INDICATIONS
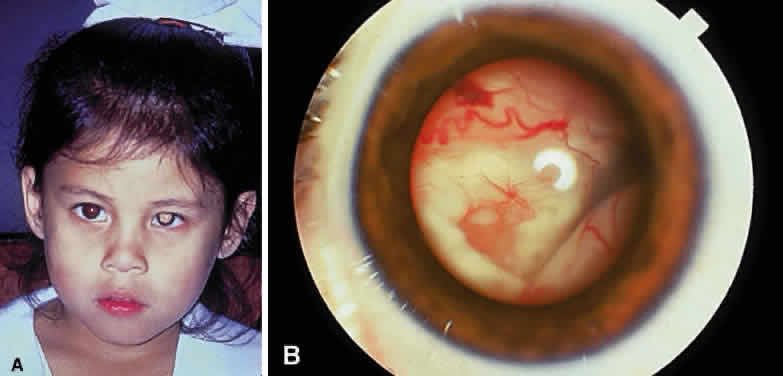
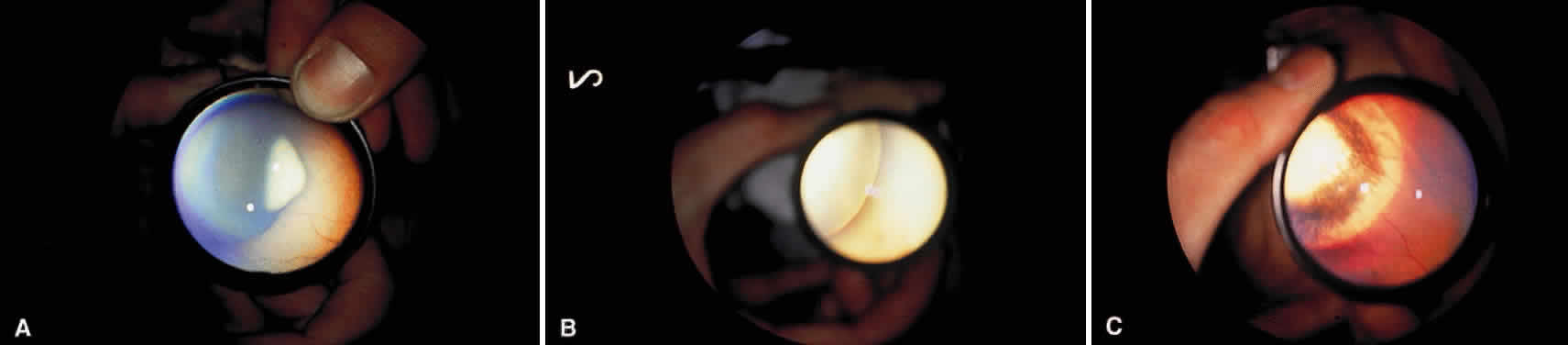
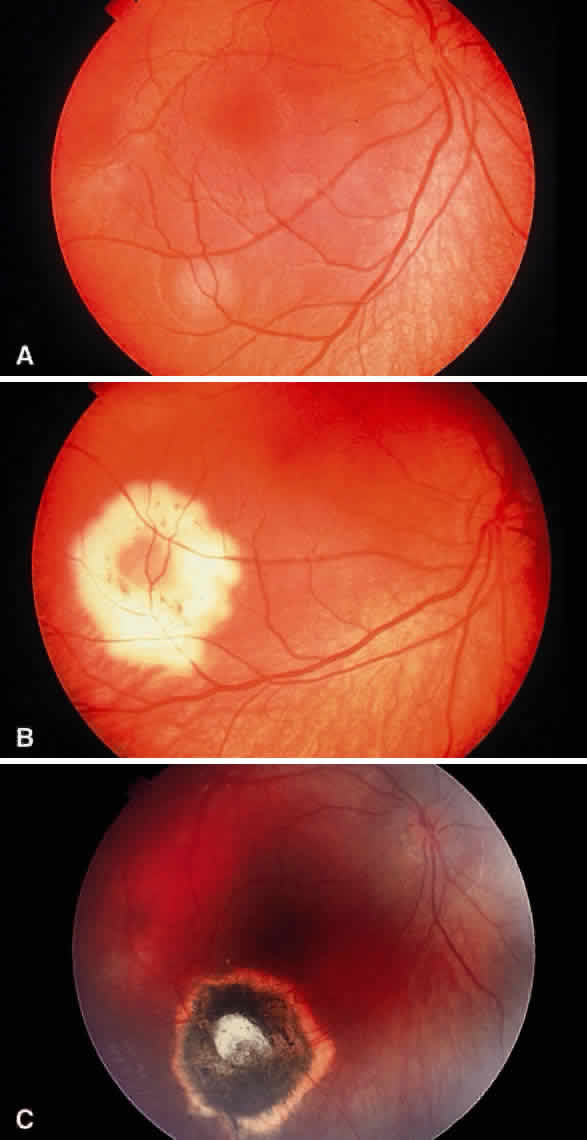
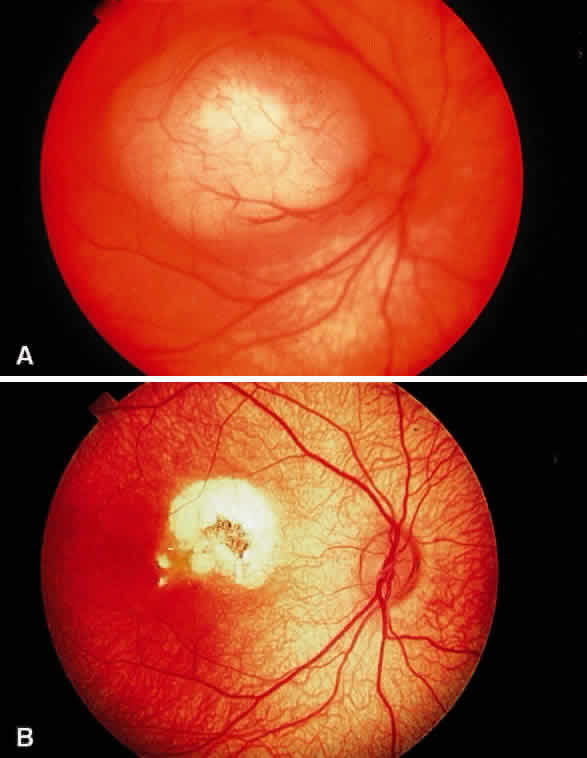
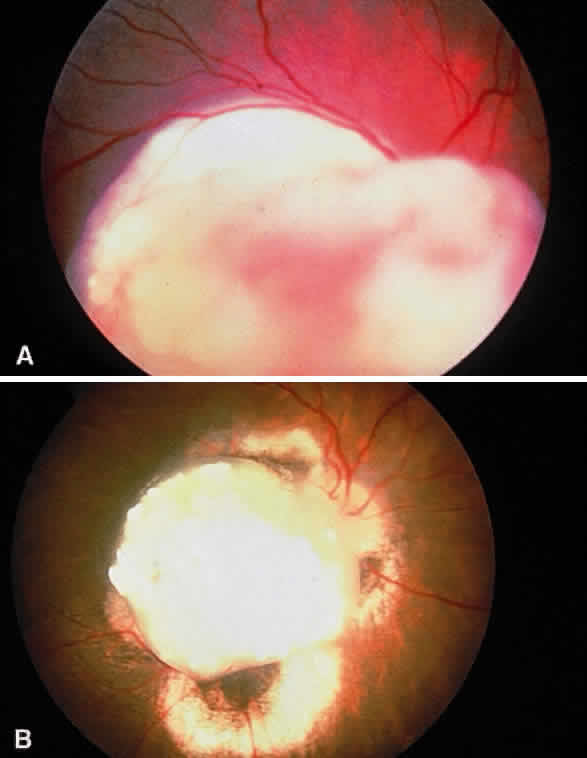
Enucleation probably is indicated for all unilateral cases in which the tumor fills most of the globe and in which there is little hope of salvaging any viable retina or useful vision (Fig. 1). If half of the retina is free from tumor, then other methods of treatment can be considered, as long as parents have been fully informed as to the possibilities of metastasis, the complications of treatment, and the risk for ultimate enucleation. Other indications for enucleation include the presence of neovascular glaucoma in an eye with retinoblastoma and the suspicion of optic nerve, choroidal, or orbital tumor extension. Seeding of retinoblastoma onto the pars plana or into the anterior chamber are important findings that often lead to enucleation.
In bilateral cases, the eye with the most advanced tumor traditionally has been enucleated and the less involved eye managed with irradiation or other methods. If the most advanced eye has sparing of more than half of the retina, an attempt can be made to salvage both eyes with treatment. If both eyes have far-advanced tumors and there is no hope of any vision, bilateral enucleation may be necessary. Trying chemoreduction, bilateral external beam irradiation, or both with close follow-up may be justified if the parents are fully informed and refuse bilateral enucleation.
TECHNIQUE
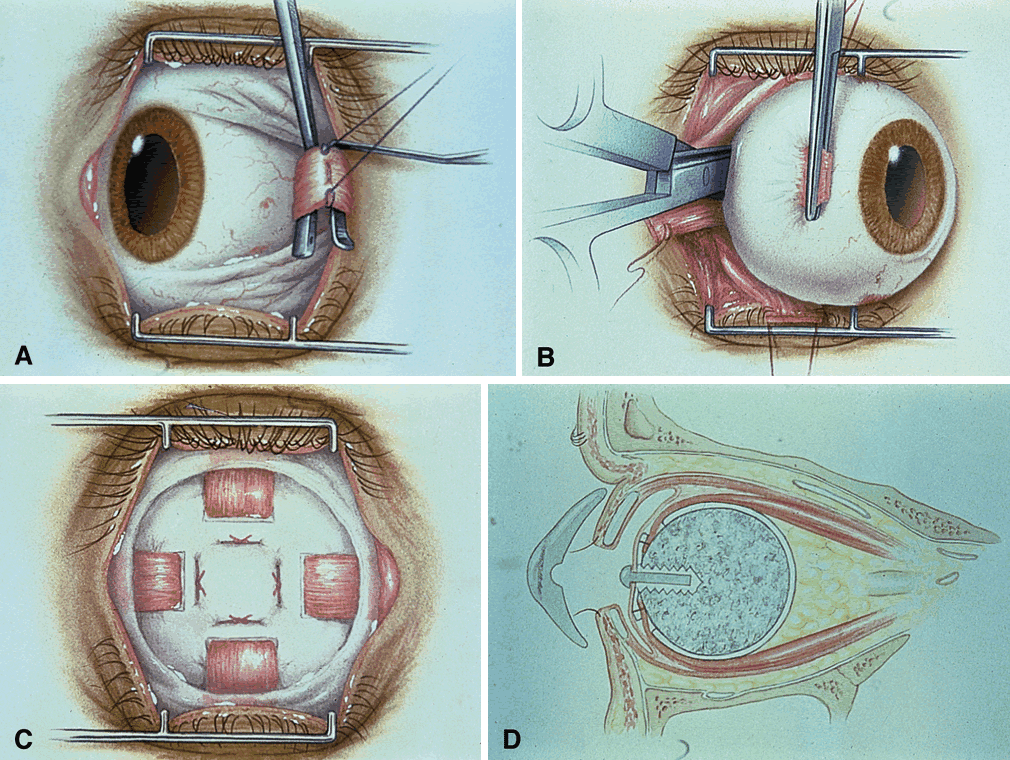
The technique of enucleation for retinoblastoma is slightly different from the standard enucleation performed by most ophthalmic surgeons.12,13 Since retinoblastoma is a loosely cohesive malignant tumor and the scleral wall in these young children can be thin, precautions should be taken to be extremely gentle during the procedure. A lateral canthotomy can be performed first if the child has a small, tight orbit. A peritomy is made for 360° at the limbus. Tenon's fascia is separated between the rectus muscles.
If the surgeon is not placing a motility implant, then the procedure is as follows (Fig. 2). The rectus muscles are individually gently hooked and cut with scissors near their insertion onto the sclera. The inferior and superior oblique muscle are sequentially hooked with two muscle hooks, clamped, and cut.
If the surgeon is placing a motility implant such as hydroxyapatite or13 polyethylene, then the technique is as follows. The rectus muscles are sequentially isolated on muscle hooks and tagged with double-armed 5-0 vicryl sutures near their insertion and cut at the insertion. The oblique muscles are isolated and cut without tagging. Orientation of the muscles and their respective sutures should be accurate.
The next step is cutting the optic nerve. Since the main route of extension of retinoblastoma is through the optic nerve and subarachnoid space, it is advisable to obtain as long a section of optic nerve as possible at the time of enucleation. This can be accomplished by placing a hemostat on the stump of the severed medial rectus muscle for traction. This permits the globe to be gently pulled forward, stretching the optic nerve when it is cut deep in the orbit. A firm grip with a hemostat provides better traction than silk sutures placed through the muscle insertions. By using this technique, the globe can be removed intact, and a long section of optic nerve generally can be obtained.
Enucleation scissors with long tips that have a slight curve are better than short scissors with a sharp curve for obtaining a sizable section of optic nerve. Snares or clamps on the optic nerve are not recommended, since they induce more trauma and can produce crush artifact in the optic nerve. This can lead to difficulty for the pathologist in differentiating meningothelial cells from crushed retinoblastoma cells.
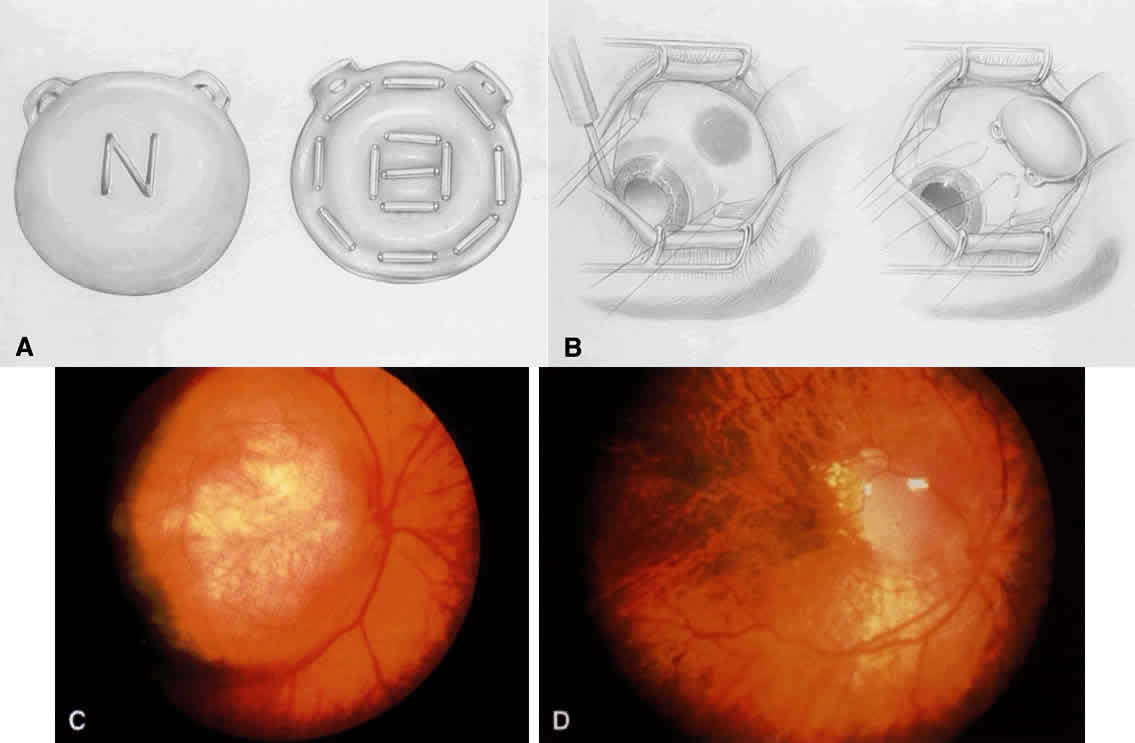
The optic nerve is cut and the eye removed. The socket is compressed with gauze until adequate hemostasis is obtained. An appropriately sized implant measuring 18 to 20 mm in diameter is placed in posterior Tenon's capsule. If there is a standard ball implant, then the horizontal rectus muscles along with overlying Tenon's capsule are tied horizontally to each other, and the vertical rectus muscle and Tenon's capsule are then tied vertically to each other. If there is a motility implant, then the four rectus muscles are attached with anatomic orientation to the implant. The cut rectus muscles are pulled through windows that have been cut in the scleral wrap, covering the implant. Currently, we use the hydroxyapatite orbital motility implant, and it is wrapped with betadine-soaked sclera13 or prepared bovine pericardium.
Closure of the socket includes 5-0 vicryl sutures placed in an interrupted vertical fashion in the deep and superficial layer of Tenon's capsule. The conjunctiva is closed with running 5-0 vicryl sutures. A conformer is placed between the lids and the conjunctiva. Antibiotic ointment is instilled, and an absorbent pressure patch is applied for 24 hours. In 6 weeks, the patient visits an ocularist for permanent prosthesis fitting.
Motility implants can provide remarkable prosthesis movement. In some cases, a peg can be placed into the motility implant to improve coupling of the movement of orbital implant with the prosthesis. The peg placement generally is delayed for at least 6 months after enucleation to allow adequate fibrovascularization of the implant. In children with retinoblastoma, we generally postpone the peg placement until the teen-age years, when the patient is more cooperative and the procedure can be performed under local anesthesia.
Some authors recommend that frozen sections of the optic nerve be performed at the time of enucleation to detect tumor extension.14 Since posterior optic nerve extension is rare in our experience and since histopathologic interpretation can be difficult, we do not routinely perform frozen sections in such cases. However, it seems reasonable to consider frozen sections in cases where optic nerve extension is strongly suspected.
COMPLICATIONS
There are few complications of enucleation.15 Hemorrhage at the time of surgery may be controlled with orbital compression. Postoperative ecchymosis and edema of the eyelids usually subside with use of a pressure patch and ice compresses postoperatively. Chemosis of the conjunctiva may occur and cause the conformer to temporarily protrude or become displaced. This problem is remedied by pressure patching and topical ointment. Patients who are receiving chemotherapy may develop socket infection, which can be managed with appropriate antibiotic therapy. Long-term complications include ptosis, orbital fat atrophy, or superior sulcus fat atrophy. These problems can be managed by adjustment of the prosthesis by the ocularist or by reconstructive plastic surgery of the eyelid or orbit.
Motility implants pose a higher risk for postoperative problems such as conjunctival thinning, implant exposure, implant infection, and implant extrusion.15–17 The hydroxyapatite peg system can extrude and lead to granulation tissue.18 However, careful attention to the surgical placement of the hydroxyapatite-integrated implant minimizes complications.15
RESULTS
The cosmetic results of enucleation for retinoblastoma generally are excellent. If the child had external beam radiotherapy in addition to enucleation, then the cosmetic result often is less satisfactory, related to radiation-induced orbital fat atrophy and a sunken appearance to the prosthesis, as well as decreased tear production with chronic discharge mucous drying on the prosthesis. The use of the integrated hydroxyapatite implant with rectus muscles attached improves the motility of the prosthesis.
HANDLING OF THE GLOBE AFTER ENUCLEATION
Retinoblastoma research, including genetic DNA analysis, requires fresh tumor tissue. The ophthalmologist who performs enucleation for retinoblastoma should be familiar with a safe technique to harvest adequate tissue for research within minutes after enucleation and still provide good histopathologic sections.12 Fresh tumor tissue or other ocular tissue should be harvested immediately after enucleation before cytolysis occurs. This technique requires close communication and cooperation with the researchers and the ophthalmic pathologist.
Immediately after the eye is enucleated, a piece of optic nerve is transected, and the proximal portion (toward the brain) of the optic nerve is marked with an indelible marking pencil and placed in 10% buffered formalin. This should be done immediately, before the globe is manipulated or opened. The pathologist is instructed to study the first sections through the proximal end of the nerve looking for tumor extension.
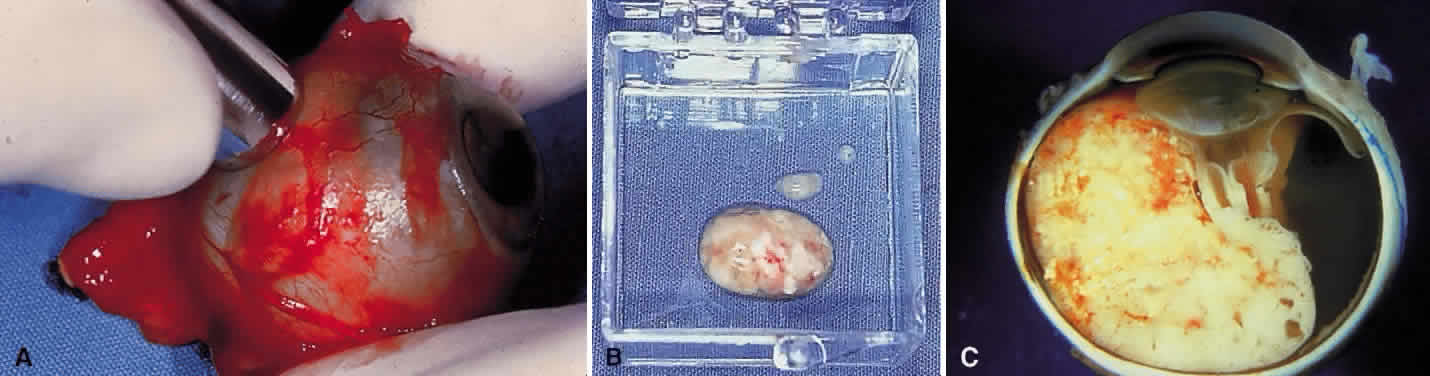
The area of the base of the tumor then is accurately marked on the sclera, and a scleral window is outlined to slightly overlap the tumor margin. A scleral window measuring 8 mm in diameter then is cut with a trephine (Fig. 3). The scleral opening should reveal typical soft white tumor tissue, which is partially removed with scissors or curretted without spillage and placed in tissue culture or submitted fresh. The tissue is immediately taken to the genetic research laboratory and prepared for DNA analysis or other studies. The sclera generally retains its spherical shape without collapse. The opened globe then is gently immersed in 10% formalin and submitted for routine histopathologic studies. It is important that the window be almost 90° from the center of the tumor base. This allows the pathologist to later make a pupillary-optic nerve section through the center of the tumor, avoiding the scleral window, which is left in the minor calotte. If done properly, the quality of the histopathologic sections will be excellent. The tray, instruments, and gloves used to harvest fresh tissue are contaminated with tumor and should be properly disposed.