PRENATAL DEVELOPMENT
Almost all the sclera develops from the neural crest, except a small temporal portion which develops from mesoderm.1,2 (Table 1). The neural crest, mesoectoderm, or ectomesenchyme is the cellular mass situated on either side of the invaginating neural folds. Interestingly, other connective tissues are also of neural crest–mesodermal origin including cartilages, bones, ligaments, tendons, dermis, leptomeninges, and perivascular smooth muscles; this may explain, at least in part, the frequent association of scleritis and arthritis in many systemic connective tissue diseases.3
TABLE 1. Embryology of Ocular Structures
| Neuroectoderm | Surface ectoderm | Neural crest | Mesoderm |
| Retina | Corneal epithelium | Choroid | Striated extraocular muscles |
| Fibers of optic nerve | Conjunctival epithelium | Iris | Vascular endothelia |
| Glia of optic nerve | Lens | Ciliary musculature | Small portion of the sclera |
| Smooth muscle of iris | Lacrimal gland | Part of vitreous | |
| Tarsal glands | Corneal stroma | ||
| Epidermis of eyelids | Corneal endothelium | ||
| Optic nerve meninges | |||
| Most of the sclera |
The developmental process of the human sclera progresses from anterior to posterior and from inside to outside.4 The human eye develops early in week 4 as an evagination from the ventral lateral aspect of the neural tube or neuroectoderm at the level of the forebrain in the diencephalon. The end of the evagination becomes slightly dilated to form the optic vesicle. At the same time a small area of surface ectoderm overlying each optic vesicle thickens, forming the lens placode, which invaginates to become the lens vesicle. At this time there are three waves of neural crest mesenchymal invasion. The first is responsible for the corneal endothelium and trabecular meshwork, the second for the corneal and scleral fibrocytes, and the third for iris and choroidal stroma. By week 5 of development each optic vesicle invaginates to form the double-layered optic cup or neuroectoderm that is surrounded by neural crest which is also of ectodermal origin. The differentiation of neural crest cells into sclera and choroid occurs by week 6 of development in humans in the region anterior to the equator (about day 43 of development),5 progresses backward to the equator by week 8, and reaches the posterior pole by week 12.1 This differentiation is induced by the retinal pigment epithelium.6–8 The sclera as well as the choroid and the retinal pigment epithelium requires the presence of the developing lens for normal growth and change in shape, structure, and function.1 By the 4th month circularly oriented scleral fibers form the scleral spur, and by the 5th month scleral fibers around the axons of the optic nerve form the lamina cribrosa.1,4 Arrest of fetal development at this stage or the failure to lay down new collagen on the inner aspect of the posterior sclera might well account for some of the staphylomatous changes found in congenital myopia and disc changes found in some patients with congenital glaucoma.
Ultrastructural studies show that developmental events of the sclera begin in the region anterior to the equator at approximately day 43.4,5 The late mesenchymal cells or very early fibroblasts of the anterior portion possess elongated nuclei and many glycogen granules and lipid vacuoles whereas those of the posterior portion possess round-to-oval nuclei and few glycogen granules and lipid vacuoles. The late mesenchymal cells or very early fibroblasts of the anterior and posterior portions contain many free ribosomes and polyribosomes, as well as immature rough-surfaced endoplasmic reticulum and Golgi complex. The early fibroblasts begin the synthesis of glycoproteins, glycosaminoglycans (especially hyaluronic acid), collagen, and elastin between day 43 and 50, thus filling the intercellular space. Developmental events directed from inside outward begin at week 7.2 with a marked increase in the inner portion in glycogen granules and lipid droplets of the cells, and in number and average diameter of collagen fibrils. Cytodevelopment of the sclera is characterized by decrease of ribosomes, polyribosomes, glycogen granules, and lipid vacuoles, and by increase of rough-surfaced endoplasmic reticulum and Golgi complex components. Development of intercellular substances is characterized by an increase in the number and average diameter of collagen fibrils (Fig. 1) and in the amount of elastic deposits with electron-translucent central cores. By week 10.9 there are no more differences between the inner and outer portions. By week 13 there are no more differences between the anterior and posterior portions. By week 24, fetal sclera has the same ultrastructural characteristics as adult sclera. Between week 6 and week 24 there is a threefold increase in thickness, possibly by progressive laying down of collagen fibrils on its inner aspect as more mature collagen fibrils are found in the outer part of the sclera and the younger smaller collagen fibrils on its inner aspect; thereafter the rate of increase diminishes rapidly.
Defects in synthesis of extracellular matrix components during scleral development may account for conditions such as Marfan syndrome, osteogenesis imperfecta, pseudoxanthoma elasticum, Ehlers-Danlos syndrome, congenital myopia, and nanophthalmos.
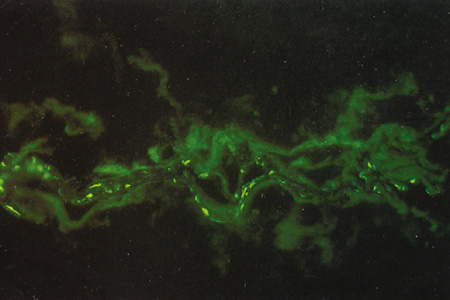
Immunohistochemical studies show that the extracellular matrix of fetal sclera has the collagens I, III, IV, V, and VI, the glycosaminoglycans dermatan sulfate, heparan sulfate, hyaluronic acid, and chondroitin sulfate, and the glycoproteins fibronectin, vitronectin, and laminin.9 Collagens II and VII are not present. Collagens play a major role in strength (collagen I), resiliency (collagen III) and structural integrity (collagens IV, V, VI); glycosaminoglycans in distensibility; and glycoproteins in cell adhesion, growth, differentiation and migration, as well as in other extracellular matrix component organization. Collagen VIII may also be present in human fetal sclera.10 Development of scleral extracellular matrix from fetus into the adult includes increase of collagens I, III, and VI, and decrease of collagen IV and VIII, hyaluronic acid (Fig. 2), fibronectin, vitronectin, and laminin.9,10 By the time the tissue is mature, collagen IV, although abundant in fetal tissue, has almost completely disappeared, except in blood vessels. Heparan sulfate is identified in fetal and adult sclera in small amounts. Increase of collagens I, III, and VI may account for the increase in strength, resiliency, and structural integrity of adult sclera. Decrease of hyaluronic acid may explain the decrease of distensibility of adult sclera. Decrease of fibronectin, vitronectin, and laminin may suggest they play a major role in directing developmental events during the younger gestational periods, including cell and extracellular matrix organization.
POSTNATAL DEVELOPMENT AND AGE-RELATED CHARACTERISTICS
Scleral development is determined by the genetic signaling to the fibrocytes of the sclera, and by the concomitant development of the adjacent structures such as lens, retina, choroid, and the production of aqueous by the ciliary body. During this period of growth there is an increase in axial length in order to acquire a state of emmetropia. This increase in axial length occurs in two stages, an infantile phase which is up to 3 years and then a slower juvenile phase which is up to 13 years; after this age the eye is fully developed.
The postnatal sclera is relatively thin, allowing the pigment cells of the choroid to show through, giving a bluish color. It is also somewhat distensible, allowing the sclera to stretch as a result of increased intraocular pressure in infantile glaucoma (buphthalmic globe). The relatively thin, bluish, distensible, small, and translucent postnatal sclera gradually becomes thicker, whiter, less distensible, larger, and more opaque as the eye goes through childhood and puberty.
Although the adult sclera is poorly distensible, ectasias (localized protrusions of thin sclera) or staphylomas (localized protrusions of thin sclera lined by uveal tissue) can appear at any age after damage (trauma or inflammation). The water content of the adult sclera ranges from 65% to 75%.11 The sclera appears opaque if the water content is between 40% and 80% but becomes translucent if it falls below 40% or rises above 80%.12 This is especially evident in surgical procedures such as strabismus surgery or retinal detachment in which conjunctiva and extraocular muscles are temporally removed from the underlying sclera. The exposed sclera becomes dry and therefore appears translucent unless it is continuously moistened. Similar changes occur after removal of perilimbal conjunctiva in surgical procedures such as excision of pterygium or other limbal lesions. The exposed sclera, adjacent to small elevations of conjunctiva, becomes dry because of the interference in the lubricating effect of the tear film. The dry spots, called dellen, dissappear after rehydration of the area with artificial tears or eye patching.
The increase in transparency after inflammation is the result of rearrangement of the fibrils of the sclera and physiochemical changes; it is only rarely caused by true thinning of the sclera.
In the elderly, the sclera is even less distensible, has decreased water content, and contains fewer glycosaminoglycans.13 This is the result of a progressive cross-linking of the lysine residues of collagen and a decrease in the size of the interfibrillar spaces, possibly as the result of changes within the proteoglycans. Other age-related changes are the subconjunctival deposition of lipids such as cholesterol esters, free fatty acids, triglycerides, and sphingomyelin, which give the sclera a yellowish color.14,15 Calcium phosphate may also be deposited in small rectangular or ellipsoid areas with a vertical axis longer than the horizontal one (approximately 6 mm high and 1 mm wide) just anterior to the insertions of medial or lateral rectus muscles. These slate-gray areas are called senile scleral plaques and usually occur in individuals over 70 years of age.16,17 The cause is uncertain but some etiologic possibilities taken either individually or in combination include ischemia secondary to atherosclerosis of the anterior ciliary arteries,18 dehydration,19 constant stress by the rectus muscles,20 and actinic damage from solar irradiation.17 The collagen fibrils themselves become thicker and less uniform, especially in the region of the muscle insertions. Here the sclera becomes progressively thinned, increasing the color contrasts between one part of the sclera and the next.
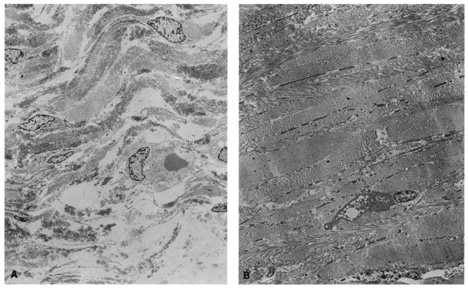
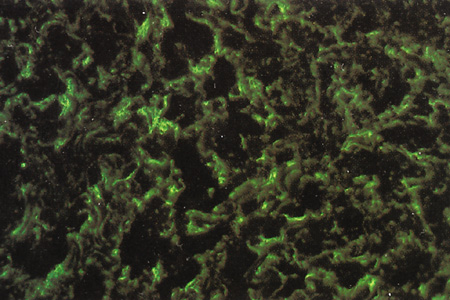

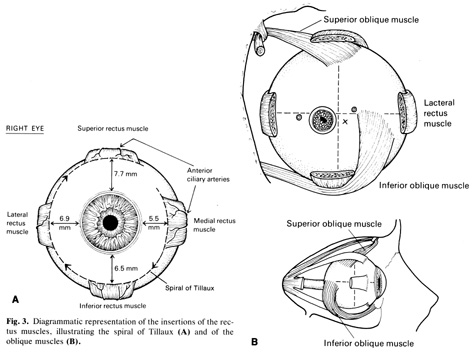
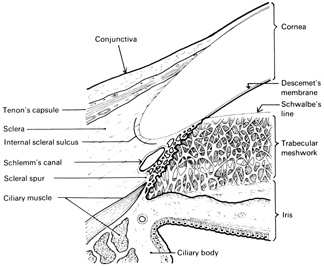
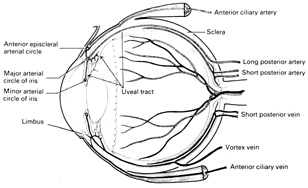
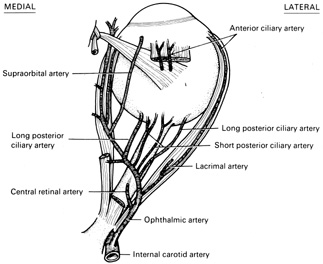
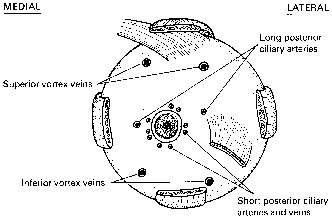
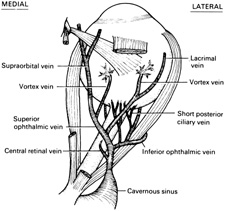

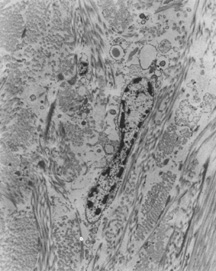
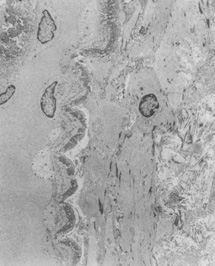
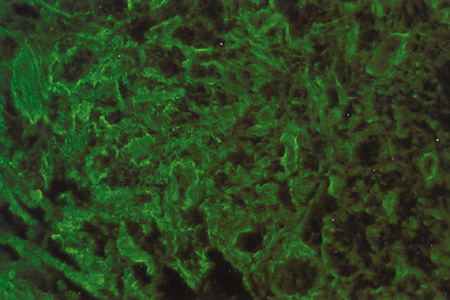

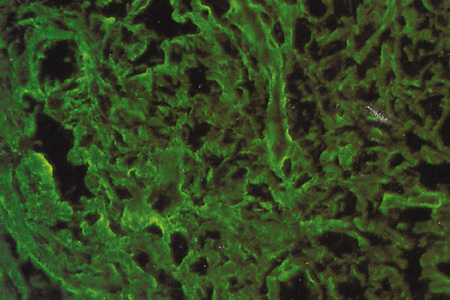 Fig. 15.
Fig. 15.