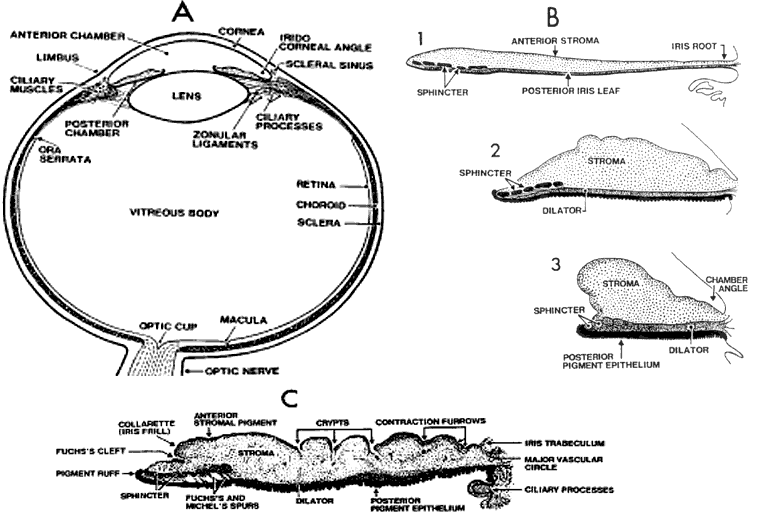
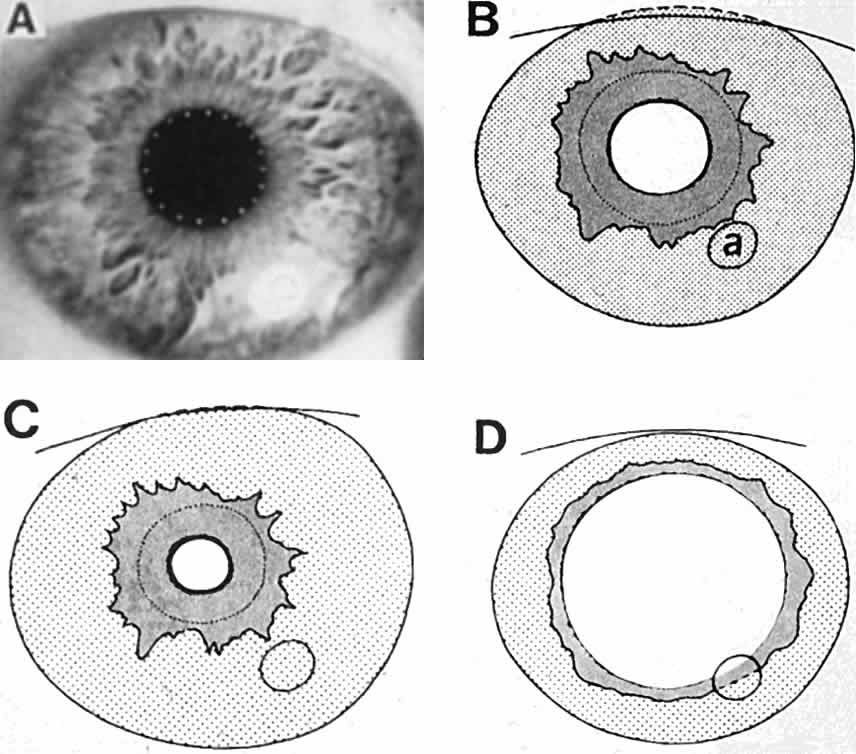
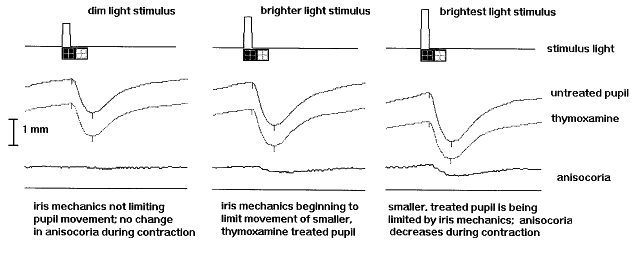
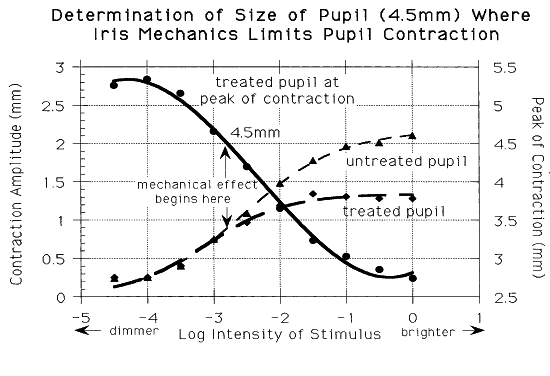
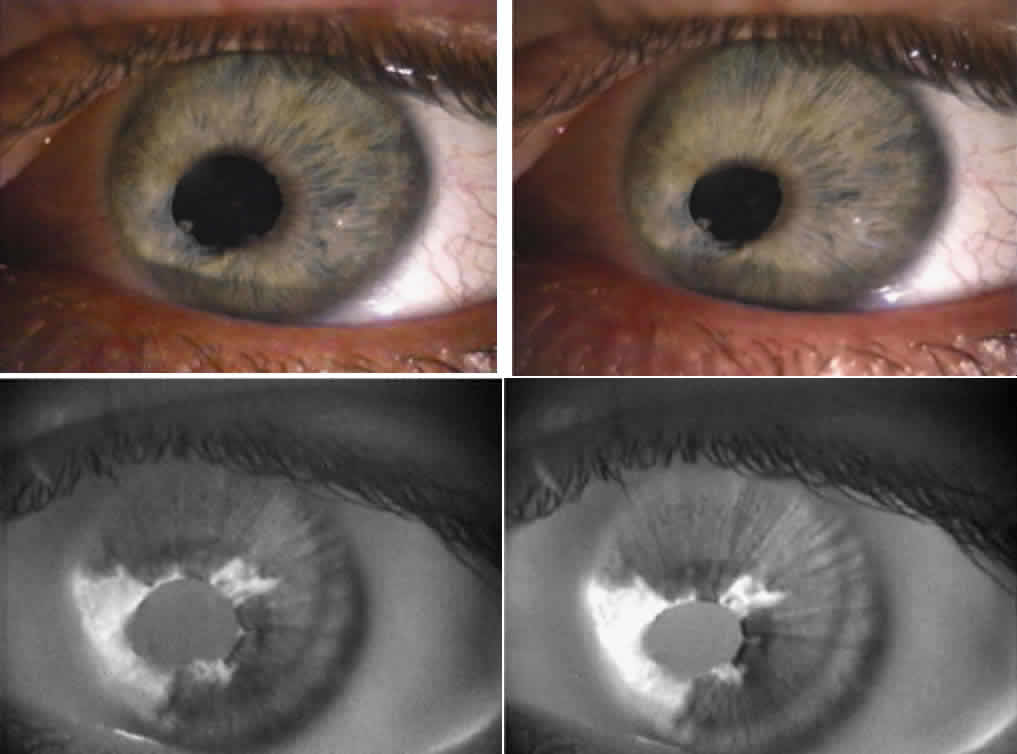
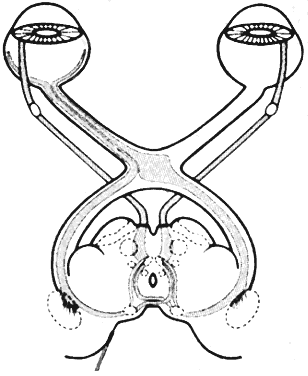
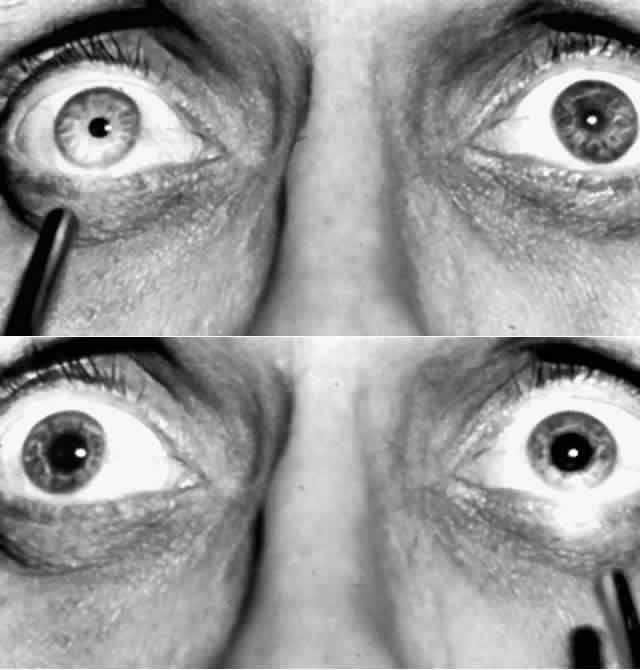
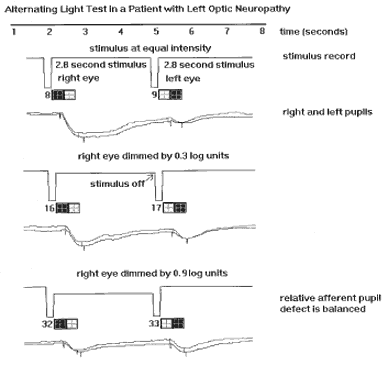
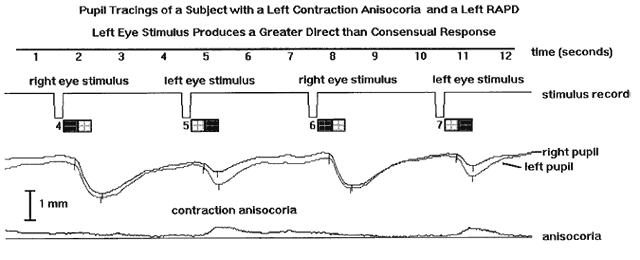
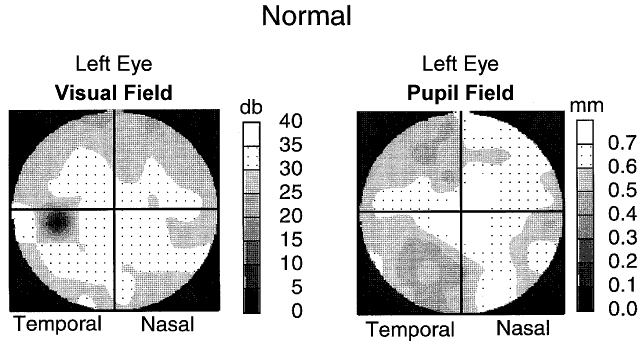
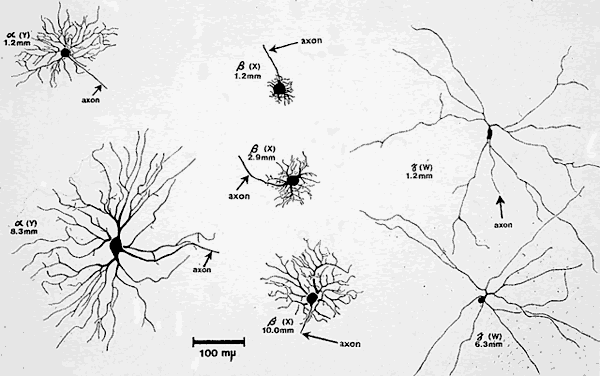
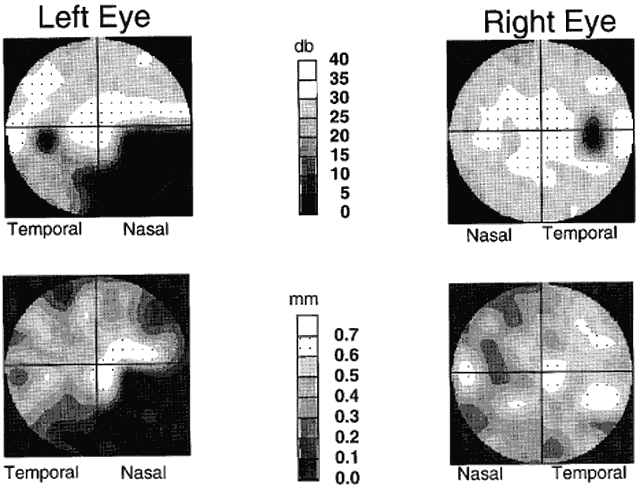
1. Newsome DA, Loewenfeld IE: Iris mechanics: II. Influence of pupil size on details of iris structure. Am J Ophthalmol 71:553, 1971 2. Loewenfeld IE, Newsome DA: Iris mechanics: I. Influence of pupil size on dynamics of pupillary movements. Am J Ophthalmol 71:347, 1971 3. Kestenbaum A: Clinical methods of neuro-ophthalmological examination, p 289. New
York, Grune & Stratton, 1946 4. Levatin P: Pupillary escape in disease of the retina or optic nerve. Arch Ophthalmol 62:768, 1959 5. Fineberg E, Thompson HS: Quantitation of the afferent pupillary defect. In
Smith JL (ed): Neuro-ophthalmology Focus, pp 25–29. New York, Masson, 1980 6. Thompson HS, Corbett JJ: Asymmetry of pupillomotor input. Eye 5:36, 19916a.Thompson HS: Putting
a number on the relative afferent pupillary defect. In Thompson HS (ed): Topics
in Neuro-ophthalmology, pp 157–158. Baltimore, Williams & Wilkins, 19796b.Thompson HS, Corbett JJ, Cox TA: How to measure
the relative afferent pupillary defect. Surv Ophthalmol 26:39, 1981 7. Rosenberg ML, Oliva A: The use of crossed polarized filters in the measurement of the relative
afferent pupillary defect. Am J Ophthalmol 110:62, 1990 8. Lowenstein O, Kawabata H, Loewenfeld I: The pupil as indicator of retinal activity. Am J Ophthalmol 57:569, 1964 8a. Cox TA, Thompson HS, Hayreh SS, Snyder JE: Visual evoked potential and pupillary signs: a comparison in optic nerve
disease. Arch Ophthalmol 100:1603, 1990 9. Thompson HS, Montague P, Cox TA, Corbett JJ: The relationship between visual acuity, pupillary defect, and visual field
loss. Am J Ophthalmol 93:681, 1982 10. Brown RH, Zillis JD, Lynch MG, Sanborn GE: The afferent pupillary defect in asymmetric glaucoma. Arch Ophthalmol 105:1540, 1987 11. Johnson LN, Hill RA, Bartholomew MJ: Correlation of afferent pupillary defect with visual field loss on automated
perimetry. Ophthalmology 95:1649, 1988 12. Kardon RH, Haupert C, Thompson HS: The relationship between static perimetry and the relative afferent pupillary
defect. Am J Ophthalmol 115:351, 1993 13. Fison PN, Garlick DJ, Smith SE: Assessment of unilateral afferent pupillary defects by pupillography. Br J Ophthalmol 63:195, 1979 14. Cox TA: Pupillography of a relative afferent pupillary defect. Am J Ophthalmol 101:320, 1986 15. Cox TA: Pupillographic characteristics of simulated relative afferent pupillary
defects. Invest Ophthalmol Vis Sci 30: 1127, 1989 16. Kawasaki A, Moore PA, Kardon RH: Variability of the relative afferent pupillary defect. Am J Ophthalmol 120: 622, 1995 17. Loewenfeld IE: The light reflex. In: The Pupil: Anatomy, Physiology and
Clinical Applications, Vol 1, Chap 3, Section V, Pupillary reflex pathways, pp 198–240. Detroit, Iowa State University Press, Ames and
Wayne State University Press, 1993 18. Lowenstein O: Alternating contraction anisocoria. Arch Neurol Psychiatry 72:742, 1954 19. Cox TA, Drewes CP: Contraction anisocoria resulting from half-field illumination. Am J Ophthal 97:577, 1984 20. Smith SA, Ellis CJK, Smith SE: Inequality of the direct and consensual light reflexes in normal subjects. Br J Ophthalmol 63:523, 1979 21. Smith SA, Smith SE: Contraction anisocoria: nasal versus temporal illumination. Br J Ophthalmol 64:933, 1980 22. Wyatt HJ, Musselman JF: Pupillary light reflex in humans: evidence for an unbalanced pathway from
the nasal retina, and for signal cancellation in brainstem. Vision Res 21: 513, 1981 23. Loewenfeld IE: Damage to the intercalated pretectal neuron: consensual
deficit. In: The Pupil: Anatomy, Physiology and Clinical Applications, Vol 1, Chap 18, pp 945–955. Detroit, Iowa State University Press, Ames
and Wayne State University Press, 1993 24. Loewenfeld IE: The light reflex. In: The Pupil: Anatomy, Physiology and
Clinical Applications, Vol 1, Chap 3, Section III, pp 96–135. Detroit, Iowa
State University Press, Ames and Wayne State University Press, 1993 25. Young RSL, Han B, Wu P: Transient and sustained components of the pupillary responses evoked by
luminance and color. Vision Res 33:437, 1993 26. Young RSL, Kennish J: Transient and sustained components of the pupil response evoked by achromatic
spatial patterns. Vision Res 33:2239, 1993 27. Barbur JL, Harlow AJ, Sahraie A: Pupillary responses to stimulus structure, colour, and movement. Ophthalmic Physiol Opt 12:137, 1992 28. Barbur JL, Forsyth PM: Can the pupil response be used as a measure of visual input associated
with the geniculo-striate pathway? Clin Vision Sci 1:107, 1986 29. Barbur JL, Keenleyside MS, Thomson WD: Investigation of central visual
processing by means of pupillometry. In Kulikowski JJ, Dickinson CM, Murray
IJ (eds): Seeing Colour and Contour, pp 431–451. Oxford, Pergamon
Press, 1989 30. Slooter JH, van Noren D: Visual acuity measured with pupil responses to checkerboard stimuli. Invest Ophthalmol Vis Sci 19:105, 1980 31. Ukai K: Spatial pattern as a stimulus to the pupillary system. J Opt Soc Am 2:1094, 1985 32. Barbur JL, Thomson WD: Pupil response as an objective measure of visual acuity. Ophthalmic Physiol Opt 7:425, 1987 33. Cocker KD, Moseley MJ: Visual acuity and the pupil grating response. Clin Vision Sci 7:143, 1992 34. Harms H: Grundlagen, Methodik und Bedeutung der Pupillenperimetrie fur die Physiologie
und Pathologie des Schorgans. Graefes Arch Klin Exp Ophthalmol 149:1, 1949 35. Cibis G, Campos E, Aulhorn E: Pupillary hemiakinesia in suprageniculate lesions. Arch Ophthalmol 93:1322, 1975 36. Hellner K, Jensen W, Muller-Jensen A: Video-processing pupillography as a method for objective perimetry in pupillary
hemiakinesia: Proceedings of the Second International Visual
Field Symposium, Tubingen, 1976. Doc Ophthalmol 14:221, 1977 37. Hellner KA, Jensen W, Muller A: Video processing pupillographic perimetry in hemianopsia. Klin Monatsbl Augenheilkd 172:731, 1978 38. Alexandridis E, Krastel H, Reuther R: Disturbances of the pupil reflex associated with lesions of the upper visual
pathway. Graefes Arch Klin Exp Ophthalmol 209:199, 1979 39. Reuther R, Alexandridis E, Krastel H: Disturbances of the pupil reflex associated with cerebral infarction in
the posterior cerebral artery territory. Arch Psychiatr Nervenkr 229:249, 1981 40. Reuther R, Krastel H, Alexandridis E: Disturbances of the pupil reflex associated with homonymous hemianopic
paracentral scotoma. Arch Psychiatr Nervenkr 229:259, 1981 41. Hamann K, Hellner K, Muller-Jensen A, Zschocke S: Videopupillographic and VER investigations in patients with congenital
and acquired lesions of the optic radiation. Ophthalmologica 178:348, 1979 42. Narasaki S, Kawai K, Kubota S, Noguchi J: Videopupillographic perimetry and its clinical application. Jpn J Ophthalmol 18:253, 1974 43. Yoshitomi M: The examination of pupillary light reflex with a specific pupillary paralysis. Acta Soc Ophthalmol Jpn 9: 853, 1955 44. Kardon RH, Kirkali PA, Thompson HS: Automated pupil perimetry. Ophthalmology 98:485, 1991 45. Kardon RH: Pupil perimetry. Curr Opin Ophthalmol 3: 565, 1992 46. ten Doesschate J, Alpern M: Response of the pupil to steady state retinal illumination: contribution
by cones. Science 149:989, 1965 47. Gamlin PDR, Zhang H, Clarke RJ: Luminance neurons in the pretectal olivary nucleus mediate the pupillary
light reflex in the rhesus monkey. Exp Brain Res 106:177, 1995 48. Reiner A, Williams J, Gamlin PDR: Characterization of neuropeptides, calcium binding proteins and glutamate
receptors found in the olivary pretectal nucleus of the Rhesus monkey. Invest Ophthal Vis Sci 36(suppl):S292, 1995 49. Edinger L: Ueber den Verlauf der centralen Hirnnervenbahnen mit Demonstration von
Praparaten. Arch Psychiatr Nervenkr 16:858, 1885 50. Westphal C: Ueber einen Fall von chronischer progressiver Lahmung der Augenmuskeln (ophthalmoplegia
externa) nebst Beschreibung von Ganglienzellengruppen
im Bereiche des Oculomotoriuskerns. Arch Psychiatr Nervenkr 18:846, 1987 51. Spitzka EC: The oculo-motor centres and their co-ordinators. J Nerv Ment Dis 13:413, 1988 52. Warwick R: The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat 88:71, 1954 53. Carpenter MB, Harbison JW, Peter P: Accessory oculomotor nerve nuclei in the monkey: projections and effects
of discrete lesions. J Comp Neurol 140:131, 1970 54. Akert K, Glicksman MA, Lang W et al: The Edinger-Westphal nucleus in the monkey: a retrograde tracer study. Brain Res 184:491, 1980 55. Burde RM, Loewy AD: Central origin of oculomotor parasympathetic neurons in the monkey. Brain Res 198:434, 1980 56. Jampel RS: Representation of the near-response on the cerebral cortex of the macaque. Am J Ophthalmol 48: 473, 1959 57. Loewenfeld IE: The reaction to near vision. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 5, pp 295–317. Detroit, Iowa
State University Press, Ames and Wayne State University Press, 1993 58. Loewenfeld IE: The light reflex. The Pupil: Anatomy, Physiology and Clinical
Applications, Vol 1, Chap 3, Section V, pp 256. Detroit, Iowa State
University Press, Ames and Wayne State University Press, 1993 59. Loewenfeld IE: Reactions to darkness. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 4, p 282. Detroit, Iowa State
University Press, Ames and Wayne State University Press, 1993 60. File RR, Patton TS: Topically applied pilocarpine. Pupillary response as a function of drop
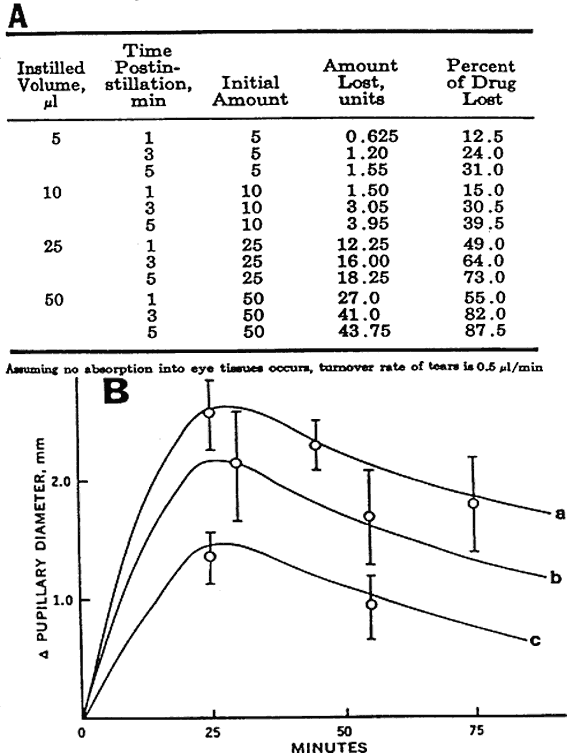
size. Am J Ophthalmol 98:112, 1980 61. Chrai SS, Makoid MC, Eriksen ST, Robinson JR: Drop size and initial dosing frequency problems of topically applied ophthalmic
drugs. J Pharm Sci 63:333, 1974 62. Apt L, Henrick A: Pupillary dilation with single eye drop combinations. Am J Ophthalmol 89:553, 1980 63. Korczyn AD, Rubenstein AE, Yahr MD, Axelrod FB: The pupil and familial dysautonomia. Neurology 31: 628, 1981 64. Sugaya N, Nagataki S: Kinetics of topical pilocarpine in the human eye. Jpn J Ophthalmol 22:127, 1978 65. Duffin RM, Camras CB, Gardener SK, Pettit TH: Inhibitors of surgically induced myosis. Ophthalmology 89:966, 1982 66. Settone MF, Glannaccini B, Savigni P, Wirth A: The effect of different ophthalmic vehicles on the activity of tropicamide
in man. J Pharm Pharmacol 32:519, 1980 67. Nagataki S, Sugaya N: Methylcellulose and ointment vehicles: their effects on ocular pharmacokinetics. Nippon Ganka Gakkai Zasshi 82:127, 1978 68. Zimmerman TJ, Leader B, Kaufman HE: Advances in ocular pharmacology. Ann Rev Pharmacol Toxicol 20:415, 1980 69. Burstein NL: Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine
digluconate and cat and rabbit corneas. Invest Ophthalmol Vis Sci 19:308, 1980 70. Lyle WM, Bobier WR: Effective topical anesthetics on phenylephrine-induced mydriasis. Am J Optometry 54: 276, 1977 71. Araie M, Takase M, Yokoyama Y, Kitagawa M: Timolol maleate: pharmacokinetic analysis of ocular penetration in the
rabbit eye and effects on human aqueous humor dynamics. Acta Soc Ophthalmol Jpn 84:2139, 1980 72. Lee VHL, Hui HW, Robinson JR: Corneal metabolism of pilocarpine in pigmented rabbits. Invest Ophthalmol Vis Sci 19:210, 1980 73. Mikkelson TJ, Chrai SS, Robinson JR: Altered bioavailability of drugs in the eye, due to drug-protein interaction. J Pharmacol Exp Ther 62:1648, 1973 74. Mikkelson TJ, Chrai SS, Robinson JR: Competitive inhibition of drug-protein interaction in the eye fluids and
tissues. J Pharm Sci 62:1942, 1973 75. Loewenfeld IE: Pupillary Pharmacology. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 14, Section IV-C2, pp 809–816. Detroit, Iowa
State University Press, Ames and Wayne State University
Press, 1993 76. Patil PN: Cocaine binding by the pigmented and the nonpigmented iris and its relevance
to the mydriatic effect. Invest Ophthalmol 11:739, 1972 77. Mishima S: Clinical pharmacokinetics of the eye. Invest Ophthalmol Vis Sci 21:504, 1981 78. Loewenfeld IE: Pupillary Pharmacology. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 14, Section IV-C3, pp 817–822. Detroit, Iowa
State University Press, Ames and Wayne State University
Press, 1993 79. Borgmann H, Worster W: Der Einfluss unterschiedlicher Konzentrationen Und Vehikel auf die Pilocarpin-Miosis: II. Unterschiedliche Vehikel. Klin Monatsbl Augenheilkd 163:51, 1973 80. Millodot M: Do blue-eyed people have more sensitive corneas than brown-eyed people? Nature 235:151, 1975 81. Koss MC: Studies on the mechanism of amphetamine in the cat. J Pharmacol Exp Ther 213:49, 1980 82. Loewenfeld IE: Pupillary Pharmacology. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 14, Section III-B, pp 774–775. Detroit, Iowa
State University Press, Ames and Wayne State University
Press, 1993 83. Dahlstrom A, Fuxe K, Hillarp NA, Malmfors T: Adrenergic mechanisms in the pupillary light reflex path. Acta Physiol Scand 62:119, 1964 84. Lee HK, Wang SC: Mechanism of the morphine-induced miosis in the dog. J Pharmacol Exp Ther 192:115, 1975 85. Sigg EB, Sigg TD: The modification of the pupillary light reflex by chlorpromazine, diazepam
and pentobarbital. Brain Res 50:77, 1973 86. Loewenfeld IE: Pupillary Pharmacology. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 14, Section III-C, pp 788–791. Detroit, Iowa
State University Press, Ames and Wayne State University
Press, 1993 87. Loewenfeld IE: Lesions in the Ciliary Ganglion and Short Ciliary Nerves: The
tonic pupil (“Adie's” syndrome). In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 24, Section
III-C, pp 1111–1116. Detroit, Iowa State University Press, Ames
and Wayne State University Press, 1993 88. Thompson HS: Adie's syndrome: some new observations. Trans Am Ophthalmol Soc 75:587, 1977 89. Jacobson DM, Olson KA: Influence of pupil size, anisocoria, and ambient light on pilocarpine miosis: implications
for supersensitivity testing. Ophthalmology 100:275, 1993 90. Jacobson DM: A prospective evaluation of cholinergic supersensitivity of the iris sphincter
in patients with oculomotor nerve palsies. Am J Ophthalmol 118:377, 1994 91. Czarnecki JC, Thompson HS: The iris sphincter in aberrant regeneration of the third nerve. Arch Ophthalmol 96: 1606, 1978 92. Burde RM, Savino PJ, Trobe JD: Clinical Decision in Neuro-Ophthalmology, pp 178–185. St. Louis, CV Mosby, 1985 93. Scinto LFM, Kirk RD, Dressler D et al: A potential noninvasive neurobiological test for Alzheimer's disease. Science 266:1051, 1994 94. Wisniewski HM, Rabe A, Wisniewski KE: In Davies P, Finch C (eds): Molecular
Neuropathology of Aging, Banbury Report, pp 399–413. Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, 1988 95. Sacks B, Smith S: People with Down's syndrome can be distinguished on the basis of cholinergic
dysfunction. J Neurol Neurosurg Psychiatry 52:1294, 1989 96. Loupe DN, Newman NJ, Green RC et al: Pupillary responses to tropicamide in patients with Alzheimers disease. Ophthalmology 103:495, 1996 97. Katz B: Letter to the Editor. Science 267:1578, 1995 98. Sitaram N, Pomara N: IRCS Med Sci 9:409, 1981 99. Forman AR: A new low-concentration preparation for mydriasis and cycloplegia. Ophthalmology 87:213, 1980 100. Sindell BD, Baker MD, Maisels MJ, Weinstein JM: A comparison of the pupillary and cardiovascular effects of various mydriatic
agents in preterm infants. J Pediatr Ophthalmol Strabismus 23:273, 1987 101. Kardon RH, Denison CE, Brown CK, Thompson HS: Critical evaluation of the cocaine test in the diagnosis of Horner's
syndrome. Arch Ophthalmol 108:834, 1990 102. Loewenfeld IE: Injury and repair in the nervous system. In: The Pupil: Anatomy, Physiology
and Clinical Applications, Vol 1, Chap 11, Section
II-D, pp 540–545. Detroit, Iowa State University Press, Ames and
Wayne State University Press, 1993 103. Cremer SA, Thompson HS, Digre KB, Kardon RH: Hydroxyamphetamine mydriasis in Horner's syndrome. Am J Ophthalmol 110:66, 1990 104. Weinstein JM, Zweifel TJ, Thompson HS: Congenital Horner's syndrome. Arch Ophthalmol 98:1074, 1980 105. Almegaard B, Stjernschantz J, Bill A: Cholecystokinin contracts isolated human and monkey iris sphincters: a
study with CCK receptor antagonists. Eur J Pharmacol 211: 183, 1992 106. Almegaard B, Bill A: C-terminal calcitonin gene-related peptide fragments and vasopressin but
not somatostatin-28 induce miosis in monkeys. Eur J Pharmacol 250:31, 1993 |