| Most clinicians use the retinoscope solely to perform the technique of “neutralization.” As discussed previously, it is unfortunate
that the retinoscope is not often used to its fullest potential. However, many
clinicians believe that they get enough information from this
one technique that they do not feel the need to become skilled in
the others. In truth, they cannot be faulted too harshly because neutralization
alone can provide the skilled retinoscopist with a great deal
of information about a patient's refractive status. This is particularly
true because most patients are either near plano or present
themselves to the examiner with an almost correct prescription in their
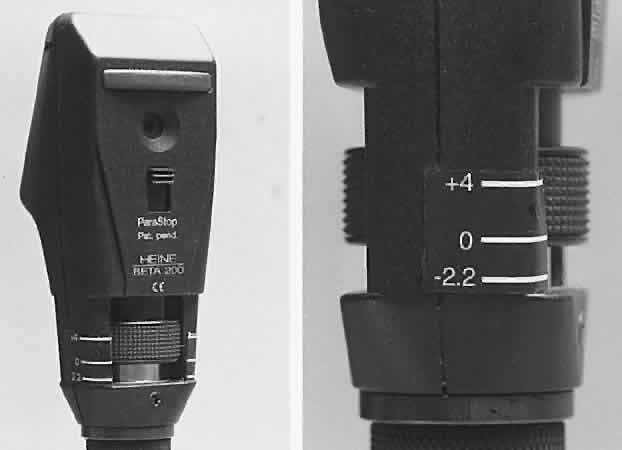
present spectacles. Neutralization is performed with the retinoscope held at a constant predetermined
distance from the patient with the sleeve all the way down (light
emitted in a diverging manner). The retinoscopist makes decisions
about the patient's refractive error based on the appearance of
the retinoscope reflex after it is reflected off the patient's
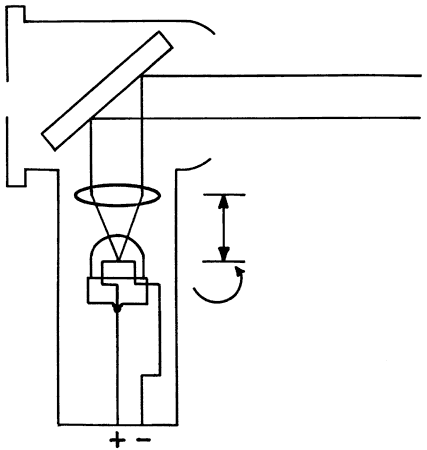
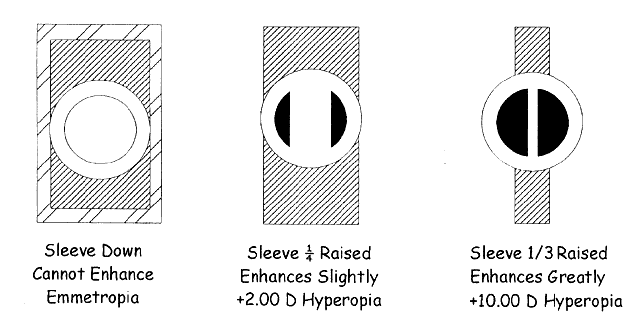
fundus and back through the pupil (Fig. 16). What the retinoscopist sees is not the image “on the retina” (which
is what she sees when performing ophthalmoscopic retinoscopy), but
rather the magnified image “of the retina.” Therefore, discussion
about neutralization retinoscopy must begin with discussion
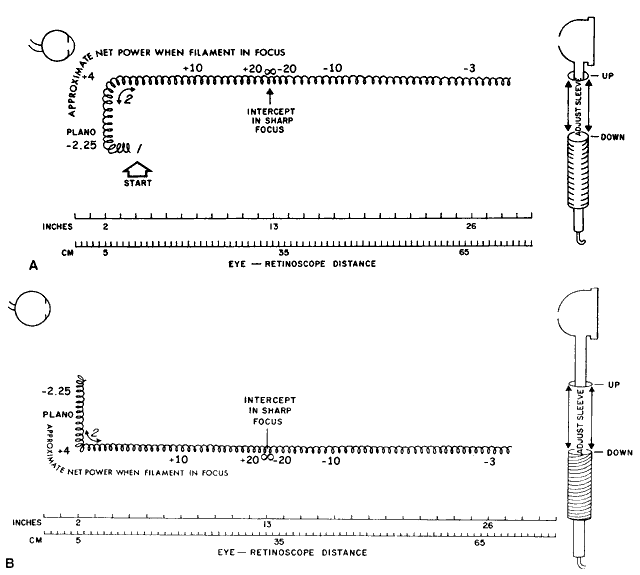
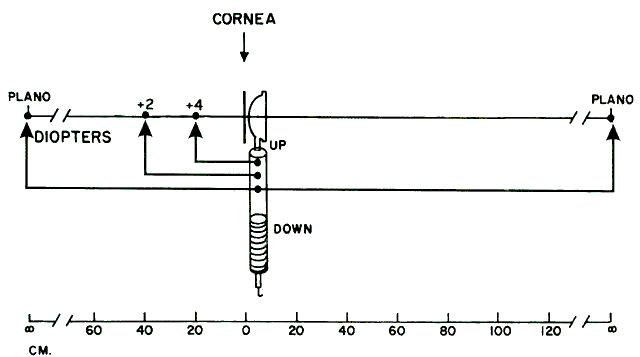
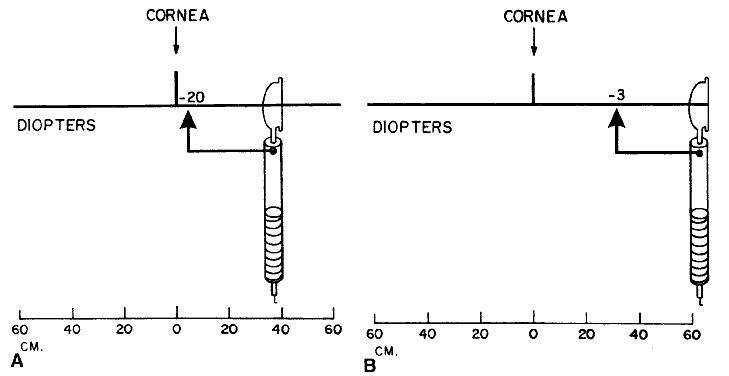
about the retinoscopic reflex at neutralization. 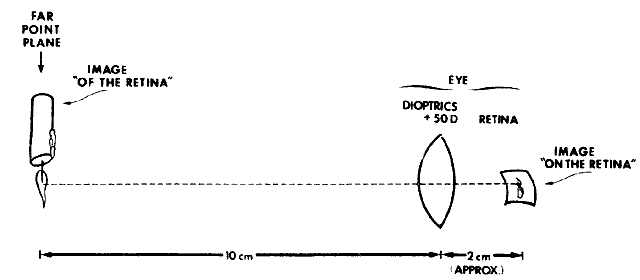 Fig. 16. A method of estimating the magnification of image “of the retina” as
compared with image “on the retina.” Eye has 10.00 D
of myopia. Magnification = Image of/Image on = 10/2 = 5. (Safir A: Retinoscopy. In Tasman W, Jaeger EA [eds]: Duane's
Clinical Ophthalmology. Philadelphia: JB Lippincott, 1982.) Fig. 16. A method of estimating the magnification of image “of the retina” as
compared with image “on the retina.” Eye has 10.00 D
of myopia. Magnification = Image of/Image on = 10/2 = 5. (Safir A: Retinoscopy. In Tasman W, Jaeger EA [eds]: Duane's
Clinical Ophthalmology. Philadelphia: JB Lippincott, 1982.)
|
THE NEUTRALIZATION REFLEX When performing neutralization retinoscopy, the retinoscopist shines diverging
light through the patient's pupil from a standard working
distance (usually 66 cm). This light is reflected off the patient's
fundus, and in this way, the fundus acts as a new point source of
light. This is called the illuminating system. The light that originates
from the luminous retina then passes through the patient's vitreous, lens, pupil, aqueous, and
cornea, until it finally exits the patient's eye on its way
back to the retinoscope. This is called the viewing system. The retinoscopist
must be able to differentiate between the illuminating and viewing systems because different techniques of retinoscopy can depend on varying the
components of one but not the other. For example, ophthalmoscopic retinoscopy, as
described previously, allows the user to vary different aspects
of the illuminating system while keeping the viewing system constant. Neutralization retinoscopy, conversely, varies
the viewing system while keeping the illumination system constant. When diverging light is shone onto an emmetrope's retina, the retina
becomes luminous and acts as a point source of light. The rays of light
then escape his eye in a parallel fashion. If this concept is not
intuitive, merely follow the standard light ray diagram backward. In
similar fashion, light starting as a point on a myope's luminous
retina is emitted as converging light, where more myopic individuals emit
more highly converging light than less myopic ones. Similarly, light
starting as a point on a hyperope's luminous retina is emitted
as diverging light, and hyperopic patients emit more diverging light
than less hyperopic ones. The vergence of the rays emitted from the eye
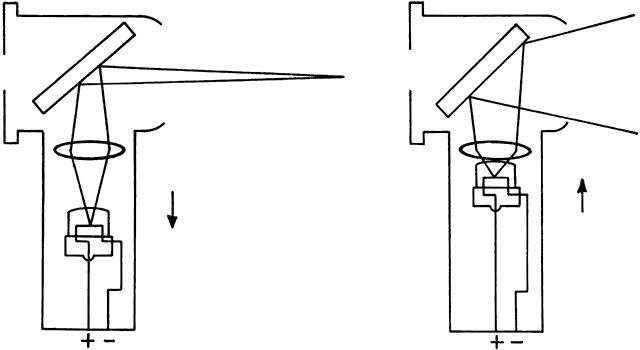
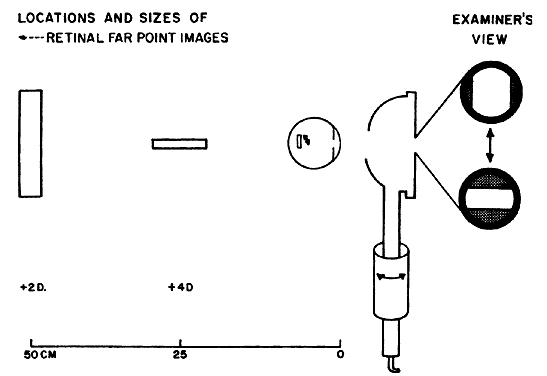
determines the qualities of the reflex seen by the retinoscopist. A neutralization reflex occurs under the circumstance when the far point of the eye correlates
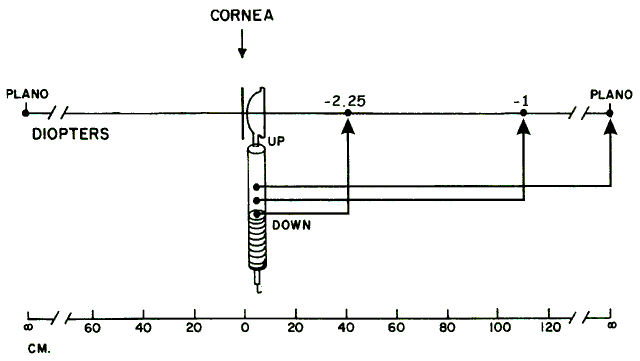
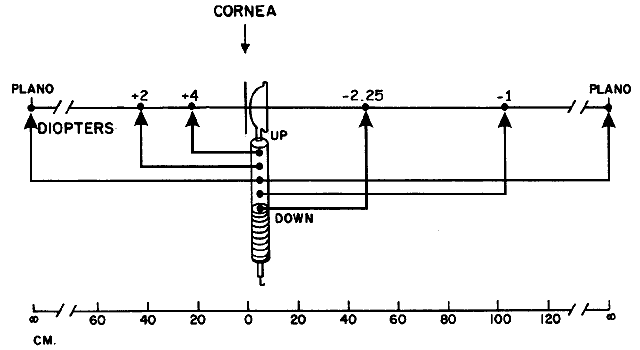
with the location of the peephole of the retinoscope (Fig. 17). 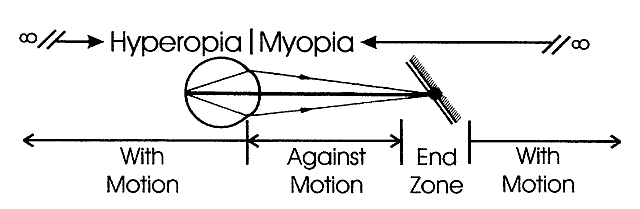 Fig. 17. The optical basis for neutralization retinoscopy. The location of far points
produces the “with” and “against” motions
for a retinoscope with a divergent beam when performing neutralization
retinoscopy. “With” motion is seen under all circumstances
except when the far point of the eye-corrective lens system is situated
between the cornea and the peephole of the retinoscope. The far
point of the illustrated eye is at the peephole and is thus neutralized. Fig. 17. The optical basis for neutralization retinoscopy. The location of far points
produces the “with” and “against” motions
for a retinoscope with a divergent beam when performing neutralization
retinoscopy. “With” motion is seen under all circumstances
except when the far point of the eye-corrective lens system is situated
between the cornea and the peephole of the retinoscope. The far
point of the illustrated eye is at the peephole and is thus neutralized.
|
If a retinoscopist were to examine an emmetropic eye at infinity, she could
make assumptions on the diverging, converging, or parallel nature
of the reflected light by sweeping the retinoscope streak back and forth
across the patient's pupil. However, it is not possible to perform
retinoscopy from an infinite distance; it is customary to adapt
a working distance of 66 cm, corresponding to + 1.50 D. By introducing + 1.5 lens
in front of the subject's eye, the far point
of a plano prescription is relocated to 66 cm (correcting the final
prescription for the working distance lens is described subsequently). In
this circumstance, what the retinoscopist is truly evaluating is whether
the retinoscope lies between the patient's eye and far point, lies
at the far point, or lies beyond it. If the patient is an emmetrope, the
far point lies on the horizon, and therefore the retinoscope
always must lie between the patient's eye and far point. If the
patient is a hyperope, the far point actually lies beyond the horizon, and
the retinoscope also lies between the patient's eye and far
point. Things are more interesting, however, when evaluating myopes
in this way. Light is emitted from a myope in a converging manner so that
the far point is somewhere in real space in front of the myope's
eye. It is possible for the retinoscope to be placed between the patient
and far point, exactly on the far point, or out beyond the far
point. This relationship depends, of course, on both the location of the
retinoscope, and the level of myopia (which determines the location
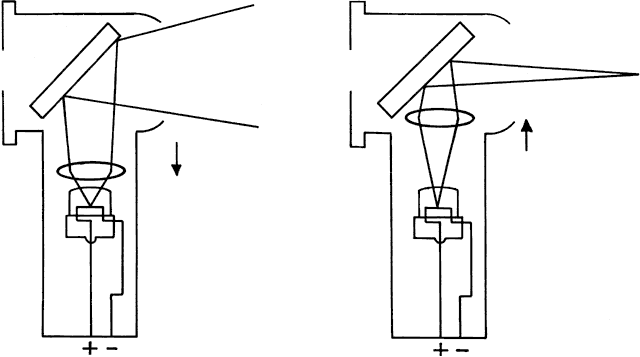
of the far point). If the retinoscope is placed between the eye and far point (as it is for
all emmetropes and hyperopes, and some myopes) and turned so that the
emitted streak is swept from side to side across the patient's
pupil, the light reflex seen inside the pupil appears to sweep in the
same direction as the light emitted from the retinoscope (seen on the
patient's iris, lids, brow, and cheek). This motion is called “with” motion
because the light that is afferent to the retinoscope
seems to move “with” the light that is efferent from
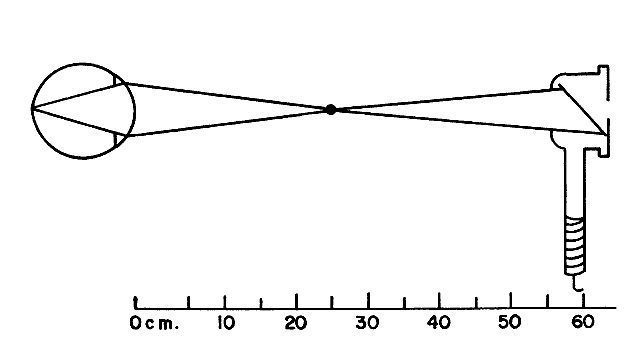
the retinoscope (Fig 18). 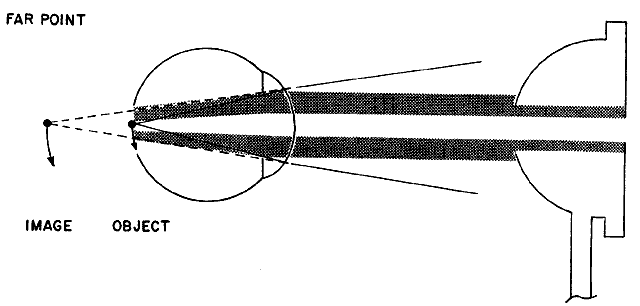 Fig. 18. “With” motion reflex in hyperopia: a “with motion” reflex
of light comes into the shadow projected in the optical system
from the aperture of the retinoscope or the examiner's pupil. The
rays from the filament to the retina are not shown. They form an
unfocused horizontal filament image on the retina that acts as a new object
with its image behind the retina. When the retinoscope is tilted
slightly, the object moves down and the image moves down and into the
shadow. This is seen as a “with motion” reflex. Light rays
from the image move up on the examiner's retina. (Weinstock SM, Wirtschafter JD: A Decision-Oriented Manual of Retinoscopy. Springfield, IL: Charles C. Thomas, 1976.) Fig. 18. “With” motion reflex in hyperopia: a “with motion” reflex
of light comes into the shadow projected in the optical system
from the aperture of the retinoscope or the examiner's pupil. The
rays from the filament to the retina are not shown. They form an
unfocused horizontal filament image on the retina that acts as a new object
with its image behind the retina. When the retinoscope is tilted
slightly, the object moves down and the image moves down and into the
shadow. This is seen as a “with motion” reflex. Light rays
from the image move up on the examiner's retina. (Weinstock SM, Wirtschafter JD: A Decision-Oriented Manual of Retinoscopy. Springfield, IL: Charles C. Thomas, 1976.)
|
If the retinoscope is placed beyond the patient's far point and swept
from side to side across the pupil, the light reflex seen inside the
pupil appears to sweep in the opposite direction as the streak emitted
from the retinoscope (Fig. 19). This motion is called “against” motion because the light
emitted from the eye appears to move “against” the light
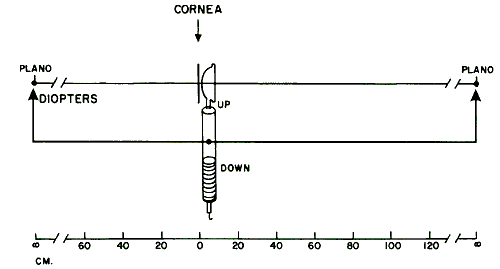
that is emitted directly from the retinoscope. 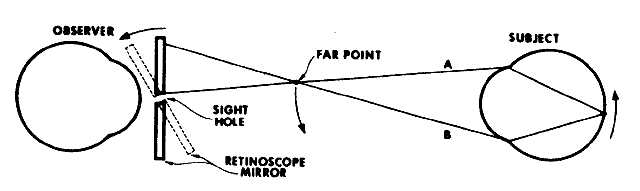 Fig. 19. Origin of the “against” movement. (Safir A: Retinoscopy. In Tasman W, Jaeger EA [eds]: Duane's
Clinical Ophthalmology. Philadelphia: JB Lippincott, 1982.) Fig. 19. Origin of the “against” movement. (Safir A: Retinoscopy. In Tasman W, Jaeger EA [eds]: Duane's
Clinical Ophthalmology. Philadelphia: JB Lippincott, 1982.)
|
When the retinoscope is placed exactly on the patient's far point, neither “with” nor “against” motion is seen. At
this point, all the light emitted from the patient's eye enters
the retinoscopist's eye simultaneously. At exact neutrality, in
a spherical eye with a small pupil, the retinoscopist may see no motion
at all; rather, the patient's pupil seems to suddenly fill with
light as the streak moves across it. This “on-off” phenomenon
is important to recognize because it serves as the end point when
performing the technique of neutralization. In addition to its direction of movement, other qualities of the reflected
retinoscope streak can be evaluated. These qualities all give the
retinoscopist clues as to how close to the far point the retinoscope is
being held. The three most important qualities of the reflex are the
speed at which it moves, its brightness, and its width. If one thinks
of the reflex at the neutralization point as infinitely fast (so fast
that it immediately fills the pupil without apparent motion), infinitely
bright, and infinitely wide, it is easy to understand what the reflex
should look like when the retinoscope is either near to, or far from, the
neutralization point (Fig. 20). When the retinoscope is held near the patient's far point, the reflex should appear fairly fast, bright, and
wide. As the retinoscope is moved farther from the far point, the
reflex appears to move slower and is dimmer and thinner. The retinoscope
can eventually be moved so far from the patient's far point
that the reflex is slow, thin, and dim enough that it is quite difficult
to recognize as a reflex at all. 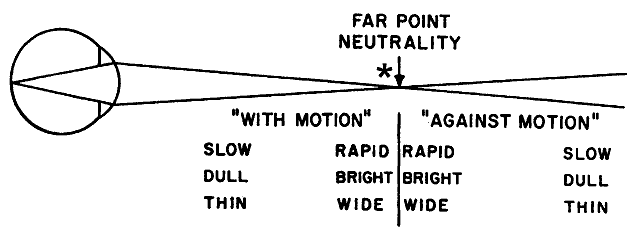 Fig. 20. Neutralization retinoscopy: diagram of changes in characteristics of reflex
as in the zone surrounding the point of neutrality. At neutrality, the
reflex motion may be so fast that it cannot be detected. The end
point or end zone should be approached from the “with” reflex
side and the judgment of neutrality made erring toward the “with” reflex
rather than the “against” reflex. *The
point within the neutralization zone where neutralization is best observed. (After Weinstock SM, Wirtschafter JD: A Decision-Oriented Manual of Retinoscopy. Springfield, IL: Charles C Thomas, 1976.) Fig. 20. Neutralization retinoscopy: diagram of changes in characteristics of reflex
as in the zone surrounding the point of neutrality. At neutrality, the
reflex motion may be so fast that it cannot be detected. The end
point or end zone should be approached from the “with” reflex
side and the judgment of neutrality made erring toward the “with” reflex
rather than the “against” reflex. *The
point within the neutralization zone where neutralization is best observed. (After Weinstock SM, Wirtschafter JD: A Decision-Oriented Manual of Retinoscopy. Springfield, IL: Charles C Thomas, 1976.)
|
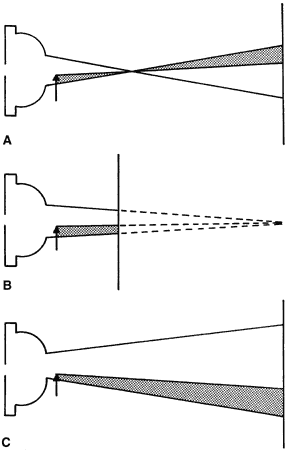
Optics of the Neutralization Reflex Five features characterize the neutralization end point, the point at which
neither a “with” nor “against” reflex can
be identified. Three of these are considered to define the end point, but
two others can also be observed. The three standard characteristics
are increases in speed, brightness, and width of the moving image. To
these can be added , the “on-off phenomenon” (the intermittent
disappearance of the observed reflex) and the scissors reflex. - Speed of the “with” or “against” motion: If the
retinoscope mirror is tilted in a highly ametropic eye, the resultant
reflex is imaged at a far point that is much closer to the eye than the
reflex of an almost emmetropic eye, the far point of which is located
at a much greater distance. With regard to the subject's pupil, movement
of the image at the far point of the almost emmetropic eye
will seem to have a greater angular velocity or speed. It should be stressed
that the direction of movement of the fundus image is not influenced
by the patient's ametropia (a downward movement of the mirror
will always produce a downward movement of the light on the fundus). The “with” or “against” movement is a function
of the observation system, thus an “against” movement occurs
only when the eye and external lens system have a far point between
the patient's eye and the retinoscope peephole.
- Brightness of the image: As neutrality is approached, all of the rays emerging
from the eye are focused at the peephole, where they provide the
brightest image that the examiner observes. Illumination increases
inversely to the square of image size. At any other focal distance, some
or all of the rays of light will not reach the peephole and the image
becomes duller (Fig. 21).
- Width: Safir1 has noted that the power of retinoscopy results from the image of the
retina being projected in space with large magnification. As neutrality
is approached, the retinoscopic reflex appears widest. The apparent
width of the moving retinoscopic image is the most difficult of the concepts
to comprehend because it relates to the concentration of light
emerging from the retina through the patient's pupil and then through
the peephole of the retinoscope. Michaels2 has discussed this subject in detail.
- The on-off phenomenon: Although the retinoscopic reflex is bright and wide
on either side of neutrality, the reflex may disappear completely
when the retinoscope peephole is exactly conjugate to the eye-corrective
lens system (see Fig. 21). Fortunately, neither the patient's eye nor the examiner's
eye and hand can maintain this exact position for long, but astute retinoscopist's
may notice the on-off phenomenon at neutrality.
- The scissors reflex: The refractive elements of the eye are not perfectly
spherical. Thus, the center of the optical path may be slightly myopic
when compared with that of the periphery. The amount of aberration
may be small, but under circumstances of perfect neutralization and a
widely dilated pupil, the center of the optical path may return a “with” motion
while the periphery returns an “against” motion. This
pattern of opposing central and peripheral retinoscopic
movements is known as a scissors reflex. There is only a small dioptric
distance over which the scissors reflex can be detected. The entire
reflex returns to all “with” or all “against” motion
within about 0.50 D on either side of neutralization.
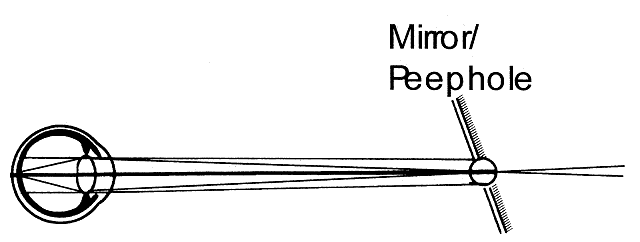 Fig. 21. The origin of the on-off phenomenon at neutrality. The far point of the
eye is situated at the peephole of the retinoscope. Either all or none
of the rays will pass through the peephole with the slightest shift
in the subject's eye or the retinoscope or the retinoscopist's
eye, causing the retinoscopist to see the contents of the pupil as
either filled with light or black. Fig. 21. The origin of the on-off phenomenon at neutrality. The far point of the
eye is situated at the peephole of the retinoscope. Either all or none
of the rays will pass through the peephole with the slightest shift
in the subject's eye or the retinoscope or the retinoscopist's
eye, causing the retinoscopist to see the contents of the pupil as
either filled with light or black.
|
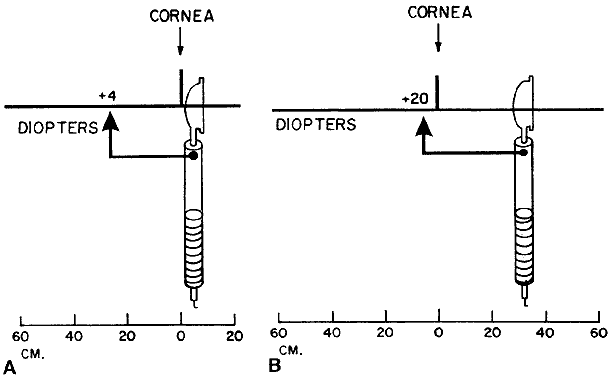
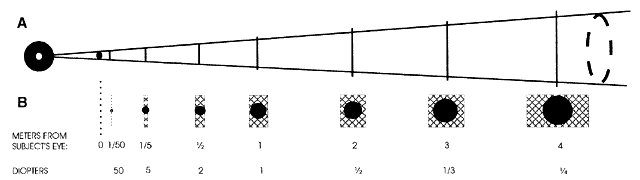
Estimating Low Myopes via Neutralization Without Lenses By now the reader should have determined that it is in fact quite possible
to neutralize low myopes without the use of lenses. The trick is to
place the retinoscope directly on the patient's far point, sweep
the retinoscope streak across the patient's pupil with the sleeve
down, recognize the “on-off” phenomenon of the neutralization
reflex, measure the distance from the patient's eye to the
retinoscope in meters, take the reciprocal—thus converting from
meters (distance) to diopters (vergence)—and voilà, the patient's refractive error has been determined. For example, neutralization for a -2.00-D myope can be seen by placing
the retinoscope 50 cm from the patient's eye, and for a -4.00-D myope
by placing the retinoscope 25 cm from this patient's eye. Neutralization
for an emmetrope can only be done in this fashion by placing
the retinoscope infinitely far from the patient's eye—theoretically
possible, but not practically feasible. Because the far points
of hyperopes do not lie in real space (they lie beyond infinity), hyperopes
cannot be neutralized in this way. The patients who are best served by this estimation technique of neutralization
retinoscopy are those whose net refractive errors lie between -1.50 and -3.00 D, and
conveniently this is exactly the group that lies
outside the range of estimation possible with ophthalmoscopic retinoscopy. NEUTRALIZATION RETINOSCOPY OF SPHERICAL EYES The aforementioned technique describes a way to estimate a low myope's
refractive error without the use of lenses. The key to this method
is that the retinoscopist must change the distance that the retinoscope
is held from the patient's eye when trying to find the far point. When
performing neutralization retinoscopy, she does exactly the
opposite—she holds the retinoscope at a constant specific working
distance and uses lenses to bring the patient's far point to the
retinoscope. The first thing that a retinoscopist must do is choose a comfortable working
distance. She wants to be as far from her patient as possible while
still being close enough to comfortably manipulate lenses in front
of his eye. Thus, the working distance usually is described as “arms
length” away from the patient. For the average retinoscopist, this
distance works out to about 66 cm. Taller retinoscopists may
prefer 75 cm, whereas shorter ones may use 50 cm. It is not uncommon for
retinoscopists to work closer than their usual working distance in
difficult cases, such as small children, or adults with cataracts or small
pupils. The actual working distance does not matter as long the retinoscopist
is aware of the distance and adjusts her calculations accordingly. The retinoscopist should be able to sit at her comfortable working distance
while using lenses to bring the patient's far point to her. The
retinoscopist accomplishes this feat by sweeping the retinoscope streak
across the patient's pupil and evaluating the direction, speed, brightness, and
width of the retinoscopy reflex. If she observes “against” motion, the retinoscope must lie beyond the patient's
far point, and the retinoscopist can move the far point toward
the retinoscope by placing a minus lens in front of her patient's
eye. If the reflex is fast, bright, and wide, the retinoscope must
have been near to the patient's far point, and a weak minus lens
should be chosen. However, if the reflex is slow, dim, and narrow, the
retinoscope probably lies a greater distance from the far point, and
a stronger minus lens should be chosen. If “with” motion
is observed after a minus lens is placed before the patient's eye, the
patient's far point has been moved beyond the retinoscope
because too strong of a minus lens was chosen. This lens should be removed
and replaced with a weaker minus one. Similar manipulations are performed if “with” motion is initially
seen when neutralization is begun. In such cases, the far point
must lie beyond the retinoscopist's comfortable working distance. Again, how
far away the far point lies can be estimated by judging the
quality of the reflex. A plus lens then is chosen to bring the far
point forward toward the retinoscope. Whenever possible, the retinoscopist should try to manipulate the far point
in such a way that “with” motion is being observed. A “with” reflex
typically is sharper and easier to judge than
an “against” reflex. Thus, if “against” motion
is seen, neutralization will be easier to perform if a strong enough
minus lens is placed to push the far point beyond the retinoscope, so
that the retinoscopist can observe “with” motion. Care
must always be taken, however, when putting minus lenses in front of younger
patients because they can easily “eat up” this minus
by accommodating, thus leading the less careful retinoscopist down the
wrong path. It should also be noted that the neutralization end point is not exactly
an end point—rather it is an end zone that measures about half a diopter in depth (see Fig. 20). The true size of this “zone of doubt” varies with pupil
size and working distance—it is narrowest with a small pupil and
close working distance. Best results are achieved when entering the zone
of doubt from the plus side, by watching the “with” motion
reflex get faster, brighter, and wider until the retinoscopist is
convinced the neutralization reflex has been achieved. If the zone of
doubt is entered from the minus side (through “against” motion), there
is a greater chance for error. Eventually, after just a few different lenses are placed before the patient's
eye, the retinoscopist can observe the neutralization reflex. At
this point the goal is achieved, and the retinoscopist has managed
to bring the patient's far point to the retinoscope (which is
being held at the working distance). The retinoscopist is now ready to
write a spectacle correction. However, the lenses currently in front
of the patient's eye do not represent the correction needed to see
clearly at infinite distance; rather, the lenses represent the correction
needed to see clearly at 66 cm. The patient will be quite dissatisfied
if given a prescription for a pair of glasses that allows for
clear vision only 66 cm away or closer. CORRECTING THE PRESCRIPTION FOR THE WORKING DISTANCE LENS The retinoscopist must always remember to modify the prescription for distance
vision, a mathematical manipulation called correcting for the working distance. The gross power is that which the retinoscopist is holding when retinoscopy is completed. This
corresponds to the power that brings light from the patient's
luminous retina to focus at the working distance. The net power is that which neutralizes the patient's refractive error for
good distance vision—the power that focuses light from the luminous
retina to a point at the horizon. The mathematical computation is
simple. The retinoscopist merely subtracts the working distance (in
diopters) from the gross to get the net. For example, when the working
distance is 66 cm (+ 1.50 D) and the patient is neutralized with
a -2.5 lens, the gross minus the working distance equals the net, or: -2.5 - (+ 1.5) = -4. The retinoscopist will give a prescription
for a -4 lens. NEUTRALIZATION RETINOSCOPY OF ASTIGMATIC EYES The previous discussion describes neutralization of spherical patients. Further
steps need to be taken in a patient with astigmatism. In patients
with astigmatism, the retinoscopy reflex seen in the pupil has one
more quality in addition to speed, brightness, and width. The reflex
in patients with astigmatism also appears to “break” as the
light filament is rotated (Fig. 22). The retinoscope reflex seen in the patient's pupil will not be
continuous with the streak lying on the cornea, lids, forehead, and cheek; it
will appear broken. There will be, however, two meridians where
the retinoscope reflex will be continuous with the streak—where
it will not appear broken. These meridians correspond to the two axes
of the patient's astigmatism. The retinoscopist merely needs to
neutralize these two meridians separately and combine them to come up
with the desired spectacle correction. This can be done using only spherical
lenses (as is best when neutralizing children with loose lenses), spherical
and plus cylindrical lenses (using a plus cylinder phoropter
or loose lenses and trial frames), or spherical and minus cylindrical
lenses (using a minus cylinder phoropter or loose lenses and trial
frames). Let us further explore the methods of neutralizing astigmatic
individuals in whom the less plus (or more minus) axis is neutralized
first and the more plus (or less minus) axis is neutralized second. When
neutralizing the axes in this order, the retinoscopist can use either
only spherical lenses, or spherical and plus cylindrical lenses. 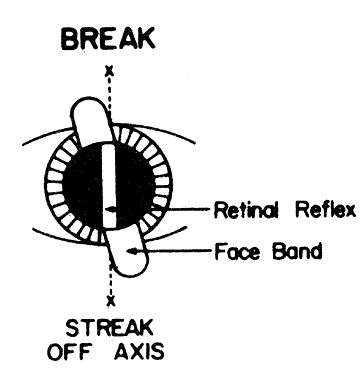 Fig. 22. Break. The line between the streak in the pupil and outside the pupil is
broken when the streak is off the correct axis. (Corboy JM: Refining the cylinder. In Corboy JM [ed]: Retinoscopy, p 87. 4th
ed. Thorofare, NJ: Slack, 1996.) Fig. 22. Break. The line between the streak in the pupil and outside the pupil is
broken when the streak is off the correct axis. (Corboy JM: Refining the cylinder. In Corboy JM [ed]: Retinoscopy, p 87. 4th
ed. Thorofare, NJ: Slack, 1996.)
|
Spherical Lens Technique The first step is for the retinoscopist to find the least plus axis. The
retinoscope streak is swept back and forth across the pupil while it
is rotated 360 degrees by rotating the light filament in the handle. The
retinoscopist then observes at which two meridians the retinoscope
reflex does not appear broken—in cases of regular astigmatism, these
two meridians should be 90 degrees apart. The retinoscopist then
compares the reflex in one meridian to the reflex in the other, noting
which meridian's streak exhibits more “against” (slower, thinner, dimmer) or
less “with” (faster, broader, brighter) qualities
than the other. This meridian is neutralized first. If
the reflex in one meridian shows “with” motion and in the
other shows “against” motion, the meridian with the reflex
that shows “against” is neutralized first. The more minus meridian of the astigmatic person is then merely neutralized
much as the spherical myope or hyperope described previously. The
axis of the streak is held along the meridian line and swept in a direction
perpendicular to it (i.e., if the 90-degree axis is being neutralized, the streak is oriented straight
up and down and swept from side to side). At first, it is not intuitive
that the streak be held in the same orientation as the axis meridian
because one is searching for the power of the astigmatism, and
the power lies not along the axis, but perpendicular to it. Here the
retinoscopist must remember that the power is found not by holding the
streak still, but rather by sweeping it across the pupil. Another way
of saying this is that for each meridian to be neutralized, the axis lies in the orientation of the streak, and the power lies in the
direction of the sweep. Once the proper lens is placed before the patient's eye so that the
neutralization reflex is observed for that meridian, the retinoscopist
merely subtracts the working distance and records the power needed
to correct the patient for that particular axis. She then addresses the
other meridian. The retinoscope streak is rotated 90 degrees, and the reflex is re-examined. The
reflex should not appear broken in the new meridian—a
broken reflex signifies that either the retinoscope streak is not exactly
aligned along the patient's second axis or that the patient has
irregular astigmatism. If the reflex is not broken, it is neutralized
with lenses. If spherical lenses are to be used, the second meridian
is neutralized in exactly the same manner as the first. Once the neutralization
reflex has been found, the retinoscopist again subtracts the
working distance and records the lens power needed to correct the patient
for that particular axis. A simple conversion then needs to be
performed before presenting the patient with the proper spectacle prescription, as
follows: Q: A patient is neutralized with the following lenses at a working distance
of 66 cm: [+ 3.50 axis 90] and [+ 4.25 axis 180]. What
is the eyeglasses prescription?
A: Step 1: Subtract the working distance. In this case, the working distance
is 66 cm, which is equal to 1.50 D:
[+ 3.50 axis 90] - 1.50 = + 2.00 axis 90 [+ 4.25 axis 180] - 1.50 = + 2.75 axis 180
Step 2: Transpose from cross-cylinder notation to plus-cylinder notation:
+ 2.00 sphere + ([+ 2.75 - 2.00] axis 180)
= + 2.00 + 0.75 × 180
Plus-Cylinder Technique If the second meridian is to be neutralized with a plus-cylinder lens (as
is done with a plus-cylinder phoropter or loose lenses and trial frames), the
first spherical lens should be left in the phoropter or trial
frames. The axis of the cylindrical lens is oriented in the direction
of the axis of the streak for the second meridian. Because a cylinder
lens is being used, no power is being added along the axis of the second
meridian (which, of course, corresponds to the power of the first
meridian). When the neutralization reflex is found for the second meridian, the
streak is rotated 90 degrees to ensure that the first meridian
is still neutralized. The working distance is then subtracted from
the spherical lens, and the spectacle prescription is easily determined
as follows: Q: A patient is neutralized with the following lenses at a working distance
of 66 cm: [+ 3.50 sphere] and [+ 0.75 axis 180]. What
is the eyeglasses prescription?
A: Step 1: Subtract the working distance from the spherical lens only. In
this case, the working distance is 66 cm, which is equal to 1.50 D:
[+ 3.50 sphere] - 1.50 = + 2.00 sphere
Step 2: Add the cylindrical lens to the new power of the spherical lens:
+ 2.00 sphere + [+ 0.75 axis 180]
= + 2.00 + 0.75 × 180
Minus-Cylinder Technique Some clinicians prefer to work in minus cylinder. Patients are neutralized
in the same aforementioned manner, except that the more “with” or
less “against” meridian is neutralized first with
spherical lenses. Then the less “with” or more “against” meridian
is neutralized with a minus-cylinder in much the
same way as the previous example used a plus-cylinder lens. The transposition
is done as follows: Q: A patient is neutralized with the following lenses at a working distance
of 66 cm: [+ 4.25 sphere] and [-0.75 axis 90]. What
is the eyeglasses prescription?
A: Step 1: Subtract the working distance from the spherical lens only. In
this case, the working distance is 66 cm, which is equal to 1.50 D:
+ 4.25 sphere minus 1.50 = + 2.75 sphere
Step 2: Add the minus cylindrical lens to the new power of the spherical
lens:
+ 2.75 sphere + (-0.75 axis 90)
= + 2.75 - 0.75 × 90
|