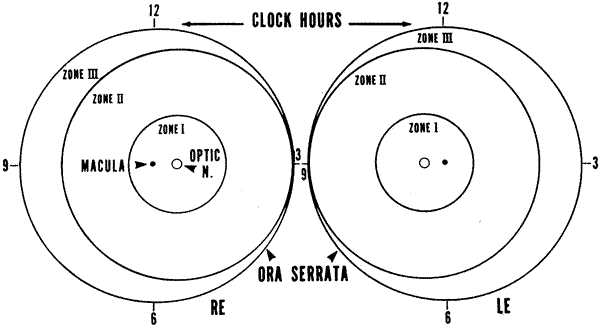
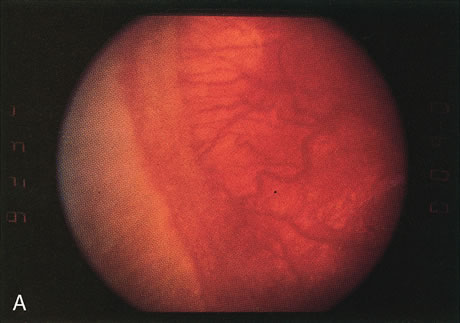
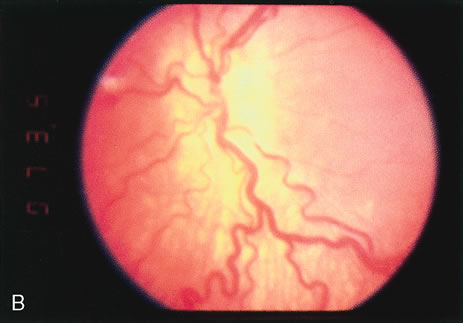
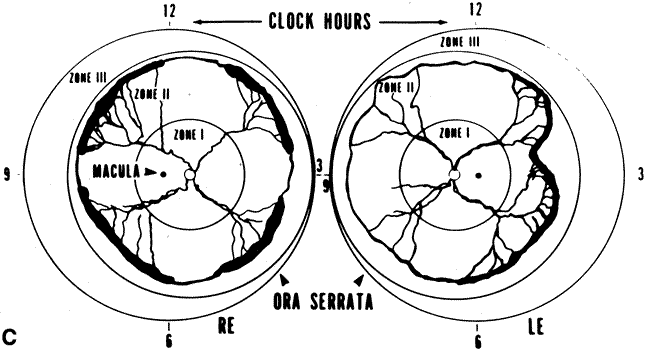
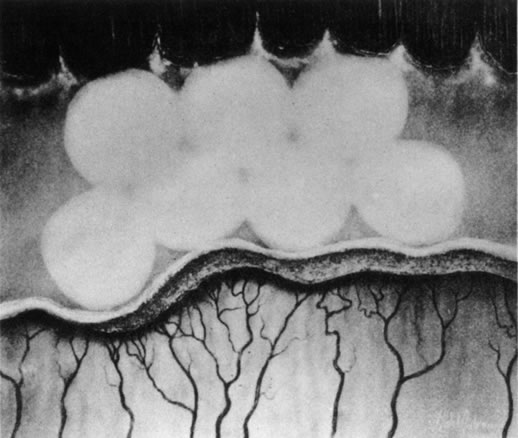
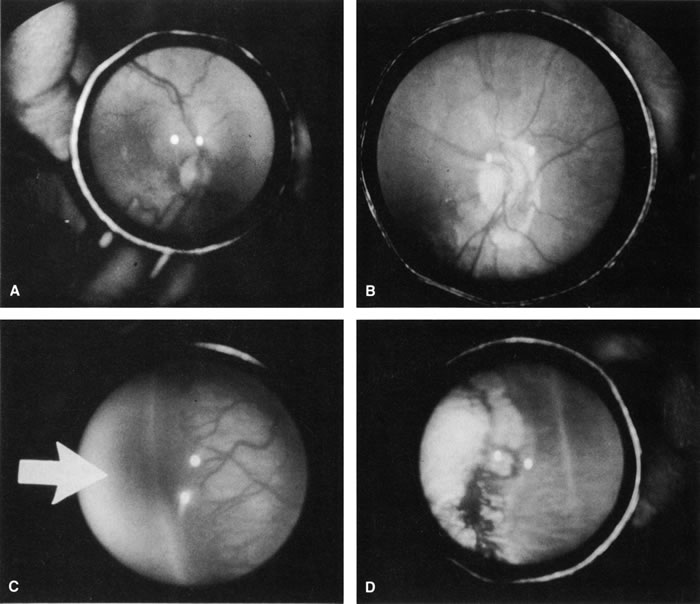
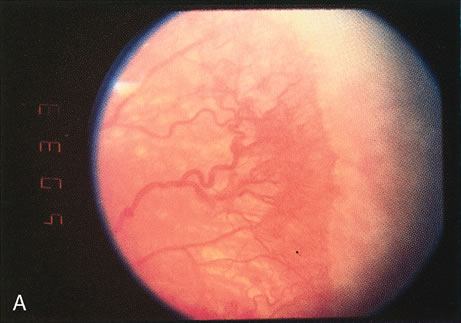
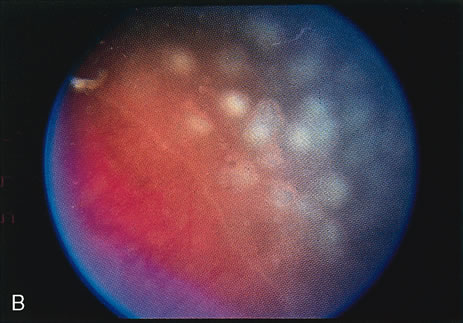
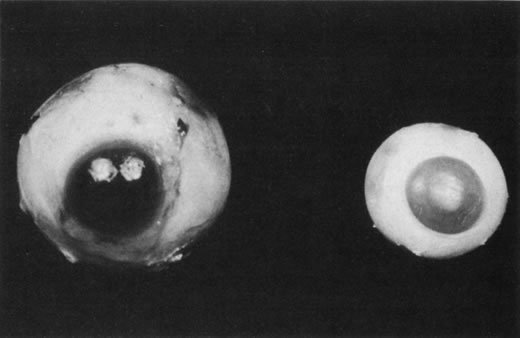
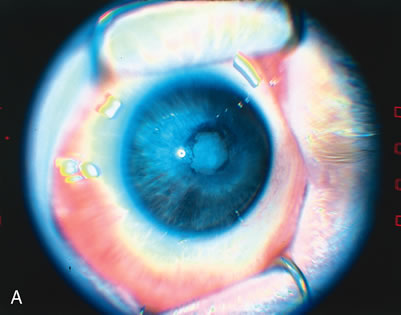
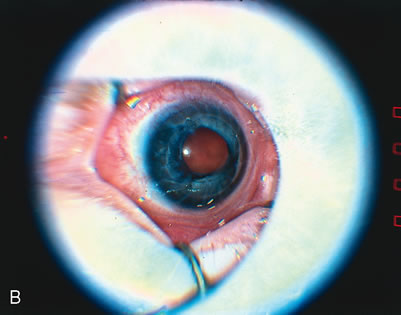
1. Rowlands E, Ionides AC, Chinn S et al: Reduced incidence of retinopathy of prematurity. Br J Ophthalmol 85:933-935, 2001 2. Fledelius HC, Dahl H: Retinopathy of prematurity, a decrease in frequency and severity. Trends over 16 years in a Danish county. Acta Ophthalmol Scand 78:359, 2000 3. Bullard SR, Donahue SP, Feman SS et al: The decreasing incidence and severity of retinopathy of prematurity. J Aapos 3:46, 1999 4. Hussain N, Clive J, Bhandari V: Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics 104:e26, 1999. 5. Gould JB, Benitz WE, Liu H: Mortality and time to death in very low birth weight infants: California, 1987 and 1993. Pediatrics 105:E37, 2000. 6. Campbell PB, Bull MJ, Ellis FD et al: Incidence of retinopathy of prematurity in a tertiary newborn intensive
care unit. Arch Ophthalmol 101:1686, 1983 7. Kalina RE, Karr DJ: Retrolental fibroplasia. Experience over two decades in one institution. Ophthalmology 89:91, 103, 1982. 8. Repka MX, Palmer EA, Tung B: Involution of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 118:645, 2000 9. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 102:1130, 1984 10. Multicenter trial of cryotherapy for retinopathy of prematurity: Ophthalmological
outcomes at 10 years. Arch Ophthalmol 119:1110, 2001 11. Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen
visual acuity and structural outcome at 5 ½ years after randomization. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 114:417, 1996 12. Multicenter trial of cryotherapy for retinopathy of prematurity: 3 ½-year
outcome—structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 111:339, 1993 13. Multicenter trial of cryotherapy for retinopathy of prematurity: Preliminary
results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics 81:697, 1988 14. Vander JF, Handa J, McNamara JA et al: Early treatment of posterior retinopathy of prematurity: a controlled trial. Ophthalmology 104:1731; discussion 1735, 1997 15. Flynn JT: An International Classification of Retinopathy of Prematurity: development
of the classification of the late stages of retinopathy of prematurity. Birth Defects Orig Artic Ser 24:175, C172, 1988 16. Haigh PM, Chiswick ML, O'Donoghue EP: Retinopathy of prematurity: systemic complications associated with different
anaesthetic techniques at treatment. Br J Ophthalmol 81:283, 1997 17. Brown GC, Tasman WS, Naidoff M et al: Systemic complications associated with retinal cryoablation for retinopathy
of prematurity. Ophthalmology 97:855, 1990 18. Multicenter trial of cryotherapy for retinopathy of prematurity: Three-month
outcome. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 108:195, 1990 19. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year
outcome—structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 108:1408, 1990 20. Greven CM, Tasman W: Rhegmatogenous retinal detachment following cryotherapy in retinopathy
of prematurity. Arch Ophthalmol 107:1017,1989 21. Topilow HW, Ackerman AL: Cryotherapy for stage 3+ retinopathy of prematurity: visual and anatomic
results. Ophthalmic Surg 20:864, 1989 22. Kaiser RS, Trese MT, Williams GA, Cox MS Jr: Adult retinopathy of prematurity: outcomes of rhegmatogenous retinal detachments
and retinal tears. Ophthalmology 108:1647, 2001 23. Quinn GE, Dobson V, Kivlin J et al: Prevalence of myopia between 3 months and 5 ½ years in preterm infants
with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 105:1292, 1998 24. Quinn GE, Dobson V, Siatkowski R et al: Does cryotherapy affect refractive error? Results from treated versus control
eyes in the cryotherapy for retinopathy of prematurity trial. Ophthalmology 108:343, 2001 25. Effect of retinal ablative therapy for threshold retinopathy of prematurity: results
of Goldmann perimetry at the age of 10 years. Arch Ophthalmol 119:1120, 2001 26. Repka MX, Summers CG, Palmer EA et al: The incidence of ophthalmologic interventions in children with birth weights
less than 1251 g. Results through 5 ½ years. Cryotherapy for Retinopathy of Prematurity
Cooperative Group. Ophthalmology 105:1621, 1998 27. Nagata M: [Therapeutic possibility in retrolental fibroplasia in the premature
infant with light coagulation]. Ganka 10:719, 1968 28. Nagata M, Kobayashi Y, Fukuda H et al: Photocoagulation for the treatment of retinopathy of prematurity. Jpn J Clin Ophthalmol 22:419, 1968 29. Yamashita Y: Studies on retinopathy of prematurity: III. Cryocautery for retinopathy of prematurity. Jpn J Clin Ophthalmol26:385, 1972 30. McNamara JA, Tasman W, Brown GC, Federman JL: Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology 98:576, 1991 31. Iverson DA, Trese MT, Orgel IK, Williams GA: Laser photocoagulation for threshold retinopathy of prematurity. Arch Ophthalmol 109:1342, 1991 32. Hunter DG, Repka MX: Diode laser photocoagulation for threshold retinopathy of prematurity. A
randomized study. Ophthalmology 100:238, 1993 33. McGregor ML, Wherley AJ, Fellows RR et al: A comparison of cryotherapy versus diode laser retinopexy in 100 consecutive
infants treated for threshold retinopathy of prematurity. J Aapos 2:360, 1998 34. Pearce IA, Pennie FC, Gannon LM et al: Three year visual outcome for treated stage 3 retinopathy of prematurity: cryotherapy
versus laser. Br J Ophthalmol 82:1254, 1998 35. Seiberth V, Linderkamp O, Vardarli I: Transscleral vs transpupillary diode laser photocoagulation for the treatment
of threshold retinopathy of prematurity. Arch Ophthalmol 115:1270, 1997 36. Christiansen SP, Bradford JD: Cataract in infants treated with argon laser photocoagulation for threshold
retinopathy of prematurity. Am J Ophthalmol 119:175, 1995 37. Seiberth V, Linderkamp O, Vardarli I et al: Diode laser photocoagulation for threshold retinopathy of prematurity in
eyes with tunica vasculosa lentis. Am J Ophthalmol 119:748, 1995. 38. Steinmetz RL, Brooks HL Jr: Diode laser photocoagulation to the ridge and avascular retina in threshold
retinopathy of prematurity. Retina 22:48, 2002 39. Banach MJ, Ferrone PJ, Trese MT: A comparison of dense versus less dense diode laser photocoagulation patterns
for threshold retinopathy of prematurity. Ophthalmology 107:324, discussion 328, 2000 40. Landers MB3rd , Toth CA, Semple HC, Morse LS: Treatment of retinopathy of prematurity with argon laser photocoagulation. Arch Ophthalmol 110:44, 1992 41. McNamara JA, Tasman W, Vander JF, Brown GC: Diode laser photocoagulation for retinopathy of prematurity. Preliminary
results. Arch Ophthalmol110:1714, 1992 42. DeJonge MH, Ferrone PJ, Trese MT: Diode laser ablation for threshold retinopathy of prematurity: short-term
structural outcome. Arch Ophthalmol 118:365, 2000 43. Ling CS, Fleck BW, Wright E et al: Diode laser treatment for retinopathy of prematurity: structural and functional
outcome. Br J Ophthalmol 79:637, 1995 44. Axer-Siegel R, Snir M, Cotlear D et al. Diode laser treatment of posterior retinopathy of prematurity. Br J Ophthalmol 84:1383, 2000 45. Seiberth V, Linderkamp O, Vardarli I et al: Diode laser photocoagulation for stage 3+ retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 233:489, 1995 46. Foroozan R, Connolly BP, Tasman WS: Outcomes after laser therapy for threshold retinopathy of prematurity. Ophthalmology 108:1644, 2001 47. Connolly BP, McNamara JA, Regillo CD et al: Visual outcomes after laser photocoagulation for threshold retinopathy
of prematurity. Ophthalmology 106:1734, ; discussion 1737, 1999 48. Ng EY, Connolly BP, McNamara JA et al: A comparison of laser photocoagulation with cryotherapy for threshold retinopathy
of prematurity at 10 years:part 1. Visual function and structural outcome. Ophthalmology 109:928, discussion 935, 2002 49. Shalev B, Farr AK, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy
for threshold retinopathy of prematurity: seven-year outcome. Am J Ophthalmol 132:76, 2001 50. Kaiser RS, Trese MT: Iris atrophy, cataracts, and hypotony following peripheral ablation for
threshold retinopathy of prematurity. Arch Ophthalmol 119:615, 2001 51. Rundle P, McGinnity FG: Bilateral hyphaema following diode laser for retinopathy of prematurity. Br J Ophthalmol 79:1055, 1995 52. Simons BD, Wilson MC, Hertle RW, Schaefer DB: Bilateral hyphemas and cataracts after diode laser retinal photoablation
for retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 35:185, 1998 53. Capone A Jr, Drack AV: Transient lens changes after diode laser retinal photoablation for retinopathy
of prematurity. Am J Ophthalmol 118:533, 1994 54. Drack AV, Burke JP, Pulido JS, Keech RV: Transient punctate lenticular opacities as a complication of argon laser
photoablation in an infant with retinopathy of prematurity. Am J Ophthalmol 113:583, 1992 55. Pogrebniak AE, Bolling JP, Stewart MW: Argon laser-induced cataract in an infant with retinopathy of prematurity. Am J Ophthalmol 117:261, 1994 56. Lambert SR, Capone A Jr, Cingle KA, Drack AV: Cataract and phthisis bulbi after laser photoablation for threshold retinopathy
of prematurity. Am J Ophthalmol 129:585, 2000. 57. Noonan CP, Clark DI: Acute serous detachment with argon laser photocoagulation in retinopathy
of prematurity. J Aapos 1:183, 1997 58. Algawi K, Goggin M, O'Keefe M: Refractive outcome following diode laser versus cryotherapy for eyes with
retinopathy of prematurity. Br J Ophthalmol 78:612, 994 59. Connolly BP, Ng EY, McNamara JA et al: A comparison of laser photocoagulation with cryotherapy for threshold retinopathy
of prematurity at 10 years:part 2. Refractive outcome. Ophthalmology 109:936, 2002 60. An international classification of retinopathy of prematurity:II. The classification of retinal detachment. The International Committee
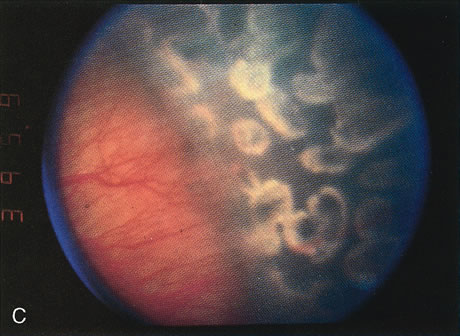
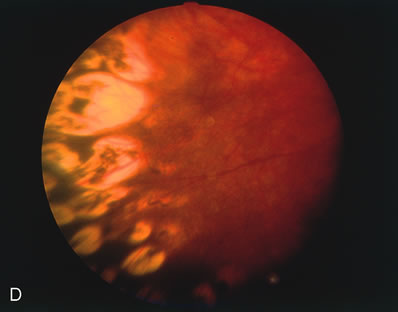
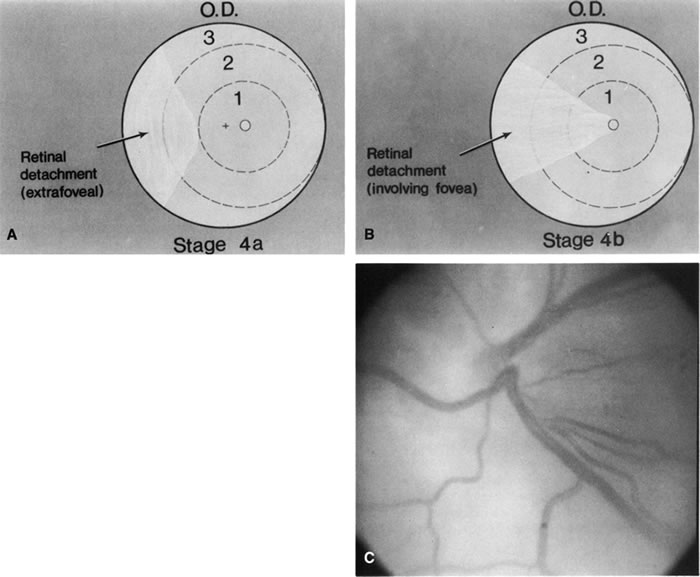
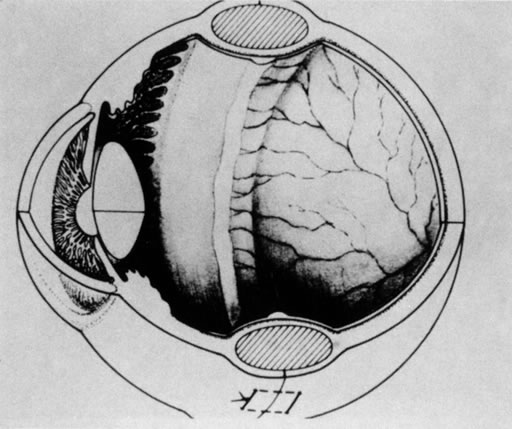
for the Classification of the Late Stages of Retinopathy of Prematurity. Arch Ophthalmol 105:906, 1987. 60a. Capone A Jr, Trese MT: Lens-sparing vitreous surgery for tractional stage 4A retinopathy
of prematurity retinal detachments. Ophthalmology 108:2068, 2001 61. Gilbert WS, Quinn GE, Dobson V et al: Partial retinal detachment at 3 months after threshold retinopathy of prematurity. Long-term structural and functional outcome. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative
Group. Arch Ophthalmol 114:1085, 1996 62. Trese MT. Scleral buckling for retinopathy of prematurity. Ophthalmology 101:23, 1994 63. Hinz BJ, de Juan E Jr, Repka MX: Scleral buckling surgery for active stage 4A retinopathy of prematurity. Ophthalmology 105:1827, 1998 64. Chuang YC, Yang CM: Scleral buckling for stage 4 retinopathy of prematurity. Ophthalmic Surg Lasers 31:374, 2000 65. Choi MY, Yu YS: Efficacy of removal of buckle after scleral buckling surgery for retinopathy
of prematurity. J Aapos 4:362, 2000 66. Noorily SW, Small K, de Juan E Jr, Machemer R: Scleral buckling surgery for stage 4B retinopathy of prematurity. Ophthalmology 99:263, 1992 67. Ricci B, Santo A, Ricci F et al: Scleral buckling surgery in stage 4 retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 234(Suppl 1):S38, 1996 68. Greven C, Tasman W: Scleral buckling in stages 4B and 5 retinopathy of prematurity. Ophthalmology 97:817, 1990 69. Ryan EH Jr, Arribas NP, Olk RJ et al: External argon laser drainage of subretinal fluid using the endolaser probe. Retina 11:214, 1991 70. McPherson AR, Hittner HM, Lemos R: Retinal detachment in young premature infants with acute retrolental fibroplasia. Thirty-two new cases. Ophthalmology 89:1160, 1982. 71. Quinn GE, Dobson V, Barr CC et al: Visual acuity of eyes after vitrectomy for retinopathy of prematurity: follow-up
at 5 ½ years. The Cryotherapy for Retinopathy
of Prematurity Cooperative Group. Ophthalmology 103:595, 1996 72. Trese MT: Surgical results of stage V retrolental fibroplasia and timing of surgical
repair. Ophthalmology 91:461, 1984 73. Hirose T, Katsumi O, Mehta MC, Schepens CL: Vision in stage 5 retinopathy of prematurity after retinal reattachment
by open-sky vitrectomy. Arch Ophthalmol111:345, 1993 74. Tasman W, Borrone RN, Bolling J: Open sky vitrectomy for total retinal detachment in retinopathy of prematurity. Ophthalmology 94:449, 1987 75. Clarkson JG, Jacobson SG, Frazier-Byrne S, Flynn JT: Evaluation of eyes with stage-5 retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 227:332, 1989. 76. Trese MT: Surgical therapy for stage V retinopathy of prematurity. A two-step
approach. Graefes Arch Clin Exp Ophthalmol 225:266, 1987 77. Schepens CL: Clinical and research aspects of subtotal open-sky vitrectomy. XXXVII Edward Jackson Memorial Lecture. Am J Ophthalmol 91:143, 1981 78. Hirose T, Schepens CL, Lopansri C: Subtotal open-sky vitrectomy for severe retinal detachment occurring
as a late complication of ocular trauma. Ophthalmology 88:1, 1981 79. Treister G, Machemer R: Results of vitrectomy for rare proliferative and hemorrhagic diseases. Am J Ophthalmol 84:394, 1977 80. Charles S: Retinopathy of prematurity. In Vitreous Microsurgery. Baltimore: Williams & Wilkins, 1987:158. 81. Trese MT: Visual results and prognostic factors for vision following surgery for
stage V retinopathy of prematurity. Ophthalmology 93:574, 1986 82. Zilis JD, deJuan E, Machemer R: Advanced retinopathy of prematurity. The anatomic and visual results of vitreous surgery. Ophthalmology 97:821, 1990 83. deJuan E Jr, Machemer R: Retinopathy of prematurity. Surgical technique. Retina 7:63, 1987 84. Capone A Jr, Trese MT: Lens-sparing vitreous surgery for tractional stage 4A retinopathy
of prematurity retinal detachments. Ophthalmology 108:2068, 2001 85. Trese MT: Two-hand dissection technique during closed vitrectomy for retinopathy
of prematurity. Am J Ophthalmol 101:251, 1986 86. Maguire AM, Trese MT: Visual results of lens-sparing vitreoretinal surgery in infants. J Pediatr Ophthalmol Strabismus 30:28, 1993 87. Ferrone PJ, Harrison C, Trese MT: Lens clarity after lens-sparing vitrectomy in a pediatric population. Ophthalmology 104;273, 1997 88. Quinn GE, Dobson V, Barr CC et al: Visual acuity in infants after vitrectomy for severe retinopathy of prematurity [published
erratum appears in Ophthalmology 98:1005, 1991]. Ophthalmology 98:5, 1991 89. Choi MY, Yu YS: Anatomical and visual results of vitreous surgery for advanced retinopathy
of prematurity. Korean J Ophthalmol 12:60, 1998 90. Chong LP, Machemer R, de Juan E: Vitrectomy for advanced stages of retinopathy of prematurity. Am J Ophthalmol 102:710, 1986 91. Fuchino Y, Hayashi H, Kono T, Ohshima K: Long-term follow up of visual acuity in eyes with stage 5 retinopathy
of prematurity after closed vitrectomy. Am J Ophthalmol 120:308, 1995. 92. Trese MT, Droste PJ: Long-term postoperative results of a consecutive series of stages 4 and 5 retinopathy
of prematurity. Ophthalmology 105:992, 1998 93. Blacharski PA, Charles ST: Thrombin infusion to control bleeding during vitrectomy for stage V retinopathy
of prematurity. Arch Ophthalmol 105:203, 1987 94. Good WV, Hardy RJ: The multicenter study of early treatment for retinopathy of prematurity (ETROP). Ophthalmology 108:1013,2001 95. Fujii GY, De Juan E Jr, Humayun MS et al: Initial experience using the transconjunctival sutureless vitrectomy system
for vitreoretinal surgery. Ophthalmology 109:1814, 2002. 96. Penn JS, Rajaratnam VS, Collier RJ, Clark AF: The effect of an angiostatic steroid on neovascularization in a rat model
of retinopathy of prematurity. Invest Ophthalmol Vis Sci 42:283, 2001. |