Ophthalmol 94:782, 1982 60. Woillez M, Dufour ABD, Guilbert M: Le decollement annulaire inflammatoire. Bull Soc Ophtalmol Fr 67:822, 1967 61. McGrand JC: Choroidal detachment in aphakic uveitis. Br J Ophthalmol 53:778, 1969 62. Kanter PJ, Goldberg MF: Bilateral uveitis with exudative retinal detachment. Arch Ophthalmol 91:13, 1974 63. Gamringer H, Schreck E, Wollensak J: Zur pathogenese und therapie der cyclitis anularis ersudative pseudotumorosa. Klin Monatsbl Augenheilkd 135:638, 1959 64. DeLuise VP, Clark SW III, Smith JL et al: Syphilitic retinal detachment and uveal effusion. Am J Ophthalmol 94:757, 1982 65. Green WR: The uveal tract. In Spencer WH (ed): Ophthalmic Pathology, Vol 3, pp 1778–1791. Philadelphia, WB Saunders, 1986 66. Groenouw A: Choroiditis disseminata sympathetica nach Iridectomie des anderen Auges. Klin Monatsbl Augenheilkd 76:694, 1962 67. de Veer JA: Sympathizing eye in sympathetic ophthalmia. Arch Ophthalmol 48:723, 1952 68. Hurd ER, Snyder WM, Ziff M: Choroidal nodules and detachments in rheumatoid arthritis. Am J Med 48:273, 1970 69. Brockhurst RJ, Schepens CL, Okamura ID: Uveitis: II. Peripheral uveitis: Clinical description, complications and
differential diagnosis. Am J Ophthalmol 49:1257, 1960 70. Brockhurst RJ, Schepens CL, Okamura ID: Uveitis: III. Peripheral uveitis: Etiology and treatment. Am J Ophthalmol 51:19, 1961 71. Kimura R, Sakai M, Okabe H: Transient shallow anterior chamber as initial symptom of Harada's
syndrome. Arch Ophthalmol 99:1604, 1981 72. Muirhead WM: Disease of the conjunctiva: Case of subconjunctival abscess associated
with a detachment of the choroid. Trans Ophthalmol Soc UK 54:578, 1934 73. Ryan SJ, Zimmerman LE, King FM: Reactive lymphoid hyperplasia: An unusual form of intraocular pseudotumor. Trans Am Acad Ophthalmol Otolaryngol 76:652, 1972 74. Preisler E: Spontaneous choroidal detachment. Acta Ophthalmol 42:657, 1964 75. Simpson ER, Adams ST: Spontaneous uveal effusions. Can J Ophthalmol 5:41, 1970 76. Van Alphen GWHM: On emmetropia and ametropia. Ophthalmologica 142:49, 1961 77. Velzeboer J Jr: Spontaneous choroidal detachment. Am J Ophthalmol 49:898, 1960 78. Sears ML: Choroidal and retinal detachments associated with scleritis. Am J Ophthalmol 58:764, 1964 79. Austin D, Green WR, Sallyer DC et al: Peripheral corneal degeneration and occlusive vasculitis in Wegener's
granulomatosis. Am J Ophthalmol 85:311, 1978 80. Nanjiani MR: Ocular manifestations of polyarteritis nodosa. Br J Ophthalmol 51:696, 1967 81. Vancea P, Popa G, Vaighel V, Gavrilita L: Périartérite noueuse a localisation oculaire. Ann Ocul 195:632, 1962 82. Suan EP, Rubsamen PE, Byrne SF: Bilateral ciliochoroidal detachments after Valsalva maneuver. Arch Ophthalmol 111:304, 1993 83. Wald KJ, Brockhurst RJ, Roth S, Bodine SR: Choroidal effusions in two patients with glomerulonephritis. Ann Ophthalmol 24:64, 1992 84. Harbison JW, Guerry D, Weisinger H: Dural arteriovenous fistula and spontaneous choroidal detachment: New cause
of an old disease. Br J Ophthalmol 62:483, 1978 85. Woillez M, Dufour ABD: Decollement annulaire anterieure de la chorio-retine après fistule
carotidocaverneuse. Bull Soc Ophtalmol Fr 67:819, 1967 86. Guerry III D, Harbison JW, Weisinger H: Bilateral choroidal detachment and fluctuating proptosis secondary to bilateral
dural arteriovenous fistulas treated with transcranial orbital
decompression with resolution: Report of a case. Trans Am Ophthalmol Soc 73:64, 1975 87. Kreiger AE, Meyer D, Smith T, Reimer K: Metastatic carcinoma to the choroid with choroidal detachment. Arch Ophthalmol 82:209, 1969 88. Brockhurst RJ: Nanophthalmos with uveal effusion. Trans Am Ophthalmol Soc 72:371, 1974 89. Brockhurst RJ: Nanophthalmos with uveal effusion: A new clinical entity. Arch Ophthalmol 93:1289, 1975 90. Good WV, Stern WH: Recurrent nanophthalmic uveal effusion syndrome following laser trabeculoplasty. Am J Ophthalmol 106:234, 1988 91. Brockhurst RJ: Vortex vein decompression for nanophthalmic uveal effusion. Arch Ophthalmol 98:1987, 1980 92. Gass JDM: Uveal effusion syndrome: A new hypothesis concerning pathogenesis and technique
of surgical treatment. Retina 3:159, 1983 93. Singh OS, Simmons RJ, Brockhurst RJ et al: Nanophthalmos: A perspective on identification and therapy. Ophthalmology 89:1006, 1982 94. Gass JDM, Jallow S: Idiopathic serous detachment of the choroid, ciliary body and retina (uveal
effusion syndrome). Ophthalmology 89:1018, 1982 95. Richardson J, Walsh M: Uveal effusion as a sign of myxedema. Br J Ophthalmol 53:557, 1969 96. McDonald PR, de la Paz V Jr, Sarin LK: Nonrhegmatogenous retinal separation associated with choroidal detachment. Trans Am Ophthalmol Soc 62:226, 1964 97. Capper SA, Leopold IH: Mechanism of serous choroidal detachment. Arch Ophthalmol 55:101, 1956 98. Chylack LT Jr, Bellows AR: Molecular sieving in suprachoroidal fluid formation in man. Invest Ophthalmol 17:420, 1978 99. Wilson RS, Hanna C, Morris MD: Idiopathic chorioretinal effusions: An analysis of extracellular fluids. Ann Ophthalmol 9:647, 1977 100. Lam WK, Lee PF, Ray GS et al: Albumin-bound bilirubin in subchoroidal fluid. Arch Ophthalmol 97:149, 1979 101. Chandler PA, Maumenee AE: A major cause of hypotony. Am J Ophthalmol 52:609, 1961 102. Brubaker RF, Pederson JE, Mayo Clinic, Personal communication, August 1985 103. Bill A: The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with
evidence for unconventional routes. Invest Ophthalmol 4:911, 1965 104. Suguro K, Toris C, Pederson JE: Uveoscleral outflow following cyclodialysis in the monkey eye using a fluorescent
tracer. Invest Ophthalmol Vis Sci 26:810, 1985 105. Brubaker RF, Pederson JE: Ciliochoroidal detachment. Surv Ophthalmol 27:281, 1983 106. Johnson MW, Gass JDM: Surgical management of the idiopathic uveal effusion syndrome. Ophthalmology 97:778, 1990 107. Schwartz A: Retinal detachment surgery. Ophthalmol Dig 13(2), 1975 108. Forrester JV, Lee WR, Kerr PR et al: The uveal effusion syndrome and trans-scleral flow. Eye 4:354, 1990 109. Ward RC, Gragoudas ES, Pon DM et al: Abnormal scleral findings in the uveal effusion syndrome. Am J Ophthalmol 106:139, 1985 110. Vine AK: Uveal effusion in Hunter's syndrome: Evidence that abnormal sclera
is responsible for the uveal effusion syndrome. Retina 6:57, 1986 111. Bill A: Movement of albumin and dextran through the sclera. Arch Ophthalmol 74:248, 1965 112. Bill A, Phillips CL: Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res 12:275, 1971 113. Trelstad RL, Silbermann NN, Brockhurst RJ: Nanophthalmic sclera: Ultrastructural, histochemical and biochemical observations. Arch Ophthalmol 100:1935, 1982 114. Yue BYJT, Duvall J, Goldberg MF et al: Nanophthalmic sclera: Morphologic and tissue culture studies. Ophthalmology 93:534, 1986 115. Stewart DH, Streeten BW, Brockhurst RJ et al: Abnormal scleral collagen in nanophthalmos: An ultrastructural study. Arch Ophthalmol 109:1017, 1991 116. Birk DE, Silver FH: Kinetic analysis of collagen fibrillogenesis: II. Corneal and scleral type
I collagen. Coll Relat Res 4:265, 1984 117. Kawamura M, Tajima S, Azuma N et al: Biochemical studies of glycosaminoglycans in nanophthalmic sclera. Graefes Arch Clin Exp Ophthalmol 233:58, 1995 118. Myers DB, Highton TC, Ryans DG: Ruthemium red-positive filaments interconnecting collagen fibrils. J Ultrastruct Res 42:87, 1973 119. Borcherding MS, Blacik LJ, Sittig RA et al: Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res 21:59, 1975 120. Yue BYJT, Kurosawa A, Duvall J et al: Nanophthalmic sclera: Fibronectin studies. Ophthalmology 95:56, 1988 121. Verhoeff FH: Scleral puncture for expulsive choroidal hemorrhage following sclerectomy. Ophthalmic Res 24: 55, 1915 122. Valone J Jr, Moser D: Management of rhegmatogenous retinal detachment with macula detached: Steroids, choroidal
detachment, and acuity. Ophthalmology 93:1413, 1986 123. Grant WM, Chandler PA: An arrangement for gonioscopy during surgery. Arch Ophthalmol 52:454, 1954 124. Harbin TS Jr: Treatment of cyclodialysis clefts with argon laser photocoagulation. Ophthalmology 89:1082, 1982 125. Partamian LG: Treatment of a cyclodialysis cleft with argon laser photocoagulation in
a patient with a shallow anterior chamber. Am J Ophthalmol 99:5, 1985 126. Maumenee AE, Stark WJ: Management of persistent hypotony after planned or inadvertent cyclodialysis. Am J Ophthalmol 71:320, 1971 127. Shea M, Medrick EB: Ciliary body reattachment in ocular hypotony. Arch Ophthalmol 99:278, 1981 128. Simmons RJ, Kimbrough RL: Shell tamponade in filtering surgery for glaucoma. Ophthalmic Surg 10:17, 1979 129. Bellows AR, Chylack LT Jr, Epstein DL, Hutchinson BT: Choroidal effusion in glaucoma surgery in patients with prominent episcleral
vessels. Arch Ophthalmol 97:493, 1979 130. Berrocal J: Adhesion of the retina secondary to large choroidal detachment as a cause
of failure in retinal detachment surgery. Mod Probl Ophthalmol 20:51, 1979 131. Cadera W, Willis R: Sodium hyaluronate for postoperative aphakic choroidal detachment. Can J Ophthalmol 17: 274, 1982 132. Gitter KA, Houser BP, Sarin LK et al: Toxemia of pregnancy: An angiographic interpretation of fundus changes. Arch Ophthalmol 57:217, 1973 133. Davies EWG: Annular serous choroidal detachment. Mod Probl Ophthalmol 20:2, 1979 134. Casswell AG, Gregor ZJ, Bird AC: The surgical management of uveal effusion syndrome. Eye 1:115, 1987 135. Brockhurst RJ: Ciliochoroidal (uveal) effusion. In Ryan SJ (ed): Retina, Vol 2, pp 729–734. St. Louis, CV Mosby, 1989 136. Pollalis S, Tragakis M: Idiopathic choroidal detachment. Mod Probl Ophthalmol 20:23, 1979 137. Allen KM, Meyers SM, Zegarra H: Nanophthalmic uveal effusion. Retina 8:145, 1988 138. Morita H, Funata M, Kusakari T et al: Recurrence of nanophthalmic uveal effusion. Ophthalmologica 207:30, 1993 139. Brockhurst RJ: Cataract surgery in nanophthalmic eyes. Arch Ophthalmol 108:965, 1990 |