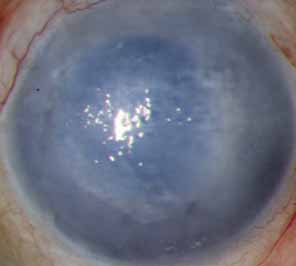
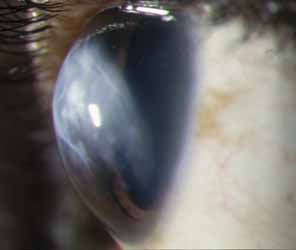
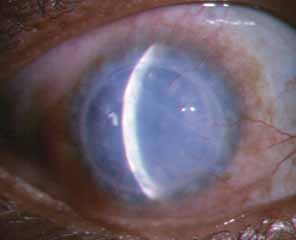
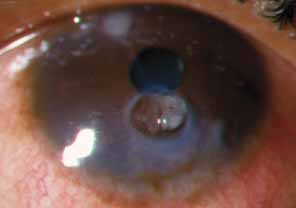
The current number of procedures performed on an annual basis is decreasing slightly; a total of 32,744 corneal transplants were performed in 2002, marking the first time since 1986 that the number of procedures performed has been less than 33,000.1 This downward trend has coincided with the decrease in incidence of pseudophakic corneal edema (PCE) (Fig. 1) and aphakic corneal edema (ACE). The percentage of PKs performed to treat PCE has dropped from 19.8% in 2001 to 18.4% in 2002, which was the lowest percent reported in this category in 15 total years of tracking by the Eye Bank Association of America (EBAA). It is possible that this decrease reflects improved cataract removal technique and technology, such as phacoemulsification and posterior chamber intraocular lens placement. The indications for PK have shifted over the past several decades, with PCE the most common indication since 1980, whereas keratoconus (Fig. 2) and ACE were the most common indications prior to 1980.2 Data is tracked for a total of 16 indications for transplant by the EBAA. Pseudophakic corneal edema, keratoconus (particularly in younger patients), Fuchs' endothelial dystrophy (particularly in older patients), and regrafting secondary to rejection or other reasons currently sit atop the list of indications for PK.
|
|
Although the overall number of penetrating keratoplasties has decreased, there are certain indications that have increased. Patel3 examined the incidence and indications at a major university setting in the northeastern United States and noted that the incidence of regraft was increasing. The number of regrafts increased from 9% to 16%, comparing the years 1983 to 1988 with 1989 to 1995. Within the regraft category, the number of multiple regrafts had also increased (11% from 1989–1991, jumping to 29% from 1992–1995). Regrafting and multiple regrafting were associated with an increased failure rate during the study period. A subsequent study at the same institution by Cosar4 showed that the incidence of regraft had increased to 18.1% from 1996 to 2000. The leading indications in their study population also included PCE (27.2%), keratoconus (15.4%), and Fuchs' endothelial dystrophy (15.2%). Differing incidences of regraft have been noted in other reports.5–7 One analysis noted only an 8.7% incidence of regraft.8 In this population, keratoconus (rather than PCE) was the leading indication for transplantation (45.6%).
With regards to availability of tissue, the transplant surgeon enjoys a relative abundance of donor corneal tissue, so the waiting-list experience endured by other transplant candidates (e.g., kidney or heart transplant) does not occur for the corneal transplant patient, with few exceptions. A total of 42,143 donations were made in 2002. The pool of suitable donor corneas will likely decrease in the future given the increasing incidence of refractive procedures. Donor screening (in the form of potential donor questionnaires and eye tissue analyses to detect evidence of prior refractive surgery) will likely become more important as the popularity of these corneal refractive procedures continues to increase.9–11
The prognosis for graft clarity can be divided into four groups (Table 1). This grouping system takes into account wound healing ability, presence of vascularity at the graft-host junction, and underlying infectious processes. Keratoconus, in group 1, has an excellent prognosis for graft clarity, and may account for the lower total number of regrafts reported by Edwards.8 The majority of PKs are performed for optical indications, so as to improve vision by removing an optically opaque or dysfunctional cornea. Tectonic (to preserve globe integrity), therapeutic (to remove progressive conditions, such as an expanding fungal infiltrate) palliative, (to improve comfort in a painful eye with bullous corneal edema), and cosmetic (to improve appearance in a blind eye) indications are less frequently encountered reasons for transplantation.
TABLE 1. Prognosis for Graft Clarity*
| Diagnosis | Morphology | Prognosis | |
| Group 1 | Keratoconus | Avascular central corneal thinning, scarring, or edema surrounded by healthy corneal tissue | Excellent, 90% or more |
| Central or paracentral inactive scarsLattice or granular Dystrophies | |||
| Central Fuchs' dystrophy (early) | |||
| Rotating grafts or autografts | |||
| Group 2 | Advanced Fuchs' dystrophy | Lesion that extends partially or totally to | Very good, 80%–90% |
| Pseudophakic corneal edema | periphery with an adequate surface and | ||
| Aphakic corneal edema | mild-to-moderate vascularity | ||
| Inactive herpes simplex keratitis | |||
| Iridocorneal endothelial syndromes | |||
| Interstitial keratitis | |||
| Macular dystrophy | |||
| Group 3 | Active bacterial keratitis | Extremes of corneal thickness, perforations, | Fair, 50%–80% |
| Active herpes simplex keratitis | peripheral descemetoceles, active disease | ||
| Congenital hereditary endothelial dystrophy | |||
| Active fungal keratitis | |||
| Mild chemical burns | |||
| Moderate keratitis sicca | |||
| Group 4 | Severe chemical burns | Severe fibrovascular replacement of cornea, | Poor, 0–50% |
| Radiation burns | conjunctival ischemia, anterior chamber | ||
| Ocular cicatricial pemphigoid | obliteration, limbal stem cell deficiency | ||
| Stevens-Johnson syndrome | |||
| Neuroparalytic disease | |||
| Congenital glaucoma | |||
| Epithelial downgrowth | |||
| Anterior chamber cleavage syndromes | |||
| Limbal stem cell deficient states | |||
| Multiple graft failures† |
*This table is meant to be a guideline and is not absolute. The prognosis for each group is worsened by the presence of elevated intraocular pressure, intraocular inflammation, and lid and conjunctival defects.
†Failed grafts generally have the prognosis for the group of their primary diagnosis or slightly less. (From Buxton JN, Norden RA: Indications and contraindications. In Brightbill FS (ed): Corneal Surgery: Theory, Technique, and Tissue. St Louis: CV Mosby, 1986)
PREOPERATIVE EVALUATION
A complete patient and family history must be taken, including vision prior to onset of symptoms, presence of amblyopia, timing of prior surgery, medical treatments of the underlying condition, current ocular and systemic medications, presence of glaucoma, presence of systemic illness, and vision in the contralateral eye. The patient's desire and ability to adhere to a prolonged postoperative course must be ascertained. A social work consultation can be extremely helpful to this end. Family and/or supportive caregiver situation, transport access, ability to receive help with administration of medications if necessary, and patient motivation can help determine likelihood of patient compliance. Previous medical records from referring physicians detailing prior ocular heath, phakic status, prior medical treatments, and prior ocular surgery can be helpful.
A complete ocular examination must be performed by the surgeon to determine prognosis and to identify factors that may need to be addressed at the time of transplantation. Preoperative visual acuity, as well as rigid gas permeable contact lens refraction for selected patients, such as those with keratoconus or central scarring, are measured. Intraocular pressure (IOP) and gonioscopy, if possible, are performed, with the knowledge of current medications and potential need for concomitant glaucoma tube shunt surgery. Given the potential exposure to long-term corticosteroid use and possible steroid sensitivity, the possibility and status of glaucoma must be addressed. The presence or absence of a relative afferent pupillary defect in the operative eye is also documented, which is especially helpful in the glaucomatous or traumatized eye.
The patient's underlying corneal disorder or disorders are identified during slit-lamp examination. The status of the limbus and presence of corneal neovascularization or conjunctivalization is noted. This may aid in the evaluation of stem cell function and re-epithelialization potential after transplantation. Ocular surface disease, as seen in patients with limbal stem cell deficient processes (e.g., alkali burns, Stevens-Johnson syndrome, and aniridia), can be associated with impaired healing and may require adjunctive therapy at the time of corneal transplantation. In addition to limbal stem cell deficiency, abnormalities of the tear film and decreased mucin production (as seen in ocular cicatricial pemphigoid, Stevens-Johnson syndrome, and chemical burns) present a relative contraindication to PK. Adequate ocular lubrication is essential to the proper healing and functioning of a corneal graft. In these select patients, it has beenfound that limbal stem cell transplantation and systemic immunosuppression combined with penetrating keratoplasty has been a successful approach to ocular surface rehabilitation. Keratoprostheses, which replace diseased corneal tissue with an integrated clear plastic device, have also been used to improve visual function in patients with severe limbal stem cell deficiency and are addressed fully in a separate chapter.
The centration and extent of corneal pathology are noted, and the graft size needed to treat the condition is estimated. The Haag-Streit slit lamp is equipped with a variable height beam, and this beam is helpful in estimating the size of corneal lesions.
The patient's lenticular status is also very important in the preoperative evaluation. The presence of an anterior chamber lens and its role in possible contribution to the need for transplantation should be determined, with possible lens removal or exchange at the time of transplantation. If the patient is phakic, lenticular opacity is assessed, being aware that corticosteroid use in the postoperative period may hasten the progression of a cataract. Therefore, combined corneal transplantation, lens removal, and intraocular lens placement or “triple procedure” may be considered. If lens removal and exchange are indicated, axial length measurement is performed.
Ocular inflammation must be controlled prior to consideration of surgery. The presence of anterior chamber cell and flare, keratic precipitates, peripheral anterior synechiae (PAS), and posterior synechiae are noted. In addition to controlling inflammation, inciting agents must also be identified and addressed. Herpetic disease, including stromal inflammation, must be adequately controlled, and we recommend a time interval of at least 6 months prior to consideration of keratoplasty in these eyes.
Eyelid function and presence of scarring are noted, especially in the patient with concomitant ocular and facial burns. Inadequate lid closure and lagophthalmos can result in corneal drying and exposure, thereby placing the graft at increased risk. Patients with advanced corneal scarring and cicatrization of the fornix are also at risk for exposure keratopathy. Excessive ectropion or entropion with trichiasis can oftentimes be adequately treated by oculoplastic consultation and surgical repair. A prerequisite to oculoplastic surgical assistance, though, is control of ocular and conjunctival inflammation. Lid margin telangiectasia, meibomian gland dysfunction, lid margin hypertrophy, and other signs of rosacea are noted and treated to decrease inflammation and potential stimuli for limbal neovascularization.
Retinal status must be evaluated but may be limited by the view to the posterior pole through a diseased cornea. Contralateral retinal examination may yield information regarding glaucoma, macular degeneration, and overall retinal status. If the view is unsatisfactory, B-scan ultrasonography is indicated.
Informed consent to the procedure is obtained only after a frank discussion with the patient (and his or her family members or caregivers, if the patient so desires). The transplant surgeon should take time to discuss the diagnosis, risks, and potential complications, benefits, alternative treatment options, and details of the procedure and postoperative care. Given the prolonged convalescence, realistic expectations regarding visual recovery, and the timetable for possible recovery, are key points in the discussion. It is important to mention the potential need for rigid gas permeable contact lens wear to treat postoperative irregular astigmatism and to identify the patient's (and/or caregiver's) ability to handle such a lens. Dedication and commitment to follow-up examination must also be stressed. Again, social work evaluation can be helpful in gauging both the patient's and the family's understanding of the procedure and of the follow-up care needed for keratoplasty patients.
PREOPERATIVE MEDICATIONS
Penetrating keratoplasty has been found to be a safe procedure based on the patient's overall medical health and can often be performed utilizing regional anesthesia. At our institution, topical fluoroquinolone antibiotic drops are given every 5 minutes for three drops total preoperatively as a prophylactic measure. In phakic patients undergoing PK only, pilocarpine 2% is given every 5 minutes for three drops total to produce a miotic pupil, thus protecting the lens. Posterior chamber pseudophakic eyes also receive pilocarpine to maintain lenticular position.
In older patients, or in those with higher intrathoracic pressures (such as individuals with stout necks), intravenous mannitol 20% is given preoperatively. Prior to administration, it is essential for the anesthesia staff to assess the patient's overall medical status. Mannitol acts as an osmotic diuretic to help deturgesce the vitreous and thereby decrease posterior vitreous pressure. In combined (keratoplasty and cataract extraction) cases, it may be prudent to place a condom-style catheter on male patients and to have a bedpan ready due to the added length of the case. A Honan balloon can be placed on the eye and inflated to a pressure of 30 mm Hg for 10 minutes, or digital massage can be performed to lower IOP. In combined procedures, we also use the cycloplegic-mydriatic combination of cyclopentolate hydrochloride 1%, phenylephrine 5%, and tropicamide 0.5% one drop every 5 minutes for three doses total for pupil dilation.
SURGICAL TECHNIQUE
There are multiple accepted ways of performing penetrating keratoplasty, and it continues to be an evolving procedure. Surgeons may utilize various techniques in achieving the same goal of a successful transplant and improved visual acuity for the patient. We will present one such approach to the procedure, and we recognize that there are many other acceptable and successful approaches. We will also discuss penetrating keratoplasty in the aphakic and pseudophakic patient, as well as concurrent PK, lens removal, and intraocular lens placement.
Patient positioning is a vital step in ensuring patient comfort for a relatively lengthy procedure while maximizing surgeon access. A tense, uncomfortable patient is apt to move unpredictably during the procedure, and even the most cooperative patient may experience fatigue and strain, resulting in a Valsalva maneuver. This can lead to a rise in posterior pressure, increasing the likelihood of anterior chamber shallowing, vitreous loss, and even suprachoroidal hemorrhage. By placing the head of the bed slightly higher than the foot of the bed in a reverse-Trendelenburg position, posterior pressure can be minimized, especially in obese patients with increased intrathoracic pressure. Also essential is surgeon comfort; the surgeon should also have direct access to both a preparation table and the patient. Placing the microscope height at a level to keep the surgeon's back and shoulders erect can help minimize surgeon fatigue. A wrist rest can be used to aid in surgeon comfort, by providing a place to rest one's hands. It can also aid in patient comfort, by minimizing pressure placed by the surgeon on the forehead and neck. A wrist rest also improves stability and helps minimize the possibility of inadvertently applying pressure on the globe or orbit during the procedure. At some surgical facilities, an ergonomist is available to examine ideal heights for microscope, bed, and wrist rest placement. A good general rule to follow is that a more comfortable position can be maintained for longer periods of time, thereby lessening fatigue.
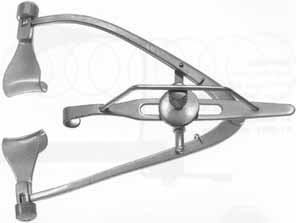
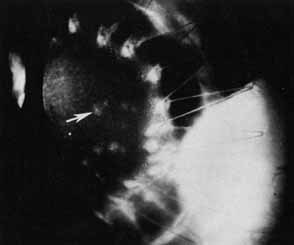
Adequate anesthesia is also important in maintaining patient comfort. Both general and local regional anesthetics can be used in penetrating keratoplasty. Pediatric or uncooperative patients as well as those with tremors, claustrophobia, high level of anxiety, or a history of ocular trauma with possible penetrating injury, are all candidates for general anesthesia. The majority of patients with nontraumatic indications for PK, however, can receive adequate anesthesia and akinesia with local regional approach. After the administration of mild intravenous sedation by the anesthesia team while in the operating room, a local lid block and retrobulbar block can be performed. Local anesthesia consists of both retrobulbar block and lid block, such as an O'Brien or Van Lint block, to minimize orbicularis function and to decrease the risk of elevated intraorbital pressure while the globe is open. An O'Brien lid block, which consists of a 3 to 5 cc injection of an equal mix of lidocaine 2% and bupivacaine 0.75% into the orbicularis muscle along the inferior and superior orbital rim, can provide adequate lid akinesia, minimizing the posterior pressure that can occur with a vigorous lid squeeze. Retrobulbar anesthesia, consisting of 3 to 5 cc of the same mix of lidocaine and bupivacaine, can provide excellent pain control and minimize globe movement during the procedure. It is important to withdraw the plunger on the syringe immediately prior to administering the block to identify inadvertent vascular canalization. Also, monitoring the globe position by retracting the upper lid (often done by an assistant) helps to minimize the risk of optic nerve trauma. It is important to not inject too large an amount of retrobulbar anesthetic, as this increase in orbital volume can result in increased posterior pressure. After the retrobulbar block, ocular massage, if the globe is intact, can be performed. Alternatively, a Honan balloon, which consists of an inflatable chamber applied over the eye and secured with an adjustable headband, can be placed for several minutes. The eyelids and skin around the eye are then cleansed with betadine antimicrobial solution. In the setting of betadine allergy, benzalkonium chloride can be used as an alternative. A fenestrated drape is then placed over the operative eye. A variety of lid specula have been devised for penetrating keratoplasty; a common goal in all of these devices is maximal exposure with minimal pressure on the globe. A locking lid speculum, such as a Maumenee-Park speculum (Fig. 3), with adjustable fissure width and blade angulations, is suitable for PK because it minimizes globe pressure. Positioning the head and neck so as to make the plane of the visual axis perpendicular to the floor and to the operating microscope is helpful in establishing a symmetric trephination. Also, tilting the head slightly away from the operative eye aids exposure.
|
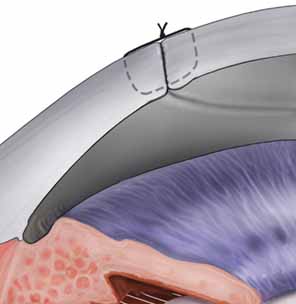
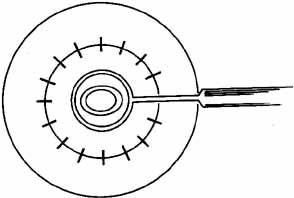
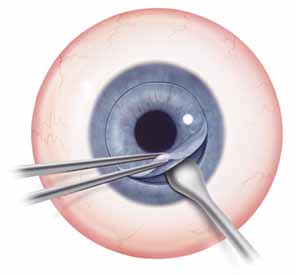
In preparing the eye for trephination, the use of a scleral fixation ring, such as a Flieringa's ring, sized to a diameter slightly larger than the corneoscleral limbal junction, can be helpful in maintaining the form of the globe. Although we do not routinely use these rings in adults, we have found scleral fixation rings to be helpful in the pediatric population, when, oftentimes, the globe and sclera are soft and easily distensible. This ring is secured with a series of 7-0 vicryl sutures and is removed at the completion of the procedure. A caliper, such as a Castroviejo caliper, is used to measure the corneal diameter and the diameter of trephination needed to clear the visual axis. Paton considered the ideal graft size to be between 6.5 and 9.0 mm.12 The smaller the trephination, the greater the distance from limbal vascularity and from the drainage structures in the angle. Therefore, the risk of rejection of a smaller graft may be lessened, in theory, given the greater distance from the peripheral limbal vasculature and the resultant immunologic response to the donor tissue. Also, by increasing the distance from the angle structures, the risks of surgeon-induced secondary angle closure, PAS, and iris incarceration are theoretically lessened. However, a smaller diameter graft is more susceptible to decentration and higher amounts of surgically-induced astigmatism. We have found that, for the majority of patients, the visual axis may be cleared with host trephinations of 7.5 to 8.0 mm. There are instances, such as infectious keratitis and peripheral corneal melting, in which an oversized therapeutic graft is indicated. Keratoconus patients with significant peripheral and inferior thinning may also benefit from a larger trephination. We often mark the visual axis with a methylene blue marking pen to help determine centration and trephine placement. Some surgeons prefer to slightly decenter the graft inferonasally so as to approximate the usual functional visual axis. The corneal periphery is marked with an eight-blade radial keratotomy marker, and these marks can be extended manually with a methylene blue marking pen to maintain orientation and identification of the corneoscleral limbal junction. These marks should extend beyond the trephination diameter, so as to help guide the radial placement of the transplant sutures. In maximizing the radial orientation, postoperative wound torque can be minimized, with a possible reduction in postoperative astigmatism. The operative microscope light is then turned off, the light source obturator is engaged, or a corneal light shield is placed on the cornea to minimize potential photic injury to the macula. The trephination of the host cornea is performed only after the preparation of the donor corneal tissue. Therefore, at this point in the procedure, attention is turned to the donor tissue.
The donor corneal tissue is now commonly available in Optisol preservative media. The tissue endothelial cell count, infectious disease status (including human immunodeficiency virus, hepatitis B and C virus, and syphilis), and overall epithelial status are reviewed prior to trephination. This information is typically shared by the supplying eye bank with the transplant surgeon 24 hours prior to the procedure, with acceptance or rejection of the tissue based on the individual patient's needs. A higher endothelial cell count is often desirable in a younger patient, whereas a lower endothelial cell count may be acceptable for a therapeutic tectonic graft (in which the need for repeat transplantation for optical improvement is more likely). Also, the donor tissue is visually inspected by the surgeon at the slit lamp for confirmation of tissue quality prior to the procedure, noting epithelial and endothelial quality and overall graft appearance. The donor tissue identification, patient identification, and operative site are confirmed with the operating room staff, and the donor tissue container is opened. The lid is removed, and the surgeon grasps the edge of the sclera with 0.3 mm Castroviejo forceps. An assistant then pours the Optisol solution into a sterile stainless steel specimen cup. Excess Optisol is removed by touching a methylcellulose sponge perpendicularly to the edge of the scleral rim, being careful to avoid touching the endothelial donor surface. By removing the excess fluid, the potential for graft slippage during trephination is minimized. The donor tissue is then centered on a Teflon trephination block. We use the Weck corneal donor trephine, and at this point, the trephination chamber guide is engaged atop the block. There are several other trephination systems available, including Hannah, Katena, Hessburg-Barron, Iowa PK Press, in addition to free-hand manual trephination, which can also be used to prepare the corneal button. The donor tissue is routinely oversized by 0.25 mm compared to the host trephination for the majority of transplants. Donor tissue is sometimes oversized by 0.50 mm in patients with shallower anterior chambers, anterior chamber intraocular lenses, and aqueous tube shunts. The donor trephine diameter is confirmed and then the donor trephine cylinder is engaged and pressed firmly against the Teflon cutting block. This trephination is done in a careful but brisk fashion to minimize endothelial crush artifact. A distinctive, firm end point is felt (“crunch” sensation), and the trephine is removed while the donor rim is grasped using 0.3 mm Castroviejo forceps. By oversizing the donor tissue by 0.25 to 0.50 mm, tissue apposition is maximized. In patients with keratoconus, in an effort to minimize postoperative myopia, the host and donor trephination may be of equal size, depending on the trephination system. The surgeon then grasps both the donor trephine and the trephination chamber guide. This stabilization minimizes the risk of the trephine dropping a second time and engaging the Teflon block. Often, the donor button remains in place on the Teflon block; however, at times, the button may become lodged in the trephine itself. If this situation is encountered, a medicine dropper filled with Optisol can be used to gently remove the button by applying soft suction to the epithelial surface. The graft is then gently placed onto the Teflon block while slowly releasing the suction created by the stopper. A drop of filtered Optisol solution is placed on the endothelial surface, the Teflon block (with donor tissue in place) is covered with a protective lid, and the donor rim and Optisol solution are cultured for bacteria and fungi. The donor tissue is then set aside on a table in a safe location. Care is taken to ensure that the sterile drapes of the table do not reach the level of the floor. If this should happen, the drapes may become caught on the wheels of the table and potentially dislodge and contaminate the prepared donor corneal tissue.
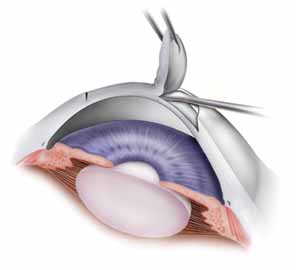
After the donor corneal tissue is safely secured, attention is then returned to the host cornea. Multiple host tissue trephines are available, and we have found success using the Hessburg-Barron vacuum trephine system. This trephine includes a central crosshair to help align trephination. The position of the trephine is stabilized using a manual vacuum system, which is activated by a handheld syringe. The diameter is confirmed by reading the printed mark on the top edge of the trephine. The blade is advanced completely by turning the trephine in a clockwise direction. A moistened fluorescein strip is then gently touched to the bottom of the trephine. The trephine is then centered on the host tissue by aligning the central crosshair with the previously placed central mark on the host cornea. The trephine is then gently touched to the cornea in a perpendicular fashion so as to leave an impression of fluorescein. The trephine is removed, and the centration of this marking is evaluated. Adjustments in the orientation of the trephine can then be made. The blade is then retreated in the trephine housing until it becomes flush. This step is done under direct visualization. Three turns of one-quarter rotation are then performed to draw the blade up into the housing. The vacuum syringe plunger is depressed, and the trephine placed onto the host cornea, making any adjustment of centration as is warranted. The plunger is then released rapidly, and the vacuum level is assessed. A satisfactory vacuum level can be noted by spontaneous trephine adherence to the cornea and host globe movement with gentle lateral pressure on the trephine. The centration is examined by viewing the crosshair and by estimating the distance from the corneoscleral limbal junction to the trephine. This estimate is done by viewing the trephine from the sides and without aid from the operating microscope. Upon confirmation of centration, the edge of the trephine is grasped and a total of 10 turns of one-quarter rotation are performed. The trephine chamber is closely monitored, and, if any aqueous egress is noted (signifying full-thickness trephination and entrance into the anterior chamber), the trephination is halted, vacuum is released, and the trephine is removed. Early entrance into the anterior chamber may be more common in patients with ectatic disorders such as keratoconus, or in patients with prior inflammation and corneal thinning.
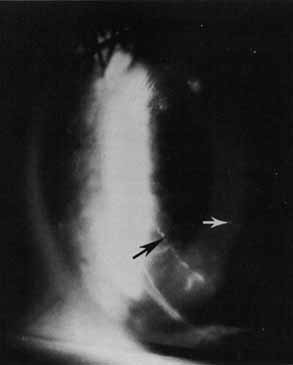
Once the trephine is removed, it is helpful to verify the depth of trephination by tracing the edge of the incision with smooth forceps. If any corneal vascularity is present, phenylephrine 2.5% can be touched to the areas affected, using a moistened methylcellulose sponge. Limbal cautery should be used sparingly as thermal injury may distort the corneal tissue. The trephined host tissue is grasped by 0.12 mm Maumenee-Park forceps (Fig. 4), and tension is gently placed toward the center of the cornea to expose the deepest extent of the trephination. A sharp 15-degree straight blade is used to enter perpendicularly into the anterior chamber, making the motion of the incision away from the surgeon. Care is also taken to not enter too deeply to avoid touching the iris or lens. The incision is extended 1 to 2 clock hours. Viscoelastic material is placed into the eye to deepen the chamber to provide space into which the blades of corneal scissors can be safely placed. Corneal scissors curved to the right and corneal scissors curved to the left are used to complete the excision of the host tissue. The blades are held parallel with the trephination to minimize asymmetric tissue overlap. The scissors are gently elevated as the blades are engaged, and the iris structure is monitored to avoid iris incarceration and trauma. Also, to help maintain tension in the area cut by the scissors, the host button is grasped 180 degrees from the site of the cut. This tension is especially helpful at the end of the tissue removal, when only 1 to 2 clock hours remain. Peripheral anterior synechiae can be lysed with a cyclodialysis spatula after the removal of host tissue.
|
The host tissue is set aside and, upon completion of the procedure, will be sent to pathology for histologic examination and special staining as warranted by the indication for corneal grafting. Until the donor tissue is securely sutured into place, however, the original host tissue should be available, in case it is needed to close the eye. Also, a temporary keratoprosthesis, such as the Cobo keratoprosthesis, should be available to help maintain the integrity of the globe. At this point of the procedure (i.e., when the host cornea is being removed and the globe pressure is equal to atmospheric pressure), the risk of suprachoroidal hemorrhage is greatest. This potentially devastating complication is caused by the rupture of a choroidal vessel, which results in rapid and uncontrolled bleeding in the suprachoroidal space. The hemorrhage may force the iris, lens, vitreous, retina, and other intraocular contents to prolapse out through the corneal trephination wound. Risk factors for surprachoroidal hemorrhage include previous intraocular surgery, elevated IOP, myopia, advanced age, and arteriosclerotic disease. Patients with large body habitus may be prone to increased intrathoracic pressure and may generate additional posterior pressure. These patients may be best approached with general anesthesia. Intraoperative risk factors include rapid decompression of IOP upon entry into the anterior chamber, Valsalva maneuver performed by the patient at the time of trephination, or compression and occlusion of the endotracheal tube resulting in a mechanical Valsalva maneuver. Management of a suprachoroidal hemorrhage is based on when it occurs during the procedure.13 Rapid recognition and response afford the best chance of salvaging the eye. If the hemorrhage occurs upon initial entry into the anterior chamber, the host cornea is sutured back into place, using 8-0 silk or 9-0 nylon sutures, which not only are easier to visualize and tie in rapid sequence, but also have greater tensile strength than 10-0 nylon sutures. Drainage of the hemorrhage can be accomplished by entering into the choroidal space by making a sclerotomy. If the hemorrhage occurs after the host cornea has been removed, the temporary keratoprosthesis is quickly placed into the wound to compress the hemorrhage and to keep intraocular contents in their correct anatomic positions. Our routine during host tissue removal is to have the assistant hold the temporary keratoprosthesis for rapid placement, and the assistant only returns it to the instrument table when all four cardinal sutures have been placed and tied. In the absence of a temporary keratoprosthesis, a surgeon may use his or her finger to tamponade the hemorrhage. Pressure is maintained until the hemorrhage stabilizes. Sclerotomy can be performed to drain the surprachoroidal hemorrhage, and then the donor or host tissue is sutured into place. Alternatively, sclerotomy and drainage of the suprachoroidal hemorrhage can be done at a later date if the intraocular contents can be successfully reposited at the the time of surgery. If possible, irrigating fluid can be placed into the anterior chamber via keratoprosthesis attachment or anterior chamber maintenance port. This fluid can help deepen the anterior chamber, resulting in more anatomic orientation of intraocular contents and may also facilitate evacuation of the hemorrhage.
Upon successful entry into the anterior chamber and removal of the host tissue, the donor tissue can then be secured to the patient. We will describe standard phakic keratoplasty and will then discuss specific situations, such as combined keratoplasty and cataract removal and also keratoplasty in the pseudophakic patient. Up to this point in the description of the procedure, the steps taken for combined procedures are similar to those in standard phakic keratoplasty.
Simple Penetrating Keratoplasty
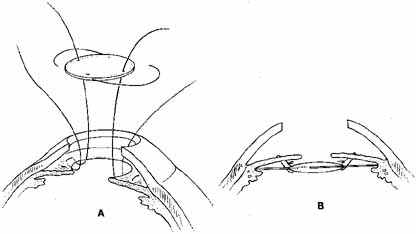
In a simple PK, such as in patients with keratoconus, the donor cornea is transferred from the Teflon block to the surface of the eye using a Paton spatula. This transfer is done immediately adjacent to the patient's eye in the operating field to minimize the likelihood of inadvertent tissue loss onto a nonsterile field. The surgeon's dominant hand is used to lift the donor graft. Viscoelastic substance is placed on both the endothelial side of the donor button and on the host corneal tissue rim. The donor tissue is brought to the host rim, and it is tipped slightly by touching the cut edge of the graft to the 6 o'clock position on the viscoelastic-coated host rim.
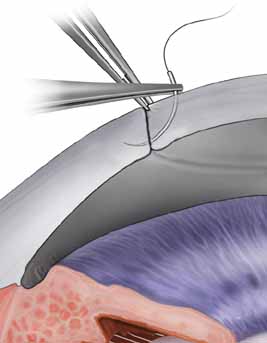
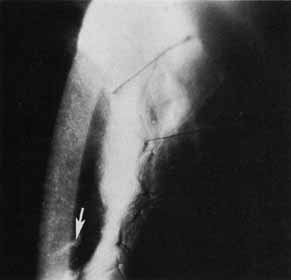
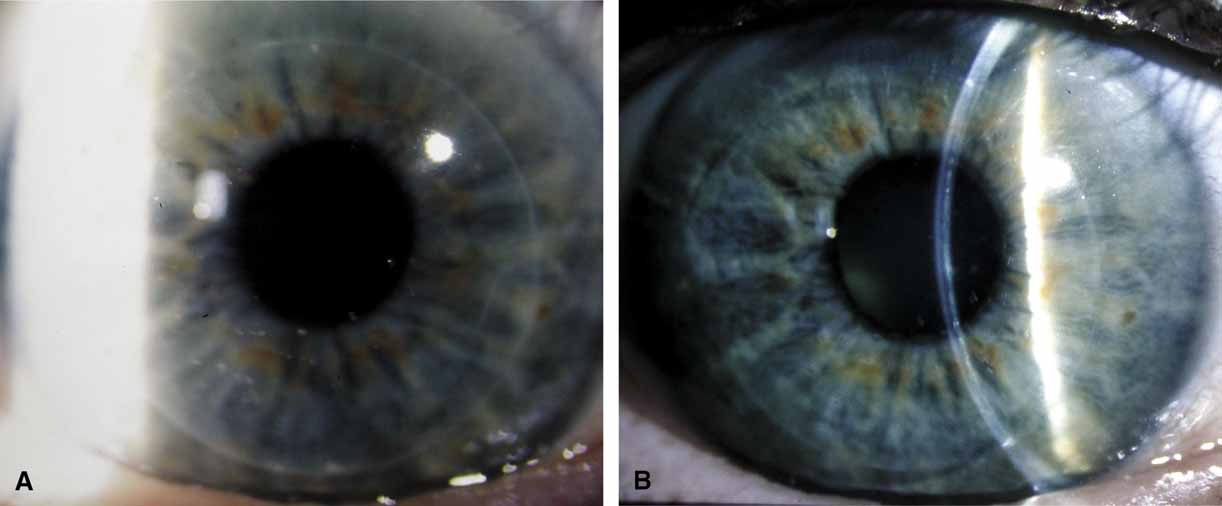
The tissue is tipped by rotating the wrist and the Paton spatula so that the graft is positioned almost perpendicularly to the host cornea. This position facilitates a precise grasping of the top edge of the donor tissue by split-tined Hofmann-Polack forceps (Fig. 5). These speciallydesigned forceps allow for the uninterrupted passage of a needle between the two toothed tines. After the elevated edge of the graft is grasped by the Hofmann-Polack forceps, the surgeon quickly exchanges the Paton spatula for a preloaded needle driver containing a 10-0 nylon suture. The suture is first placed onto the donor cornea at the 12 o'clock position. The action of suture placement involves not only the passage of the needle onto the graft tissue, but also the placement of the graft tissue onto the needle. In this sense, one can think of “placing the graft onto the needle” in addition to passing the needle through the graft. The optimal exit depth of the suture is approximately 90% thickness, with the exit path extending radially through the stromal side cut of the donor graft tissue (Fig. 6). The donor tissue is released by the Hofmann-Polack forceps, and then the host rim is grasped.
|
|
The previously placed methylene blue radial keratotomy marks are used to guide the positioning of the 12 o'clock suture pass. The suture is passed though the two tines of the forceps, making sure that the pass is completed in as radial an orientation as possible. The ratio of the distance from the entry of the needle on the donor to the exit on the host cornea is ideally one-third donor to two-thirds host. The Hofmann-Polack forceps are then exchanged for 0.12 mm Maumenee forceps. The suture is tied in place using a slipknot (two-one throw) and then cut using Vannas corneal scissors (Fig. 7). Both tags can be left long so as to be able to adjust suture tension based on intraoperative keratoscopy after the placement of the first eight sutures. The cut sutures are gently draped away from the visual axis, and the donor tissue is grasped at the 6 o'clock position, 180 degrees away from the first suture. A similar technique is used in passing the suture at this position. Symmetry of the pass can be estimated by noting the tissue alignment as well as the symmetry at 3 o'clock and 9 o'clock. Also, as gentle tension is applied in tying the suture, a straight tension line will be noted in the graft tissue. This line should be oriented from 6 o'clock to 12 o'clock. Sutures are then passed at the 3 o'clock and 9 o'clock meridians, while taking into account the amount of tissue on either side of the pass. Upon completion of the four cardinal sutures, a symmetric diamond shape should be noted on the donor tissue. A total of eight interrupted sutures are placed, the ends of which are temporarily left long. The graft is then wetted with balanced salt solution, and a manual keratoscope, such as a circular metal loop or a von Luhnen keratoscope, is used to estimate astigmatism based on the compression or expansion of the reflected rings or ovoid reflex from the corneal surface. If suture adjustment is needed, the suture is grasped proximal to the knot. The suture is cut and rotated by pulling the grasped suture. The tension of the suture can then be adjusted by retying the knot based on keratoscopy. For the slipknots not needing adjustment, a third locking throw is placed. The details of suture adjustment to minimize postoperative astigmatism are discussed more thoroughly in the suturing techniques section.
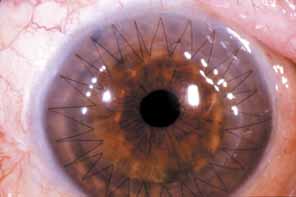
In patients undergoing simple keratoplasty, a running suture can be used (Fig. 8). A half section of 10-0 nylon suture is used, placing two suture bites between each pair of previously placed interrupted sutures. The ratio of the radial orientation is one-fourth the distance to either interrupted suture. On the final pass of the suture, the needle is left in place and the edge of the needle track is slightly expanded, using a 15-degree steel blade. This slight expansion facilitates the passage of a tied knot through corneal tissue. The suture tension is adjusted using two smooth tying forceps, grasping the suture in an “end over end” fashion to maintain tension throughout the adjustment. The suture tightening begins 180 degrees from where the knot will be placed, and two passes in both directions are made. The cornea is again wetted, and intraoperative keratoscopy is performed with appropriate suture adjustment. A knot is then tied and the suture is gently rotated to bury the knot. In patients with peripheral corneal neovascularization, multiple interrupted sutures can be placed, thereby facilitating suture removal based on local suture vascularity. A total of 16 interrupted sutures are adequate to approximate the graft to the host tissue in the majority of patients.
|
Combined Penetrating Keratoplastyand Cataract Extraction
When a cataract extraction is to be performed along with the PK, the patient's pupil is dilated preoperatively with cyclopentolate hydrochloride 1%, phenylephrine 5%, and tropicamide 0.5%, one drop of each every 5 minutes for three total doses. We favor an extracapsular technique in removing the lens nucleus. There are instances, such as in Fuchs' endothelial dystrophy, where corneal clarity is sufficient to facilitate the performance of a continuous curvilinear capsulorrhexis through a paracentesis incision prior to removal of the host corneal tissue. The anterior chamber is filled with viscoelastic substance during this procedure, which allows for additional control of the capsulorrhexis by flattening the anterior curvature of the crystalline lens. By stabilizing the anterior chamber with viscoelastic material, a capsulorrhexis is less likely to proceed radially toward the zonules, causing radial tears and potentially destabilizing the capsular support necessary for adequate intraocular lens positioning. We also favor this approach in the appropriate patient because it minimizes the duration of time spent between removal of the host cornea and placement of the donor tissue.
If, however, the cornea is too opaque to confidently visualize the anterior capsular surface, we perform a capsulorrhexis with an open anterior chamber and use an extracapsular technique for removal of the cataract unless obvious lens dislocation or significant traumatic zonular rupture necessitates an intracapsular cataract extraction. A small stab incision is made in the central capsule with a bent needle cystotome, and the edge is grasped with fine Utrata capsulorrhexis forceps. The capsule is gently torn circumferentially to create a smooth-edged, round opening. To facilitate removal of the entire lens nucleus, the opening should be 5 to 6 mm in diameter. If the tear extends toward the anterior zonules it will begin to turn outwardly and may result in a tear extending to the equator of the lens. If this occurs, the capsulotomy should be restarted in the opposite direction with fine scissors and completed using the Utrata forceps. The anterior capsule is then removed with forceps. The lens is loosened from the capsular bag by introducing a cannula just under the capsule and irrigating fluid around the periphery of the lens cortex (i.e., hydrodissection). Visualizing a wave of fluid traversing the red reflex can be helpful in confirming adequate hydrodissection. The lens is left in place until the cornea is trephined. The chamber is reformed and the paracentesis wound is secured by placing a 10-0 nylon suture. After the successful trephination of host tissue, attention can then be turned back to lens removal. The lens can be gently hydrodissected once again and rotated out of the capsular bag using a 25-gauge hydrodissection cannula. If adequate hydrodissection has been performed earlier, the lens is usually easily removed. If required, slow, steady, and gentle pressure at the 6 o'clock and 12 o'clock positions just posterior to the limbus with a muscle hook or lens loop and forceps can help express the lens nucleus.
Residual cortex is removed with either a manual irrigation-aspiration cannula, such as the Simcoe cannula, or with an automated irrigation-aspiration setup. The former allows for a central aspirating port and a peripheral irrigation port to minimize the amount of tension placed on the capsular bag. Cortical removal is more tedious and time-consuming in an open-sky technique than in a closed system because no fluid pressure can be built up and air frequently enters the aspiration port. Caution must be exercised to not tear the posterior capsule or rupture the zonules by rushing. Capsular stability provided by a successful capsulorrhexis makes cortical cleanup much easier.
The most commonly accepted method for visual rehabilitation after cataract extraction is implantation of an intraocular lens. This is also true after corneal transplantation; the combination of penetrating keratoplasty, cataract extraction, and intraocular lens implantation has become known as the triple procedure. We prefer posterior placement of the intraocular lens within the capsular bag. Placement within the capsular bag reduces contact of the optic and haptics to the pigmented, vascularized uveal tissue, reducing the risks of haptic erosion into vascular structures and the constant chafing on the uvea that may lead to chronic, low-grade uveitis, pigment dispersion, and glaucoma.
In the absence of adequate capsular support, the surgeon can place an intraocular lens in the anterior chamber. Alternatively, one can place a fixated posterior chamber intraocular lens with either iris or scleral fixation. There are anterior chamber intraocular lenses currently available that are relatively well tolerated by the corneal endothelium. These lenses are relatively easy to place and, when accompanied by a surgical peripheral iridectomy, can be a viable option in older transplant patients. However, because an anterior chamber lens is positioned anterior to the pupil, the iris cannot protect the graft endothelium from the optic. Even with the use of viscoelastic substances, significant endothelial cell loss and possible graft decompensation may result from intraocular lens touch. In younger patients (under 50) in whom longer term graft viability and endothelial function needed, implantation of a lens anterior to the capsule (in the ciliary sulcus) or a suture fixated lens may be possible and preferable to an anterior chamber lens. Intraocular lens suturing techniques are discussed in the section on aphakic and pseudophakic keratoplasty.
The technique involved in placing the posterior chamber intraocular lens into the capsular bag is relatively simple and is aided by having an 8-mm opening in the cornea through which to work. A small amount of viscoelastic is injected through a 30-gauge blunt-tipped cannula between the anterior and posterior capsule at the 12 o'clock to 6 o'clock position. The inferior haptic is then slipped into this space. The superior haptic is grasped with angled tying forceps about 1 mm from the end and swung centrally over the optic while gently depressing the intraocular lens to bring the haptic below the level of the anterior capsule. The haptic is then slowly allowed to recoil while observing it slip into position within the capsular bag. Usually, centration is excellent and rotation of the intraocular lens is unnecessary. A topical miotic agent is injected onto the surface of the iris to constrict the pupil while gently depressing the optic to minimize lens capture by the pupillary edge. We do not routinely perform an iridectomy in an otherwise quiet eye undergoing a triple procedure with a posterior chamber lens. In patients with anterior chamber intraocular lenses, a peripheral iridectomy is performed. Once the lens is adequately positioned, the donor tissue is transferred and sutured in place.
Aphakic and Pseudophakic Keratoplasty
Aphakic corneal edema and pseudophakic corneal edema are common indications for PK. Because of this, any surgeon who wishes to perform corneal transplants should be completely familiar and comfortable with the technique and instrumentation required for anterior vitrectomy, iris surgery, and intraocular lens manipulations.
As mentioned earlier, a thorough preoperative evaluation must be completed before the patient enters the surgical suite. Particular attention should be paid to the status of the opposite eye. Is it phakic, aphakic, or pseudophakic? This will often dictate which surgical procedure would be most appropriate for the second eye with regard to intraocular lens placement, removal, or exchange. The vitreous should be evaluated carefully. An intact hyaloid face that is well behind the pupil may allow the surgeon to avoid the necessity of proceeding with an anterior vitrectomy and thus to avoid the increased risk of retinal detachment or cystoid macular edema that may accompany vitreous manipulation. A broken hyaloid face or loose vitreous in the anterior chamber will require an anterior vitrectomy, as discussed below, to complete the transplant safely and reduce the potential postoperative complications associated with vitreous incarceration. Iris and pupil abnormalities, such as synechiae, corectopia, iridodialysis, or pupillary membranes, may require anterior segment reconstruction to create a more favorable anatomic or physiologic ocular status. Poorly controlled glaucoma is generally an indication for removal of an anterior chamber lens and argues against primary placement or replacement of this type of lens.
The host cornea is removed as described earlier. If the eye is to remain aphakic and the vitreous face is intact, a topical miotic agent is injected to constrict the pupil, and viscoelastic is placed onto the iris and hyaloid face before transferring the donor cornea. Based on the surgeon's preference, a peripheral iridectomy can be performed as prophylaxis against postoperative pupillary block glaucoma. When loose vitreous is present in the anterior chamber, an anterior vitrectomy must be performed. Mechanical suction-cutting units are superior to the simple cellulose sponge vitrectomy technique. An Ocutome mechanical vitrectomy unit that has a guillotine cutting action works well for our patients. The cutting rate should be set at 300 to 400 cuts per minute, and suction should be low to moderate. Care should be taken to avoid traction on the vitreous so as not to create a retinal tear. The vitrectomy is adequate when all vitreous has been removed from the anterior chamber and the iris bows backward into the vitreous cavity. In cases of preexisting cystoid macular edema or when a suture-fixated posterior chamber intraocular lens is to be placed, a deeper and more complete vitrectomy is possible and may be advantageous. Once the mechanical vitrectomy is completed, a dry cellulose sponge is touched to the anterior surface of the iris and pupillary margin for 360 degrees to ensure that no vitreous strands remain. Scissors are used to cut residual vitreous that adheres to the sponge. Balanced salt solution is gently irrigated over the iris into the posterior chamber to refill the globe.
There are a great variety of ways in which a pseudophakic eye may present. The intraocular lens may be in the anterior or posterior chamber or may be supported by the iris. The lens may be in a good, stable position; or it may be loose and dislocated; or it may be entrapped in PAS, a pupillary membrane, or even through the cataract wound. Often, a well-positioned, stable posterior chamber lens can be left in place during penetrating keratoplasty. It is unlikely that this lens will compromise graft integrity or function. However, one report indicates a slightly decreased graft survival rate in eyes requiring a corneal transplant in which a posterior chamber lens was left in place rather than exchanged at the time of the keratoplasty.14 Decreased graft survival rate has not been reported by other authors, though. Miotic agents and viscoelastic substance are injected onto the surface of the iris and lens before transferring the donor button.
We remove iris-supported lenses and closed-loop haptic anterior chamber intraocular lenses at the time of corneal transplantation. Fortunately, the current anterior chamber lens designs are often better tolerated than older anterior chamber lens designs, and fewer older lenses are encountered today. Many of the older designs of anterior chamber lenses associated with pseudophakic corneal edema can be considered a significant contributing factor in corneal endothelial decompensation. Although it is well noted that other factors, such as initial cataract surgical technique and complications, glaucoma, uveitis, and corneal dystrophy, may all influence corneal decompensation, a significant variable that may now be modified is the removal of an ill-positioned or otherwise inadequate intraocular lens. General principles will be outlined and can be applied and modified to address a given anterior chamber lens type. The reader is referred to the literature for a more detailed discussion of removal of intraocular lenses at the time of PK.15,16
Iris-supported lenses are relatively easy to remove with gentle manipulations of the pupil and lens. Often a suture is present securing the superior haptic to the iris. This should be identified and removed. The intraocular lens should be manipulated cautiously because synechiae or vitreous adhesions to the lens may be present. Blunt or sharp dissection is used to free the intraocular lens, which is then removed from the eye.
Anterior chamber intraocular lenses may be flexible, rigid with solid footplates, or have open- or closed-loop haptics. Initially, the intraocular lens may be grasped with angled tying forceps testing whether or not it may be gently removed from the eye. Some solid lenses may be removed this way, but it may sometimes be necessary to cut this type of intraocular lens in two pieces for removal. Closed-loop haptics present perhaps the most difficult challenge for a safe removal, and the reader is referred to the literature for a discussion regarding the removal of this particular type of anterior chamber lens.16 PAS are lysed with a cyclodialysis spatula by gently sweeping the angle. Large iris defects, such as an old sector iridectomy, may be repaired to bring the iris and pupil into a more anatomically correct configuration and to prevent the floppy edges of the iris from becoming incarcerated into the corneal wound. One10-0 Prolene suture on a blunt-tapered cardiovascular needle passed at the sphincter muscle is used to just approximate the tissue edges. An additional suture in the mid-portion of the iris may be required to close large defects. Care should be taken to not tie the suture too tightly because it may lead to localized iris necrosis.
Replacement of an intraocular lens is considered, if the opposite eye is phakic or pseudophakic, or if the patient has demonstrated intolerance to contact lenses or aphakic spectacle correction. In the past, the only option for intraocular lens fixation in an aphakic eye or after removal of another intraocular lens during penetrating keratoplasty was to implant an anterior chamber lens. The Multiflex-style anterior chamber intraocular lens has four-point fixation with minimal angle touch and good compression characteristics to lessen the chance of angle erosion or chronic pain but may tend to vault forward toward the corneal endothelium. The lens is relatively easy to implant through a keratoplasty opening, and the overall surgical results have been good. Hassan17 showed that 63% of patients in which this type of intraocular lens was implanted at the time of keratoplasty had a visual acuity of 20/40 or better at 2 and 3 years. Acetylcholine is used to create miosis, and viscoelastic may be used to open the angle, after which the lens should be positioned with the footplates away from preexisting iridectomies or other areas of iris defects.
The development of alternative intraocular lens fixation techniques has increased the surgeon's options regarding style and position of the pseudophakic lens after keratoplasty. Because not all eyes are good candidates for an anterior chamber intraocular lens due to PAS, iris defects, or glaucoma, suture fixation of posterior chamber intraocular lenses has become an increasingly popular technique in our surgical armamentarium.
The posterior chamber intraocular lens may be suture-fixated to the posterior iris or to the sclera18; both techniques have potential advantages and disadvantages. The surgeon who chooses to suture-fixate a posterior chamber intraocular lens should select one of the techniques and concentrate on becoming adept primarily in that technique. Contrary to what some of the literature would indicate, these are not necessarily simple procedures; they add time and complexity to the operation and require skill and forethought to successfully complete.
One of the techniques used for posterior chamber intraocular lens implantation during penetrating keratoplasty is iris fixation.19 A 6-mm, three-piece modified J-loop, 0-degree or 10-degree angulated Prolene haptic, four-hole intraocular lens can be used. It is suture-fixated to the posterior aspect of the iris with 9-0 Prolene on a blunt-tapered needle (Fig. 9). The lens is held securely in place with the haptics positioned within or posterior to the ciliary sulcus. As opposed to the old style of iris-fixated intraocular lenses, pseudophacodonesis is nearly nonexistent, and torsion of the intraocular lens along the fixational point axis does not seem to occur, probably because of the stability provided by the haptics posterior to the iris.
Suture fixation of a posterior chamber intraocular lens to the sclera requires passage of two Prolene sutures 180 degrees apart posterior to the iris, theoretically through the ciliary sulcus and out of the sclera. Either single-armed suture tied under preplaced partial-thickness scleral flaps or double-armed suture tied and rotated into the sclera can be used to avoid erosion of the Prolene knots through the conjunctiva. The sutures are tied to the tangential point of each haptic, and the intraocular lens is then implanted through the pupil. Again, there is no pseudophacodonesis, and potential lens tilt is minimized by haptic/optic approximation to the posterior iris. Both posterior lens implants with preplaced eyelets integrated to the haptics and one-piece acrylic lenses can be adequately fixated to the sclera. The interested reader is referred to the literature for more detailed descriptions of the various intraocular lens suture-fixation techniques.19–26
SUTURING TECHNIQUES
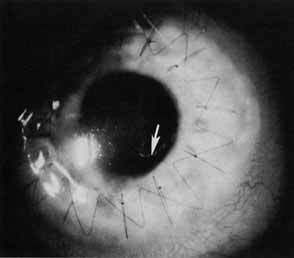
If the host cornea is not vascularized, a running suture technique can be used (Fig. 8). We often utilize an 8-bite interrupted and 12-bite running combination, but a variety of techniques can be used effectively. One alternative is a 12-bite interrupted and 12-bite running combination, and another is a 20- to 24-bite single running suture. Each provides the opportunity for modification of astigmatism in the postoperative period; the 12 and 12 combination provides selective interrupted suture removal while the running suture allows adjustment of local suture tension. We will first describe the 12 and 12 technique in which we use an Alcon CU-5 4-mil needle with 10-0 nylon for the interrupted sutures. In this technique, a 12-blade radial keratotomy marker is used to mark the peripheral cornea prior to trephination. The first suture is placed at the 12 o'clock position. The sutures are placed using the method described in the section on simple penetrating keratoplasty, proceeding to the 6 o'clock, 9 o'clock, and 3 o'clock positions, and then to all 12 clock-hour positions, alternating suture placement at 180 degrees to the previously placed suture.
It is at this point that we take what is the last of a series of intraoperative steps designed to minimize astigmatism. Before placing the running suture, we perform qualitative keratoscopy using a von Luhnen keratoscope. Other methods may be employed, such as the use of circular metal loop on a handle (Fig. 10), or an operating microscope-mounted qualitative keratometer. The Flieringa's ring, if present, is removed. Intraocular pressure is adjusted by filling the anterior chamber. The keratoscope or wire loop is held about 2 cm directly above and parallel to the plane of the cornea. Irrigation of the graft will smooth the surface enough to see a reflection of the loop on the cornea. Most commonly, the reflection is ovoid, which indicates astigmatism of the graft; the short axis represents the steepest curvature of the cornea. Suture tension is adjusted by loosening or tightening the slip knots. Keratoscopy is repeated with additional suture adjustment as needed to minimize the corneal astigmatism. When the adjustments are completed, a final throw is placed on each suture, and the knots cut short with Vannas scissors. Burying the knots in any type of corneal surgery is essential. The knots are buried into the donor stroma in an attempt to keep them as far away from any vascularization as possible. Anteroposterior graft–host disparity in alignment can also induce astigmatism and should be identified. Sometimes a meridian is flat because the donor is anteriorly displaced or steep secondary to posterior displacement. Resuturing is then necessary to correct the problem. It is not uncommon to inadvertently take superficial bites on the recipient side when suturing away from oneself.
|
The running suture is then placed, starting on the graft side at the 11:30 position. We use 10-0 nylon on an Ethicon Ultima CS 160 6-mil needle. The needle is passed radially through the graft and host, and about 10 cm of length is pulled up to allow for completion of the running suture. Each successive pass is taken halfway between the interrupted sutures, moving in a clockwise direction, taking care to create radial, deep, and equal-length bites. After the 10:30 pass, the needle is left in place and the exit track is expanded using a 15-degree steel blade as noted in the simple PK section. The two ends are cut about 3 cm long and a single two-loop throw is placed. Slack is then taken up from the running suture with microtying forceps by starting at one side of the knot and working back around the graft. Excessive tightening should be avoided. The suture is then tied with two square knots, and the ends are cut short. The knot is rotated to lie in the graft stroma, trying not to incarcerate it within the graft–host interface. While the anterior chamber is slowly refilled with balanced salt solution, the iris is observed to drop back away from the cornea. Any areas of iris incarceration or synechiae are released by introducing a cyclodialysis spatula through the wound 2 clock hours away from the area of concern. It is then passed over the iris peripheral to the incarceration and gently swept centrally to pull the iris from the graft–host interface. The chamber is again re-formed and the eye is dried with cellulose sponges. High magnification is used to look for the presence of any wound leaks, which, if present, are to be closed with single superficial 10-0 nylon sutures.
Another suture technique that allows earlier and possibly more significant adjustment of postkeratoplasty astigmatism is the use of a single running 10-0 nylon suture, as described by McNeill and Wessels27 and by Van Meter.28 Other surgeons have had considerable success with this technique and use it to reduce and initially stabilize astigmatism within 4 to 10 weeks after keratoplasty in the majority of patients. Spectacle correction can be prescribed much earlier than with other techniques, thus providing visual rehabilitation to patients, many of whom are elderly. The advantanges of astigmatism adjustment are balanced by the risk of reliance on a single suture for graft apposition and the flexibility of suture removal in interrupted techniques in the setting of peripheral neovascularization.
The suturing technique begins similarly to that just described with the placement of four interrupted cardinal sutures at the 3 o'clock, 6 o'clock, 9 o'clock, and 12 o'clock positions. A 10-0 nylon is then passed in a running fashion beginning at the 11:30 position and proceeding in a clockwise direction. Usually, 20 bites are taken at and between each of the methylene blue marks placed by the 10-blade marker. Initial experience with this technique should be gained using a 12-blade marker and 24 suture bites. The cardinal sutures are then cut and removed, and the single running suture is securely tied. Excessive tightening tends to induce a circular row of corneal compression that can resemble a “barrel top.” Sutures that are too tight induce flattening of the graft. This results in an initial hyperopic shift from the intended result followed by a (sometimes significant) myopic shift once the sutures are removed and the graft steepens to its resting curvature. The anterior chamber is refilled with balanced salt solution, and the surface of the graft is moistened. A keratoscopy ring is used to determine graft asphericity, and the running suture is manipulated to adjust the astigmatism that is present. The suture is moved toward the steep meridians by grasping the suture initially at the flat meridian with microtying forceps held parallel to the suture to avoid breaking or cutting the suture on the edge of the forceps and gently, but firmly, sliding each successive suture pass in a direction away from the flat meridian toward the steep meridian. This will redistribute suture tension more evenly, and by a series of successive adjustments and rechecking keratoscopy readings, the graft will be brought into a more spherical shape. This same type of adjustment can be performed on subsequent postoperative visits at the slit lamp to fine-tune the refractive astigmatism.
Cycloplegic agents are not routinely administered. We provide subconjunctival injections of tobramycin, 20 mg; cefazolin, 50 mg; and dexamethasone sodium phosphate, 12 mg. Subconjunctival corticosteroids are not given in patients with herpetic corneal disease. A double eye patch is taped firmly in place, and a metal Fox shield is taped over the double eye patch to protect the eye.