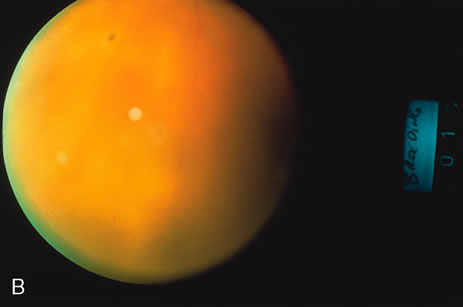
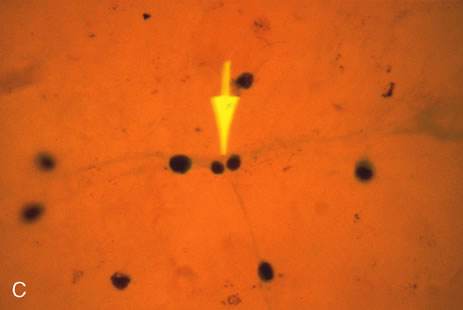
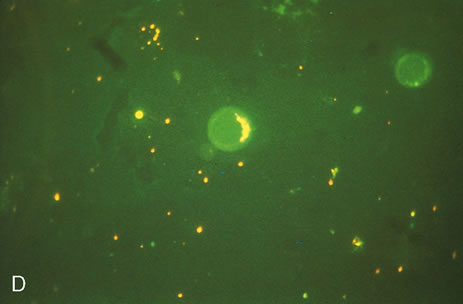
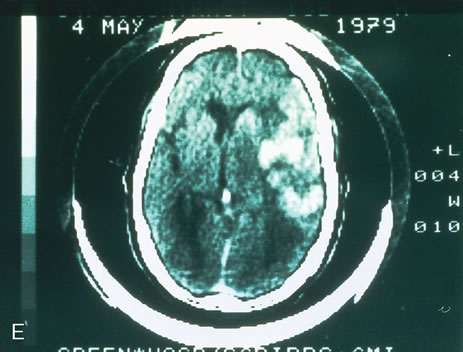
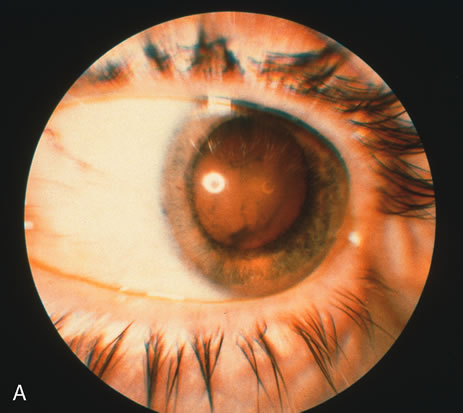
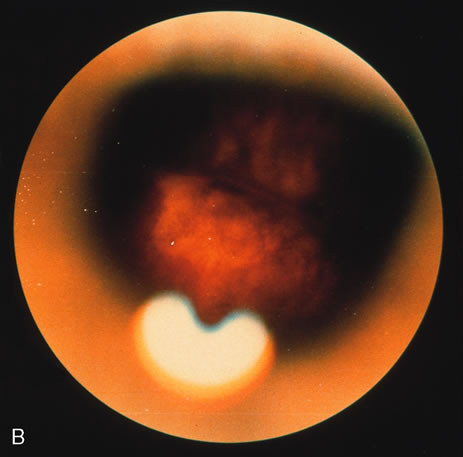
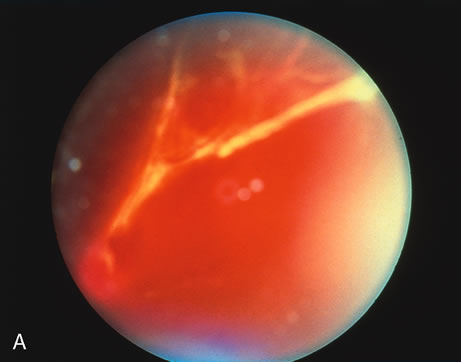
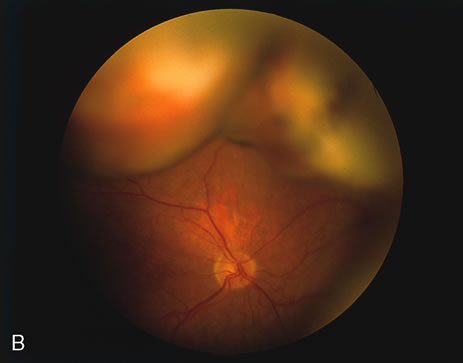
1. O'Connor OR: Calcific band keratopathy. Trans Am Ophthalmol Soc 70:58, 1972 2. Langston R, Pavan-Langston D, Dohlman CH: Penetrating keratoplasty for herpetic keratitis. Trans Am Acad Ophthalmol Otolaryngol 79:577, 1975 3. Cobo LM, Coster DJ, Rice NS, et al: Prognosis and management of corneal transplantation for herpetic keratitis. Arch Ophthalmol 98:1755, 1980 4. Intraocular Inflammation, Uveitis, and Ocular Tumors. Ophthalmology Basic and Clinical Science Course (Section 3), San Francisco, American Academy of Ophthalmology, 1981–1982 5. Smith RE, Nozik RM: Uveitis: A Clinical Approach.Baltimore, Lippincott Williams & Wilkins, 1983 6. De Roetth A Jr: Glaucomatocyclitic crisis. Am J Ophthalmol 69:370, 1970 7. Kass MA, Becker B, Kolker AE: Glaucomatocyclitic crisis and primary open-angle glaucoma. Am J Ophthalmol 75:668, 1973 8. Lieberman MF, Hoskins HD, Hetherington J: Laser trabeculoplasty and the glaucomas. Ophthalmology 90:790, 1983 9. Robin AL, Pollack IP: Argon laser trabeculoplasty in secondary forms of open-angle glaucoma. Arch Ophthalmol 101:382, 1983 10. Singh D, Singh M: Pretrabecular filtration for secondary glaucoma. Trans Ophthalmol Soc UK 98:96, 1978 11. Herschler J, Davis EB: Modified goniotomy for inflammatory glaucoma: Histologic evidence for the
mechanism of pressure reduction. Arch Ophthalmol 98:684, 1980 12. Sugar HS: Limbal trephination: A twenty-year follow-up. Int Ophthalmol Clin 21:29, 1981 13. Molteno ACB: A new implant for glaucoma: Clinical trial. Br J Ophthalmol 53:606, 1969 14. Krupin T, Rodos SM, et al: Valve implants in filtering surgery. Am J Ophthalmol 81:232, 1976 15. Schlaegel TF Jr : Essentials of Uveitis, chaps 4–12. Boston, Little, Brown and Company, 1969 16. Ceballos EM, Beck MA, Lynn MJ: Trabeculectoomy with antiproliferative agents in uveitic glaucoma. J Glaucoma 11:189, 2002 17. Foster , CS, Barrett F: Cataract development and cataract surgery in patients with juvenile rheumatoid
arthritis-associated iridocyclitis. Ophthalmology 100:809, 1993 18. Foster CS, Fong LP, Singh G: Cataract Surgery and Intraocular Lens Implantation in Patients with Uveitis. Ophthalmology 96:281, 1989 19. Foster RE, Lauder CY, Meisler DM, et al: Extracapsular cataract extraction with posterior chamber intraocular lens
implantation in uveitis patients. Ophthalmology 99:1234, 1992 20. Gee SS, Tabbara KF: Extracapsular cataract extraction in Fuchs' heterochromic iridocyclitis. Am J Ophthalmol 108:310, 1989 21. Kaufman AH, Foster CS: Cataract extraction in pars planitis patients. Ophthalmology 100:1210, 1993 22. Seamone CD, Deschenes J, Jacksoon WB: Cataract extraction in uveitis: comparison of aphakia and posterior chamber
lens implantation. Can J Ophthalmol 27:120, 1992 23. Kaplan HJ, Diamond JG, Brown SA: Vitrectomy in experimental uveitis: I. Operative technique in rabbits. Arch Ophthalmol 97:331, 1979 24. Key SN III, Kimura SJ: Iridocyclitis associated with juvenile rheumatoid arthritis. Am J Ophthalmol 80:425, 1975 25. Wolf MD, Lichter PR, Ragsdale CG: Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology 94:1242, 1987 26. Hooper PL, Rao NA, Smith RE: Cataract extraction in uveitis patients. Surv Ophthalmol 35:120, 1990 27. Franceschetti A: Heterochromic cyclitis (Fuchs' syndrome). Am J Ophthalmol 39:50, 1955 28. Jain IS, Gupta A, Gangwar DN, et al: Fuchs' heterochromic cyclitis: Some observations on clinical picture
and on cataract surgery. Ann Ophthalmol 15:640, 1983 29. Kanski JJ, Shan-Shin GA: Systemic uveitis syndromes in childhood: An analysis of 340 cases. Ophthalmology 91:1247, 1984 30. Wong IG: Fuchs' Heterochromic uveitis. eMedicine 2002 topic 432.htm 31. Jones NP: Fuchs' Heterochromic uveitis: An update. Surv Ophthalmol 37:253, 1993 32. Osman AT, Dogru M, Erturk H, et al: Transclerally fixated Intraoccular lenses in children. Ophthal Surg Lasers 33:394, 2002 33. Hazari A, Sangwan VS: Cataract surgery in uveitis. Indian J Ophthalmol 50:103, 2002 34. Abela-Formanek C, Amon M, Schauerberger J, et al: Uveal and capsular biocompatibility of two foldable acrylic intraocular
lenses in patients with uveitis or pseudo-exfoliation syndrome. J Cataract Refract Surg 28:1160, 2002 35. Abela-Formanek C, Amon M, Schauerberger J, et al: Inflalmmation after implantation of hydrophilic acrylic, hydrophobic acrylic
or silicone intraocular lenses in eyes with cataract and uveitis. J Cataract Refract Surg 28:1153, 2002 36. Abela-Formanek C, Amon M, Schauerberger J, et al: Results of hydrophilic acrylic, hydrophobic acrylic or silicone intraocular
lenses in uveitis eyes with cataract. J Cataract Refract Surg 28:1141, 2002 37. Kim CY, Kang SJ, Lee SJ, et al: Opacification of a hydrophilic acrylic intraocular lens with exacerbation
of Behcets' uveitis. J Cataract Refract Surg 28:1276, 2002 38. Kadayifcilar S, Gedik S, Elden B, et al: Cataract surgery in patients with Behcets' disease. J Cataract Refract Surg 28:316, 2002 39. Suresh PS, Jones NP: Phacoemulsification with intraocular lenses in patients with uveitis. Eye 15(Pt 5):621, 2001 40. Probst, LE, Holland EJ: Intraocular lens implantation in patients with juvenile rheumatoid arthritis. Am J Ophthalmol 122:225, 1996 41. Holland, GN Intraocular lens implantation in patients with Juvenile Rheumatoid Arthritis
associated uveitis: and unresolved management issue. Am. J. Ophthalmol. 1996 Aug; 122(2) 161–70 42. Gierek-Lipinska A, Mrukwa-Kominek E, Gierek-Ciociura S, Complicated cataract, Part 1; complicated cataract in uveitis patients. Klin Oczna 2001; 103(1)59–63. 43. Ridley H: Cataract surgery in chronic uveitis. Trans Ophthalmol Soc UK 85:519, 1965 44. Ward DM, Hart CT: Complicated cataract extraction in Fuchs' heterochromic uveitis. Br J Ophthalmol 51:530, 1967 45. Norn MS: Cataract extraction in Fuchs' heterochromia. Acta Ophthalmol 46:685, 1968 46. Smith RE, O'Connor GR: Cataract extraction in Fuchs' syndrome. Arch Ophthalmol 91:39, 1974 47. Liesengang TJ: Clinical features and prognosis in Fuchs' uveitis syndrome. Arch Ophthalmol 100:1622, 1982 48. Foster CS: Cataract surgery and intraocular lens implantation in patients with pars
planitis. Proceedings of the Third International Symposium on Uveitis. In Dernouchamps JP, Verougstraete C, Caspers-Velu L, Tassignon MJ (eds): Recent Advances in Uveitis, pp 593–595. Amsterdam, Kugler Publications, 1993 49. Fogla R, Biswas J, Ganesh SK, et al: Evaluation of cataract surgery in intermediate uveitis. Ophthal Surg Lasers 3:191, 1999 50. Smith RE, Godfrey WA, Kimura SJ: Complications of chronic cyclitis. Am J Ophthalmol 82:277, 1976 51. Diamond JG, Kaplan HJ: Lensectomy and vitrectomy for complicated cataract secondary to uveitis. Arch Ophthalmol 96:1798, 1978 52. Diamond JG, Kaplan HJ: Uveitis: Effect of vitrectomy combined with lensectomy. Ophthalmology 86:1320, 1979 53. Dugel PU, Rao NA, Ozler S, et al: Pars plana vitrectomy for intraocular inflammation-related cystoid
macular edema unresponsive to corticosteroids: A preliminary study. Ophthalmology 99:1535, 1992 54. Michelson B, Friedlander MN, Nozik RA: Lens implant surgery in pars planitis. Ophthalmology 97:1023, 1990 55. Mieler WF, Will BR, Lewis H, et al: Vitrectomy in the management of peripheral uveitis. Ophthalmology 95:859, 1988 56. Wirostko E, Spalter HF: Lens-induced uveitis. Arch Ophthalmol 78:1, 1967 57. Riise P: Endophthalmitis phacoanaphylactica. Am J Ophthalmol 60:911, 1965 58. Easom HA, Zimmerman LE: Sympathetic ophthalmia and bilateral phacoanaphylaxis: A clinicopathologic
correlation of the sympathogenic and sympathizing eyes. Arch Ophthalmol 72:9, 1964 59. de Veer JA: Bilateral endophthalmitis phacoanaphylactica. Arch Ophthalmol 49:607, 1953 60. Nobe JR, Kokoris N, Diddie KR, et al: Lensectomy-vitrectomy in chronic uveitis. Retina 3:71, 1983 61. Fitzgerald CR: Pars plana vitrectomy for vitreous opacity secondary to presumed toxoplasmosis. Arch Ophthalmol 98:321, 1980 62. Belmont JB, Michelson JB: Vitrectomy in uveitis associated with ankylosing spondylitis. Am J Ophthalmol 94:300, 1984 63. Michelson JB, Michelson PE, Bordin GM, et al: Ocular reticulum cell sarcoma: Presentation as retinal detachment with
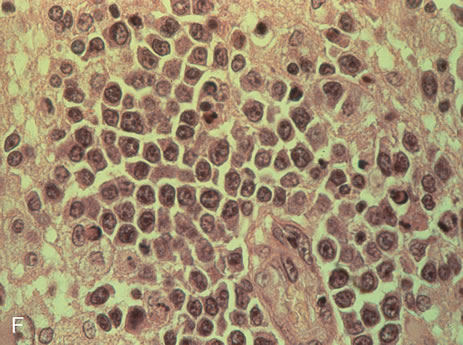
demonstration of monoclonal immunoglobulin light chains on the vitreous
cells. Arch Ophthalmol 99:1409, 1981 64. Char DH: Immunology of Uveitis and Ocular Tumors. New York, Grune & Stratton, 1978 65. Michelson JB: Color Atlas of Uveitis, 2nd ed. St. Louis, Mosby, 1992 66. Engel HM, Green WR, Michels RG, et al: Diagnostic vitrectomy. Retina 1:121, 1981 67. Robertson DM, Wilkinson CP, Murray JL, et al: Metastatic tumor to the retina and vitreous cavity from primary melanoma
of the skin. Ophthalmology 88:1296, 1981 68. Cottingham AJ Jr, Forster RK: Vitrectomy in endophthalmitis: Results of study using vitrectomy, intraocular
antibiotics, or a combination of both. Arch Ophthalmol 94:2078, 1978. 69. Eichenbaum DM, Jaffe NS, Clayman HM, et al: Pars plana vitrectomy as a primary treatment for acute bacterial endophthalmitis. Am J Ophthalmol 86:167, 1978 70. Forster RK: Etiology and diagnosis of bacterial postoperative endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol 85:320, 1978 71. Forster RK, Zachary IG, Cottingham AJ Jr, et al: Further observations on the diagnosis, cause and treatment of endophthalmitis. Am J Ophthalmol 81:52, 1976 72. Michelson JB, Freedman SD, Boyden DG: Aspergillus endophthalmitis in a drug abuser. Ann Ophthalmol 14:1051, 1982 73. Ohno S, Kimura SJ, O'Connor GR, et al: HLA antigens and uveitis. Br J Ophthalmol 61:62, 1977 74. Pettit TH, Olson RJ, Foos RY, et al: Fungal endophthalmitis following intraocular lens implantation: A surgical
epidemic. Arch Ophthalmol 98:1025, 1988 75. Peyman GA: Antibiotic administration in the treatment of bacterial endophthalmitis: II. Intravitreal injections. Surv Ophthalmol 21:332, 1977 76. Belmont JB, Irvine AR, Benson WE, et al: Vitrectomy in ocular toxocariasis. Arch Ophthalmol 100:1912, 1982 77. Halger WS, Pollard ZF, Jarrett WH, et al: Results of surgery for ocular Toxocara canis. Ophthalmology 88:1081, 1981 78. Puig-Llano M, Irvine AR, Stone RD: Pupillary membrane excision and anterior vitrectomy in eyes after uveitis. Am J Ophthalmol 98:533, 1979 79. Michels RG, Ryan SJ: Results and complications of 100 consecutive cases of pars plana vitrectomy. Am J Ophthalmol 80:24, 1975 80. Peyman GA, Huamonte FU, Goldberg MF: Vitrectomy treatment of vitreous opacities. Trans Am Acad Ophthalmol Otolaryngol 81:394, 1976 81. Ballantyne AJ, Michaelson IC: Textbook of the Fundus of the Eye, 2nd ed. Baltimore, Lippincott Williams & Wilkins, 1970 82. Hogan MJ, Zimmerman LE (eds): Ophthalmic Pathology: An Atlas and Textbook, 2nd ed, Philadelphia, WB Saunders, 1962 83. Yanoff M, Fine BS: Ocular Pathology. Hagerstown, MD, Harper & Row, 1975 84. Saari KM, Solja J, Hakli J, et al: Genetic background of acute anterior uveitis. Am J Ophthalmol 91:711, 1981 85. Hultsch E: Vitreous structure and ocular inflammation. In Silverstein AM, Michels RG, Green WR, et al: (eds): Immunology and Immunopathology of the Eye, pp 97–102. New York, Masson USA, 1979 86. Kampik A, Kenyon KR, Michels RG, et al: Epiretinal and vitreous membranes: Comparative study of 56 cases. Arch Ophthalmol 99:1445, 1981 87. Gartner J: Elekonenmikroskopische Untersuchungen uber Glaskoperrindenzellen und Zonulafasern. Z Zellforsch 66:737, 1965 88. Newsome DA, Linsenmayer TF, Trelstad RL: Vitreous body collagen: Evidence for a dual origin from the neural retina
and hyalocytes. J Cell Biol 71:59, 1976 89. Gloor BP: Mitotic activity in the cortical vitreous cells (hyalocytes) after
photocoagulation. Invest Ophthalmol 8:633, 1969 90. O'Connor GR: Basic mechanisms responsible for the initiation and perpetuation of anterior
segment inflammation. Trans Am Acad Ophthalmol Otolaryngol 79:56, 1975 91. Kanski J: Uveitic cataracts in children. Presented at the 11th Cambridge Ophthalmologic Symposium, St. John's College, Cambridge, England, September 10–11, 1981 92. Federman JL, Annesley WH Jr, Sarin LK, et al: Vitrectomy and cystoid macular edema. Ophthalmology 87:622, 1980 93. Aaberg TM, Van Horn DL: Late complications of pars plana vitreous surgery. Ophthalmology 85:126, 1980 94. Peyman GA, Huamonte FU, Goldberg MF, et al: Four hundred consecutive pars plana vitrectomies with vitrophage. Arch Ophthalmol 96:45, 1978 95. Jaffe WE: Cataract Surgery and Its Complications, p 341. St. Louis, Mosby, 1976 96. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of
immediate vitrectomy and of intravitreous antibiotics for the treatment
of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 113:1479. 1995 97. Jaffe GJ, Ben-Nun J, Guo H, et al: Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 107:2024, 2000 98. Aaberg TM, Cesarz TJ, Flikinger RR: Treatment of peripheral uveoretinitis by cryotherapy. Am J Ophthalmol 75:685, 1973 99. Dobbie JG: Cryotherapy in the management of Toxoplasma retinochoroiditis. Trans Am Acad Ophthalmol Otolaryngol 72:364, 1968 100. Ghartey KN, Brockhurst RJ: Photocoagulation of active toxoplasmic retinochoroiditis. Am J Ophthalmol 89:858, 1980 101. Spalter HF, Campbell CJ, Noyori KS, et al: Prophylactic photocoagulation of recurrent toxoplasmic retinochoroiditis: A
preliminary report. Arch Ophthalmol 75:21, 1966 102. Michelson JB, Chisari FV: Behçet's disease: A review. Surv Ophthalmol 26:190, 1982 |