The orbital apex contains a plethora of vital structures. A large number
of arteries, veins, and nerves pass through several significant foramina. The
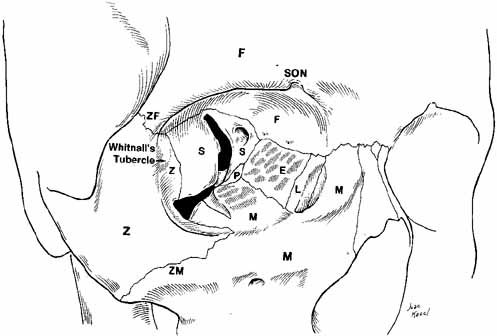
superior orbital fissure is a transverse notch between the greater
and lesser wings of the sphenoid bone that descends medially (see Fig. 1). Although the shape of the superior orbital fissure is variable, the
superior portion is usually narrower where the lacrimal, frontal, and
trochlear nerves pass (Fig. 6). The middle meningeal artery anastomosis with the ophthalmic artery
may enter here, if not through its own foramen, more anteriorly in
the roof. Most of the venous drainage from the orbit and the globe flow
through the superior orbital fissure to the cavernous sinus. Other
structures passing through the superior orbital fissure within the annulus
of Zinn include the superior and inferior divisions of the third
cranial nerve, the sixth cranial nerve, and the nasociliary branch of
the ophthalmic trigeminal nerve (Fig. 6).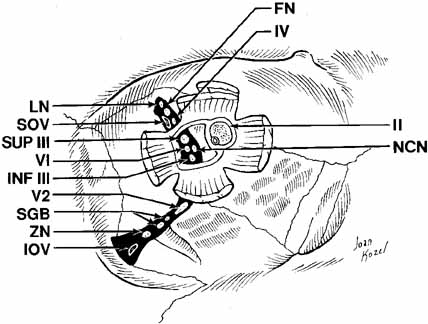 Fig. 6 Orbital apex with nerves coursing through foramina. (LN, lacrimal nerve; NCN, nasociliary nerve; FN, frontal nerve; VI, abducens nerve; IV, trochlear nerve; INF III, inferior division of cranial nerve III; SOV, superior ophthalmic vein; II, cranial nerve II; SUP III, superior division of cranial nerve III; IOV, inferior ophthalmic vein; ZN, zygomatic nerve; V2, V2 nerve; SGB, sphenopalatine ganglion branches) Fig. 6 Orbital apex with nerves coursing through foramina. (LN, lacrimal nerve; NCN, nasociliary nerve; FN, frontal nerve; VI, abducens nerve; IV, trochlear nerve; INF III, inferior division of cranial nerve III; SOV, superior ophthalmic vein; II, cranial nerve II; SUP III, superior division of cranial nerve III; IOV, inferior ophthalmic vein; ZN, zygomatic nerve; V2, V2 nerve; SGB, sphenopalatine ganglion branches)
|
Radiographic enlargement of the superior orbital fissure may accompany
pathologic processes, such as aneurysm, meningioma, chordoma, pituitary
adenoma, or tumors of the orbital apex.8 When idiopathic inflammation preferentially involves the superior orbital
fissure, the Tolosa-Hunt syndrome (painful ophthalmoplegia) results. The
nerves to the extraocular muscles may be affected
by the inflammation as they pass through the superior orbital fissure. The
pain in this syndrome results from the inflammatory involvement
of the first division of the trigeminal nerve. Interference with venous
drainage through the inflamed fissure can cause stasis edema of the
lids and orbits. Medial to the superior orbital fissure lies the optic foramen (see Fig. 1) within the lesser wing of the sphenoid, which conveys the optic
nerve and the ophthalmic artery. The optic canal attains adult dimensions
by age 3 and is symmetric in most persons. Because of the shift in
the position of the ophthalmic artery relative to the optic nerve, the
canal is horizontally oval posteriorly and more vertically oval anteriorly. In
the adult the optic canal is 8 to 10 mm long and 5 to 7 mm
wide, and the optic foramen normally measures 6.5 mm in diameter. Optic
foramen enlargement is commonly seen with optic nerve gliomas. A foramen
that measures 7 mm in diameter is usually abnormal. Among young children
whose optic canals have not yet reached adult dimensions, the
size of both foramina should be compared. In these patients, a foramen
that is 6.5 mm in diameter and at least 1 mm larger than the contralateral
foramen is considered abnormal. The optic canal is separated from
the superior orbital fissure by the bony optic strut, the inferior root
of the lesser wing of the sphenoid bone (see Fig. 1). It joins the body of the sphenoid to its lesser wing and separates
the optic foramen from the superior orbital fissure. This thin optic
strut forming the lateral and inferior borders of the optic canal is
subject to deformation by optic nerve gliomas, infraclinoid aneurysms, or
intracanalicular spread of an intracranial chiasmal tumor.9 An “optic neuritis” that is progressive over months or years
should suggest an intracanalicular meningioma.10 Other orbital diseases may cause enlargement of the optic canal. Benign
arachnoidal hyperplasia extending beyond the tumoral glial tissue can
enlarge the optic foramen. Rarely, a fungal infection, such as aspergilloma, or
a bacterial infection, such as a syphilitic gumma or tuberculoma, can
settle in the optic canal and mimic a neoplasm. Enlargement
of the canal can also occur in sarcoidosis, neurofibroma, arachnoidal
cyst, and chronic hydrocephalus. Fibrous dysplasia and ossifying fibromas
of the sphenoid bone can involve the canal and narrow its dimensions.10 The infraorbital fissure is a 20-mm bony defect bounded by the sphenoid, zygomatic, maxillary, and palatine bones, and lies between the
lateral orbital wall and orbital floor (see Fig. 1). It transmits the second (maxillary) division of the fifth
cranial nerve, the zygomatic nerve, small branches from the sphenopalatine
ganglion, and branches of the inferior ophthalmic vein leading
to the pterygoid plexus (see Figs. 1, 6, and 19). Posterior to the inferior orbital fissure, the foramen rotundum
pierces the greater sphenoid wing carrying the maxillary division of
the trigeminal nerve forward to the orbit. Arriving with the maxillary
nerve is the terminal branch of the internal maxillary artery. The structures
enter the infraorbital sulcus to become the infraorbital nerve
and artery, which then traverse the infraorbital canal and foramen to
carry sensation to the lower lid, cheek, upper lip, and upper anterior
gingiva. It is important to identify this neurovascular bundle during
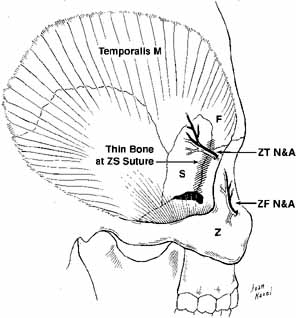
midface suborbicularis oculi fat lifts to avoid inadvertent injury. The inferior orbital fissure extends more anteriorly than the superior
orbital fissure, ending about 20 mm from the anterior orbital rim. This
structure serves as a posterior landmark in the surgical subperiosteal
dissection along the orbital floor. Immediately beneath the infraorbital
fissure lies the pterygoid space with the temporalis fossa laterally; blunt
trauma to the temporalis muscle can result in orbital hemorrhage
via this connection (see Fig. 3). Orbital Soft Tissues The soft tissues contained within the bony walls of the orbit and limited
anteriorly by the orbital septum are discussed in this section in the
following order: orbital septum, periorbita, orbital fascia, orbital
fat, lacrimal gland, extraocular muscles, levator palpebrae superioris, Müller's
muscle, optic nerve and meninges, globe, orbital
nerves, orbital vessels, and orbital lymphatic drainage. Orbital Septum The orbital septum is the anterior soft tissue boundary of the orbit and
acts as a physical barrier against pathogens and maintains the normal
posterior position of the orbital fat-pads. It is a thin, multilayered
sheet of fibrous tissue derived from the mesodermal layer of
the embryonic eyelid. The septum is covered by a thin layer of preseptal
orbicularis and skin and originates from the superior and inferior
orbital rims at a thick, white fibrous line called the arcus marginalis to insert onto the eyelid retractors. Medially, the septum covers the
posterior aspect of Horner's muscle as it inserts along the posterior
lacrimal crest. Laterally, the septum fuses with the lateral canthal
tendon and lateral horn of the levator aponeurosis to attach to the
lateral orbital rim11 (see Fig. 7). 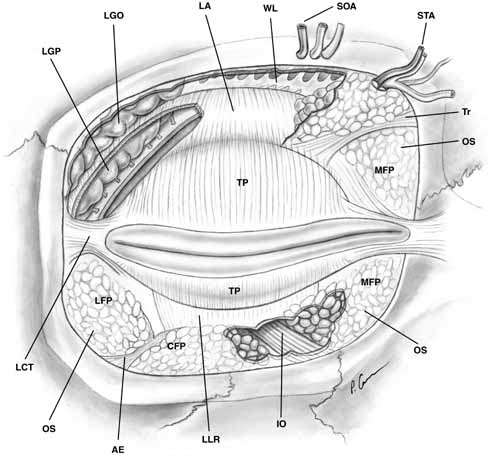 Fig. 7 Anterior view of orbital septum and related structures. The medial deep
orbital insertion of the orbicularis muscle carries the orbital septum
behind it. The septal attachments to the levator aponeurosis in the
upper lid and inferior tarsus in the lower lid are also demonstrated, as
well as the anatomic relationships to the structures of the upper lid. (AE, arcuate expansion of the inferior oblique; CFP, central fat-pad; IO, inferior oblique muscle; LA, levator aponeurosis; LCT, lateral canthal tendon; LFP, lateral fat-pad; LGO, lacrimal gland orbital lobe; LGP, lacrimal gland palpebral lobe; LLR, lower lid retractors; MCT, medial canthal tendon; MFP, medial fat-pad; OS, orbital septum; STA, supratrochlear artery, nerve, vein; SOA, supraorbital artery, nerve, vein; TP, tarsal plate; Tr, trochlea; WL, Whitnall's ligament) Fig. 7 Anterior view of orbital septum and related structures. The medial deep
orbital insertion of the orbicularis muscle carries the orbital septum
behind it. The septal attachments to the levator aponeurosis in the
upper lid and inferior tarsus in the lower lid are also demonstrated, as
well as the anatomic relationships to the structures of the upper lid. (AE, arcuate expansion of the inferior oblique; CFP, central fat-pad; IO, inferior oblique muscle; LA, levator aponeurosis; LCT, lateral canthal tendon; LFP, lateral fat-pad; LGO, lacrimal gland orbital lobe; LGP, lacrimal gland palpebral lobe; LLR, lower lid retractors; MCT, medial canthal tendon; MFP, medial fat-pad; OS, orbital septum; STA, supratrochlear artery, nerve, vein; SOA, supraorbital artery, nerve, vein; TP, tarsal plate; Tr, trochlea; WL, Whitnall's ligament)
|
In the lower eyelid, the septum inserts onto the inferior border of tarsus
after joining with the lower lid retractors 4 to 5 mm below the tarsus. The
superior orbital septum does not insert onto the superior tarsal
plate because of the intervening levator aponeurosis; rather it inserts
on the aponeurosis about 10 mm above the superior eyelid margin, or 2 to 5 mm
above the superior tarsal border in non-Asians11 (see Fig. 8). In Asian lids, the orbital septum fuses to the levator aponeurosis
at a level below the superior tarsus, allowing preaponeurotic fat
to prolapse inferior and anterior to tarsus; in the lower lid, it may
fuse directly to the inferior tarsal border rather than joining with the
retractors. An absent or lower lid crease in Asian eyelids may be due
to this fat protrusion and other subcutaneous fat tissue that inhibits
levator fibers from inserting into the subdermal skin.12 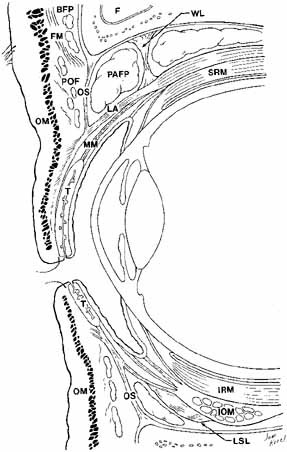 Fig. 8 Parasagittal section to show anterior orbital structures. (F, frontal sinus; SRM, superior rectus muscle; FM, frontalis muscle; MM, Müller's muscle; BFP, brow fat-pad; T, tarsus: POF, postorbicularis fascia; OM, orbicularis muscle; OS, orbital septum; LSL, Lockwood's suspensory ligament; PAFP, preaponeurotic fat-pad; IOM, inferior oblique muscle; WL, Whitnall's ligament; IRM, inferior rectus muscle; LA, levator aponeurosis) Fig. 8 Parasagittal section to show anterior orbital structures. (F, frontal sinus; SRM, superior rectus muscle; FM, frontalis muscle; MM, Müller's muscle; BFP, brow fat-pad; T, tarsus: POF, postorbicularis fascia; OM, orbicularis muscle; OS, orbital septum; LSL, Lockwood's suspensory ligament; PAFP, preaponeurotic fat-pad; IOM, inferior oblique muscle; WL, Whitnall's ligament; IRM, inferior rectus muscle; LA, levator aponeurosis)
|
The septum may attenuate with age allowing orbital fat to herniate forward, requiring
blepharoplasty. In performing levator surgery or blepharoplasty
the preaponeurotic fat is encountered just posterior to the septum. Loose
areolar tissue, termed the suborbicularis fascia, lies immediately anterior to the septum13 and shares the same plane as the eyebrow retro-orbicularis oculi
fat and malar fat-pads further from the eyelid margins. Periorbita The periorbita is the periosteal covering of the orbital bones. The periorbita
is firmly attached at the suture lines, the foramina, the fissures, the
arcus marginalis, and at the posterior lacrimal crest. Elsewhere, it
is loosely adherent to the bone and may be easily separated from
the bone by the surgeon or by accumulations of blood or pus. Posteriorly, the
periorbita is continuous with the dura of the optic nerve, where
the dura is fused to the optic canal. Likewise, the superior orbital
fissure is bound by thickened periorbita, which also blends with
intracranial dura. Anteriorly, the periorbita is continuous with the
orbital septum, which partitions the lids from the anterior orbital tissues. The
periorbita is continuous with the frontal, zygomatic, malar, and
nasal periostea, and is also continuous with the bones of the sphenopalatine
and temporal fossa through the inferior orbital fissure. The
periorbita lines the lacrimal fossa, and an extension—the lacrimal
fascia—covers the lacrimal sac between the anterior and posterior
lacrimal crest (Fig. 9). 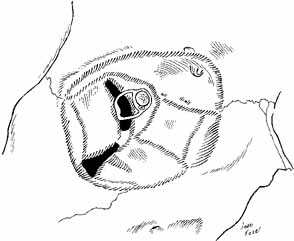 Fig. 9 Shaded areas demonstrate dense attachment sites for the periorbita. Fig. 9 Shaded areas demonstrate dense attachment sites for the periorbita.
|
The periorbita is extensively vascularized on both its bone and soft tissue
sides. These vessels are interconnected so that the periosteum does
not serve as a vascular barrier area.14 It is supplied by twigs from regional branches of the sensory intraorbital
trigeminal nerve. The periorbita has a dense layer adjacent to bone
and a more loosely packed layer next to the orbital contents. It serves
as a membrane that can restrain periosteal hematomas and temporally
provide resistance to the spread of infections and tumors from the
sinuses and bones into the orbit. However, the periorbita can be eventually
dissolved by these processes. In children, granulocytic sarcoma
has a predilection for the periosteum and bones of the orbit.10 The periorbita can often be the only separation between the orbital contents
and dermoids or mucoceles. The potential space between the periorbita
and orbital bones provides a convenient plane of dissection to
many orbital tumors or for removal of soft tissues in an exenteration. Orbital Fascia The fibrous tissue organization within the orbit may be divided into three
parts: the fascia covering the globe, the coverings of the extraocular
muscles, and the check ligament extensions of the extraocular muscle
fascia that extend to the surrounding bone and eyelids. Extensive
work by Koornneef,15 using a thick serial section technique has shown the orbital fascia to
be complex and highly organized. Tenon's capsule, the fascia bulbi, is a fibrous membrane that extends
from the posterior aspect of the globe to fuse anteriorly with the
conjunctiva slightly posterior to the corneoscleral junction. It is thinnest
at the entrance of the optic nerve. It is closely applied to the
globe but may be lifted some distance from it to reveal a fine netlike
character. The resultant space between these structures is termed
Tenon's space. Externally, Tenon's capsule is joined to the
network of fibrous septa dividing the lobules of orbital fat. Thus, the
globe is loosely related to the surrounding orbital fat, and freedom
of movement is afforded by elasticity in the septa and fat. Tunnel-like
openings in Tenon's fascia allow the extraocular muscles
to pass from the orbital fat into the Tenon's space to insert onto
the sclera (see Fig. 10). In the areas of these openings, Tenon's capsule fuses with
the intermuscular septal fascia. Orbital implants used after enucleation
are placed either within this fibrous Tenon's capsule or posterior
to it within the muscle cone. Inflammatory pseudotumor may involve
Tenon's capsule and cause a tenonitis that can produce proptosis. B-scan
ultrasonography can help identify this type of periocular
inflammation. Posterior geographic scleritis and intense choroiditis
may also cause secondary inflammations of Tenon's capsule.10 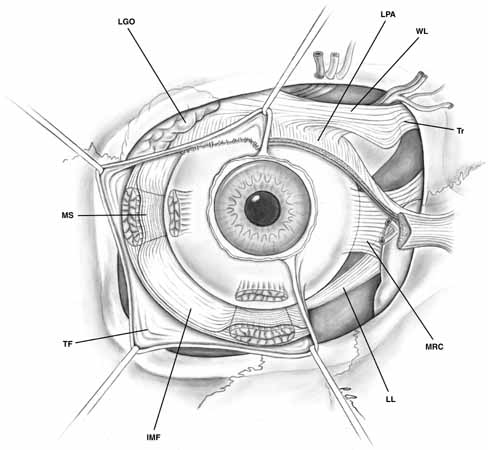 Fig. 10 Tenon's fascia, anterior view. Tenon's capsule covers the globe
and extends onto the muscular fascia. Tenon's fascia is denser
between the muscles and thinner toward the posterior aspect of the globe. The
intermuscular septal fascia connecting the muscular sheaths is
demonstrated beneath the reflected Tenon's fascia. (IMF, intermuscular fascia; LGO, lacrimal gland orbital lobe; LL, Lockwood's ligament; LPA, levator palpebrae aponeurosis; MRC, medial rectus check ligament; MS, muscular sheath; TF, Tenon's fascia; Tr, trochlea; WL, Whitnall's ligament) Fig. 10 Tenon's fascia, anterior view. Tenon's capsule covers the globe
and extends onto the muscular fascia. Tenon's fascia is denser
between the muscles and thinner toward the posterior aspect of the globe. The
intermuscular septal fascia connecting the muscular sheaths is
demonstrated beneath the reflected Tenon's fascia. (IMF, intermuscular fascia; LGO, lacrimal gland orbital lobe; LL, Lockwood's ligament; LPA, levator palpebrae aponeurosis; MRC, medial rectus check ligament; MS, muscular sheath; TF, Tenon's fascia; Tr, trochlea; WL, Whitnall's ligament)
|
The muscular fascia ensheathes the extraocular muscles and extends between
them. These muscle fascial sheaths are thin posteriorly but become
much denser anteriorly. The muscular sheaths connect from their extraconal
surface to the orbital walls and from their intraconal surface to
the fibrous septae dividing the intraconal fat lobules.16 The bulbar side of the muscular sheath is thinner than the external aspect
that forms the check ligaments, yet it is thicker than the posterior
portion of Tenon's capsule.17 Smooth muscle fibers are scattered throughout the membrane and are innervated
by the sympathetic nervous system.2
The muscles are connected to the surrounding fascia throughout the anterior
one-third of their lengths, especially where they insert onto the globe,
which prevents their retraction far posteriorly in the orbit if lost during
strabismus operation (unless the muscle has been dissected free). These
attachments account, in part, for the persistent movement of the eye socket
after enucleation when muscles have not been specifically sewn to the
implant. As noted above, each extraocular muscle sheath sends extensions
to the orbital walls. Anteriorly, they are especially prominent and are
called check ligaments. The most developed check ligaments are
those of the medial and lateral rectus muscles (see Figs.
10 and 11). The lateral
check ligament is the strongest and inserts primarily on the posterior
aspect of Whitnall's lateral orbital tubercle with lesser extensions to
the lateral conjunctival fornix and lateral orbital septum. The medial
check ligament inserts on the bone behind the posterior lacrimal crest
and to the medial orbital septum, caruncle, and plica semilunaris. The
superior rectus muscle sheath is joined anteriorly with that of the levator
palpebrae superioris by means of an intermuscular fascia.18
The superior transverse Whitnall's ligament may serve as a superior check
ligament to limit elevation by the upper eyelid19
(see Figs. 10 and 11).
The fused inferior rectus and inferior oblique muscle sheaths send fascial
connections to the inferior periorbita, which may also have some checking
function.
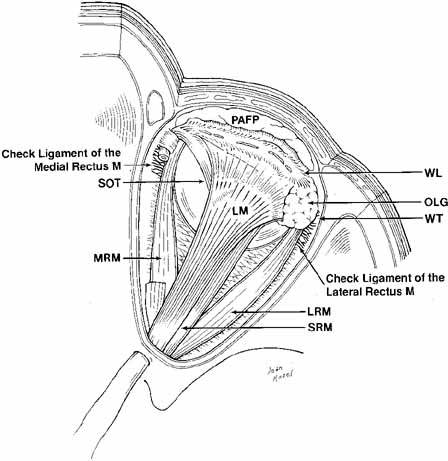 Fig. 11 Superior view of the orbit. Whitnall's ligament fuses medially with
the trochlea of the superior oblique muscle and fuses laterally with
the lacrimal gland. The medial horn of the levator aponeurosis lies directly
on top of the superior oblique reflected tendon. The lateral horn
of the levator aponeurosis splits the palpebral and orbital lobe of
the lacrimal gland. The lateral rectus check ligament attaches to Whitnall's
tubercle and is slightly denser than the medial rectus ligament. (WL, Whitnall's ligament; OLG, orbital lobe of lacrimal gland; SOT, superior oblique tendon; PAFP, preaponeurotic fat-pad; LM, levator palpebrae superioris muscle; WT, Whitnall's tubercle; MRM, medial rectus muscle; LRM, lateral rectus muscle; SRM, superior rectus muscle) Fig. 11 Superior view of the orbit. Whitnall's ligament fuses medially with
the trochlea of the superior oblique muscle and fuses laterally with
the lacrimal gland. The medial horn of the levator aponeurosis lies directly
on top of the superior oblique reflected tendon. The lateral horn
of the levator aponeurosis splits the palpebral and orbital lobe of
the lacrimal gland. The lateral rectus check ligament attaches to Whitnall's
tubercle and is slightly denser than the medial rectus ligament. (WL, Whitnall's ligament; OLG, orbital lobe of lacrimal gland; SOT, superior oblique tendon; PAFP, preaponeurotic fat-pad; LM, levator palpebrae superioris muscle; WT, Whitnall's tubercle; MRM, medial rectus muscle; LRM, lateral rectus muscle; SRM, superior rectus muscle)
|
Lockwood20 described a hammock-like structure extending from the lateral orbital
tubercle to the medial canthal tendon comprised of the fused fascia
of the inferior rectus and inferior oblique muscles. The retractor
complex of the lower eyelid is composed of aponeurotic expansions of
the inferior rectus. These expansions form the capsulopalpebral head, which
divides to extend anteriorly around the inferior oblique muscle
and then fuses into Lockwood's ligament in front of the inferior
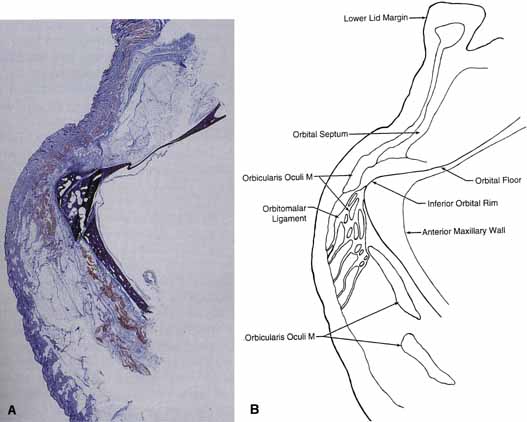
oblique to form the capsulopalpebral fascia.21 This fascia connects Lockwood's ligament to the inferior fornix, to
the inferior border of the tarsus, and to the preseptal orbicularis
muscle and skin at the level of the lid crease (see Fig. 12). It also contains the adrenergic smooth muscle fibers of the inferior
tarsal muscle, which are more diffusely distributed than in Müller's
muscle and do not insert directly onto the tarsus. Lockwood's
suspensory ligament is strongest immediately anterior to the
inferior oblique muscle and may help support the globe after removal
of the orbital floor. However, globe ptosis can occur after orbital
decompression for thyroid eye disease. 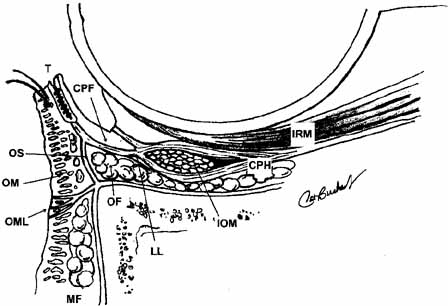 Fig. 12 Normal lower lid anatomy in cross section. (CPF, capsulopalpebral fascia; CPH, capsulopalpebral head; IOM, inferior oblique muscle; IRM, inferior rectus muscle; LL, Lockwood's ligament; MF, malar fat; OF, orbital fat; OM, orbicularis muscle; OML, orbitomalar ligament; OS, orbital septum; T, tarsus) Fig. 12 Normal lower lid anatomy in cross section. (CPF, capsulopalpebral fascia; CPH, capsulopalpebral head; IOM, inferior oblique muscle; IRM, inferior rectus muscle; LL, Lockwood's ligament; MF, malar fat; OF, orbital fat; OM, orbicularis muscle; OML, orbitomalar ligament; OS, orbital septum; T, tarsus)
|
The trabeculae of orbital fat are also part of this extensive fascial connective
tissue system of the orbit and globe. In Graves' disease
as well as early pseudotumor, the trabeculae of the orbital fat thicken, giving
the fat a rough texture.10 Nodular fasciitis is a reactive pseudosarcomatous proliferation of the
fascial connective tissues of the orbit and globe. It usually presents
as a rapidly developing nodule situated in the epibulbar region of the
anterior aponeurosis of the extraocular muscles. Although the histologic
features can be disturbing, the condition is benign. Orbital Fat The orbital structures are surrounded by orbital fat, which provides a
resilient cushion to support the globe. Anteriorly in the orbit, the fat
is fibrous, whereas the larger lobules are found posteriorly (see Fig. 13). In the upper eyelid, the orbital septum covers a central preaponeurotic
fat-pad and a smaller medial fat-pad separated
by the trochlea (see Figs. 7 and 8). The medial fat-pad of the upper eyelid is firmer and whiter
in color. The infratrochlear nerve and the medial palpebral artery
branch of the ophthalmic artery courses through the medial fat. Clinically, there
exist three areas in the inferior orbit from which fat may
protrude.22 The lateral fat pad is divided from the central third by the lateral arcuate
expansion fascial attachments of the inferior oblique passing to
the floor inferotemporally. The medial and central fat-pads of
the lower lid are separated by the inferior oblique muscle (see Fig. 14). When excising fat during blepharoplasty, excessive anterior traction
on the fat may pull the muscle forward and lead to its inadvertent
injury. 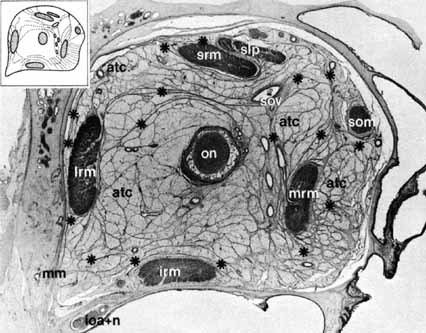 Fig. 13 Anatomic section demonstrating orbital septa 1.4 mm from behind the surface
of the eye. Diameters vertically, 2.4 cm; transversally, 2.7 cm. Enlargement
is approximately × 3.5. (ON, optic nerve; SOV, superior ophthalmic vein; SLP, superior levator palpebrae muscle; SRM, superior rectus muscle; LRM, lateral rectus muscle; IRM, inferior rectus muscle; MRM, medial rectus muscle; SOM, superior oblique muscle; *, connective tissue septa; ATC, adipose tissue
compartment; IOA + IN, infraorbital artery and nerve; MM, Müller's muscle) (From Koornneef L: Spatial aspects
of orbital musculofibrous tissue in man: A new anatomical and histological
approach. Amsterdam: Swets en Zeitlinger, 1976) Fig. 13 Anatomic section demonstrating orbital septa 1.4 mm from behind the surface
of the eye. Diameters vertically, 2.4 cm; transversally, 2.7 cm. Enlargement
is approximately × 3.5. (ON, optic nerve; SOV, superior ophthalmic vein; SLP, superior levator palpebrae muscle; SRM, superior rectus muscle; LRM, lateral rectus muscle; IRM, inferior rectus muscle; MRM, medial rectus muscle; SOM, superior oblique muscle; *, connective tissue septa; ATC, adipose tissue
compartment; IOA + IN, infraorbital artery and nerve; MM, Müller's muscle) (From Koornneef L: Spatial aspects
of orbital musculofibrous tissue in man: A new anatomical and histological
approach. Amsterdam: Swets en Zeitlinger, 1976)
|
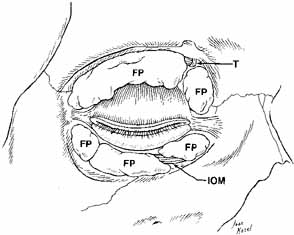 Fig. 14 Anterior view of deep dissection of orbital fat-pads to show trochlea
dividing fat-pads in the upper eyelid. The inferior oblique
muscle divides the medial from the central fat, and the arcuate expansion
fascia of the inferior oblique divides the central from the lateral
fat pads in the lower eyelid. (FP, fat pad; T, trochlea; IOM, inferior oblique muscle) Fig. 14 Anterior view of deep dissection of orbital fat-pads to show trochlea
dividing fat-pads in the upper eyelid. The inferior oblique
muscle divides the medial from the central fat, and the arcuate expansion
fascia of the inferior oblique divides the central from the lateral
fat pads in the lower eyelid. (FP, fat pad; T, trochlea; IOM, inferior oblique muscle)
|
As the fascial layers in the orbit thin with age, the orbital fat sometimes
prolapses through the weakened orbital septum into the lids. Asians
may be more predisposed to involutional entropion than whites due to
a more anterior and superior position of orbital fat within the lower
eyelid.23 The orbital fat in Asians appears to protrude anterior to the inferior
orbital rim and up to the inferior tarsus due to differences in orbital
septum insertion with the capsulopalpebral fascia. It is quite rare to find a primary tumor of the orbital fat. Prolapse of
the orbital fat must be distinguished from lipomas. Liposarcoma of the
orbit is rare and originates from primitive mesenchymal cells related
to the orbital fascia rather than from a lipoma or preexistent adipose
tissue. More commonly, inflammatory pseudotumor may involve orbital
fat to some degree. The fat cells degenerate and release their lipid
content, which further augments the inflammatory process. Eventually, fibrosis
and a sclerosing lipogranuloma occurs. Trauma to the orbit can
also cause fat necrosis and an orbital lipogranuloma. An orbital abscess
within the orbital fat can lead to fat liquefaction. All types of
chronic granulomatous disease, either infectious, such as fungal infections, or
noninfectious, such as Wegener's granulomatosis, may
involve the orbital fat. Since the orbital fat fills most of the retrobulbar space, infections and
metastatic tumors may expand at its expense. Rare parasitic conditions, such
as hydatid cyst (Echinococcus granulosus) and cysticercosis, as well as metastatic carcinoma and lymphoma
are found in the retrobulbar fat.10 Lacrimal Gland The main lacrimal gland resides in the superotemporal orbit in a shallow
lacrimal fossa of the frontal bone. The gland measures 20 mm by 12 mm
by 5 mm and is divided by the lateral horn of the levator aponeurosis
into a larger orbital lobe and a lesser palpebral lobe as shown (see Figs. 7 and 11). Division is not complete, since a posterior connection of parenchyma
persists between the lobes. The superior orbital lobe is bound anteriorly
by the orbital septum and the preaponeurotic fat-pad, behind
by orbital fat, and laterally by bone. The palpebral lobe lies
underneath the levator aponeurosis in the subaponeurotic Jones' space
and is separated from conjunctiva medially, where the superior tarsal
muscle intervenes. Pleomorphic adenomas typically involve the orbital
lobe. Secretory ducts from the palpebral lobe drain into the superotemporal conjunctival
fornix, as do those from the orbital lobe. The ducts of the
orbital lobe pass through the palpebral lobe, or on its surface, so
that damage to the latter structure may block the drainage of the entire
lacrimal gland. The scarring of the superotemporal conjunctiva may
also close the ducts of an otherwise healthy gland. Arterial blood to the lacrimal gland is supplied by the lacrimal branch
of the ophthalmic artery, often with contributions from the recurrent
meningeal artery (which may join the lacrimal artery or enter the
gland independently) and by a branch of the infraorbital artery. The
lacrimal artery then passes through the gland and provides the blood
supply to the temporal upper and lower eyelids as the lateral palpebral
arteries and subsequent arterial arcades. The lacrimal vein follows
approximately the same intraorbital course of the artery and drains
into the superior ophthalmic vein. Both artery and vein communicate
with the gland on its posterior surface. The lacrimal gland receives innervation from cranial nerves V and VII as
well as from the sympathetics of the superior cervical ganglion. The
lacrimal nerve branch of the ophthalmic trigeminal nerve travels superotemporally
in the orbit just underneath the periorbita to enter the
gland with the vessels. Like the artery, the lacrimal nerve continues
through the gland to supply more superficial eyelid structures. Sympathetic
nerves arrive with the lacrimal artery and along with parasympathetics
in the zygomatic nerve. The zygomatic branch of the maxillary trigeminal
nerve enters the orbit 5 mm behind the anterior limit of the
inferior orbital fissure and may indent the zygomatic bone (zygomatic
groove) on its anterosuperior course. The zygomatic nerve gives
off the lacrimal branch before dividing into zygomaticotemporal and
zygomaticofacial branches. This lacrimal branch anastomoses with the
lacrimal nerve of the ophthalmic trigeminal nerve or travels along the
periorbita to independently enter the gland at its posterior lateral
aspect. The lacrimal nerve is sensory, although it may carry some sympathetic fibers
gained while traversing the cavernous sinus. The parasympathetic
VII nerve supply to the lacrimal gland (via the zygomatic nerve
of V2) provides the main secretory motor function. The exact role
of sympathetic innervation in the control of lacrimal secretion is unknown.24,25 In addition to the lacrimal gland itself, there are approximately 20 accessory
glands of Krause in the superior fornix, and, perhaps, half that
number are in the inferior fornix. There are also accessory glands
of Wolfring above the tarsus. Removal of the lacrimal gland can produce
keratitis sicca, despite normally functioning accessory lacrimal glands.26 Parasympatholytic drugs may reduce lacrimal secretion. Damage to the sphenopalatine
ganglion as well as brain tumors impinging the efferent
supply to the lacrimal gland may cause hyposecretion. Hyposecretion in
central autonomic dysfunction states, such as Riley-Day syndrome, can
lead to corneal damage.27 Hyposecretion also occurs as a consequence of lacrimal gland parenchymal
loss in older persons in conditions such as age-related atrophy, Sjögren's
syndrome, sarcoidosis, and benign lymphoepithelial
lesion (seen often in postmenopausal women). Chronic inflammation
and periductal fibrosis were the most common changes seen
in a light microscopic study of lacrimal glands removed at autopsy.28 Hypersecretion is seen in cases of reflex stimulation, such as ocular trauma
or inflammation of any etiology. Damage to the facial nerve in the
vicinity of the geniculate ganglion can cause aberrant regeneration
resulting in crocodile tears in which the patient tears while masticating. This
is thought to be due to aberrant regeneration of afferent taste
fibers of the nervus intermedius into the nearby efferent parasympathetic
fibers to the lacrimal gland. A related phenomenon can be caused
by an acoustic neuroma, and the patient with this reflex may have
ipsilateral hearing loss. Tumors of the lacrimal gland can be benign or
malignant and are discussed in detail elsewhere. The tears drain through the superior and inferior puncta and canaliculi
and are pumped into the nasolacrimal sac by the orbicularis muscle sphincter
action. The nasolacrimal sac lies in a fossa between the anterior
lacrimal crest of the maxillary bone and the posterior lacrimal crest
of the lacrimal bone, and is wrapped by the thick anterior and thinner
posterior limbs of the medial canthal tendon. The puncta are 2 mm
in height, the canaliculi are 8 mm in length, and the sac is 12 to 14 mm
in height, with its fundus extending slightly above the medial canthal
tendon. The nasolacrimal duct then travels inferolaterally and slightly
posteriorly in its bony course to the inferior turbinate. The valve
of Rosenmuller is located at the junction of the common canaliculus
and sac, the valve of Krause between the sac and duct, and the valve
of Hasner at the ostium to the inferior meatus. The entry in an external
dacryocystorhinostomy is at the anterior middle meatus. Extraocular Muscles Except for the inferior oblique, the extraocular muscles all arise from
the orbital apex. The four recti muscles originate from the thick fibrous
annulus of Zinn, which surrounds the optic foramen at the orbital
apex and divides the superior orbital fissure into intraconal and extraconal
spaces (see Fig. 6). The levator and the superior oblique muscles arise more superiorly
and medially on the lesser wing of the sphenoid. The annulus of Zinn
is connected posteriorly to the dura and medially and laterally to
the lesser and greater wings of the sphenoid, respectively. Passing through
the annulus of Zinn are the oculomotor nerve divisions, the optic, the
nasociliary and abducen nerves, and the ophthalmic artery (see Fig. 6). Passing through the superior orbital fissure outside the annulus
are the trochlear, lacrimal, frontal nerves, and the superior ophthalmic
vein. The horizontal recti muscles attain a length (excluding the tendon) of
about 40.5 mm, whereas the superior rectus muscle is slightly
longer and the inferior rectus muscle shorter. The medial rectus muscle
has the greatest mass, and the superior rectus muscle has the least. The
four recti muscles course through the orbital fat and define the
muscle cone. The muscles then pass through openings in Tenon's
fascia to insert on the anterior portion of the globe in a configuration
called the spiral of Tillaux (see Fig. 15). The medial rectus inserts nearest at 5.5 mm posterior to the limbus, and
the superior rectus inserts farthest from the limbus at 7.7 mm. The
relationship of the muscle insertions and the ora serrata is clinically
important. A misdirected bridle suture passed through the insertion
of the superior rectus muscle could perforate the retina. The
medial and inferior recti and inferior oblique are supplied by the inferior
division of the oculomotor nerve, the superior rectus by the superior
oculomotor division, and the lateral rectus by the abducens nerve. Each
enters the muscle on the ocular surface at the junction of the
posterior third with the anterior two-thirds (see Fig. 19). 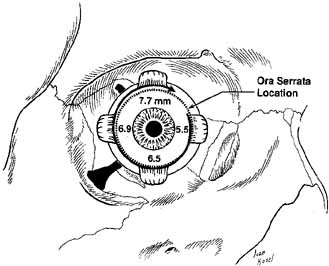 Fig. 15 Anterior view of the right globe. The spiral of Tillaux is shown with superimposed
location of the ora serrata. Fig. 15 Anterior view of the right globe. The spiral of Tillaux is shown with superimposed
location of the ora serrata.
|
The inferior rectus muscle lies juxtaposed to the orbital floor posteriorly
in the region of the palatine bone but elevates from it more anteriorly. A
series of fibrous septa radiate to the inferior periorbita, suggesting
that incarceration of this tissue alone in a floor fracture
may yield restriction of the muscle. The inferior oblique muscle courses
posterolaterally underneath the inferior rectus muscle, and their
conjoined fascias form the suspensory ligament of Lockwood (see Fig. 12). The large inferior oculomotor nerve division to the inferior oblique
muscle travels anteriorly along, and is bound to, the lateral border
of the inferior rectus muscle. The medial rectus remains close to the medial orbital wall until the anterior
third of its course when it angles laterally to insert on the eye. Just
above the medial rectus lie terminal branches of the nasociliary
nerve and ophthalmic artery. The lateral rectus muscle is separated
from the optic nerve by the ciliary ganglion, nasociliary nerve, and
the ophthalmic artery, which are embedded in the loose intraconal orbital
fat (see Fig. 6). Having arisen from the same mesoblastic mass, the superior rectus and levator
palpebrae superioris muscles remain fused at their medial borders. The
nasociliary nerve and ophthalmic artery leave the lateral orbit
to cross beneath the superior rectus. The superior oblique, the roundest of extraocular muscles, arises from
the superomedial annulus of Zinn and courses anteriorly and superiorly
for 40 mm from its origin, closely applied to the superior medial orbital
wall. Beneath it, and separating it from the medial rectus muscle, are
the ethmoidal branches of the nasociliary nerve and ophthalmic artery. The
superior oblique becomes tendinous just before it passes through
the trochlea located 5 to 10 mm posterior to the orbital rim. The
tendon then makes a 54-degree angle to continue posteriorly, laterally, and
inferiorly to the eye. The 28-mm reflected tendon
passes underneath the superior rectus and fans out to insert on the
globe in a broad-based attachment that extends to the posterior
pole. The distance between the temporal borders of the superior rectus
and superior oblique tendon averages 4.7 mm.29 The superior oblique muscle depresses, intorts, and abducts the eye (see Fig. 11). The trochlea is situated in a shallow fossa bearing its name on the anteromedial
orbital roof. Crescent-shaped cartilage is suspended
from the periorbita on either end by the fibrous pillars. The central
fibers of the reflected tendon exhibit few adhesions to the neighboring
fibers, whereas those peripheral in the tendon are connected in a loose
fashion to the fibers of the tendon. Located between the cartilage
and the tendon is a bursalike structure, presumably to reduce friction.30 The cartilage is a U-shaped ring with a grooved flange that supports
the reflected tendon posteriorly and laterally from the front of
the trochlea (Fig. 16).31 The periorbita to which the trochlea is attached can be carefully elevated
from the bone by the surgeon and replaced, if needed, although injury
to the tissues surrounding the trochlea can cause scarring and possible
superior oblique restriction or Brown's syndrome. 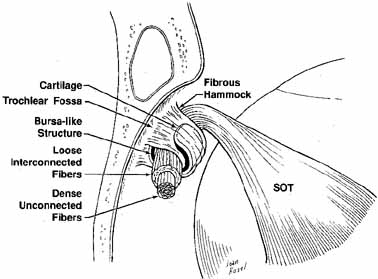 Fig. 16 Schematic drawing of the right trochlea. Tendon is supported by a layer
of cartilage suspended by fibrous supports from the periorbita. Central
fibers of the tendon are strong with dense unconnected fibers. Peripheral
tendon shows loose interconnected fibers. (SOT, superior oblique tendon) (Adapted from Helveston EM, et al: The trochlea: A study of the anatomy
and physiology. Ophthalmology 1982:89:124) Fig. 16 Schematic drawing of the right trochlea. Tendon is supported by a layer
of cartilage suspended by fibrous supports from the periorbita. Central
fibers of the tendon are strong with dense unconnected fibers. Peripheral
tendon shows loose interconnected fibers. (SOT, superior oblique tendon) (Adapted from Helveston EM, et al: The trochlea: A study of the anatomy
and physiology. Ophthalmology 1982:89:124)
|
The inferior oblique muscle arises from a shallow depression in the orbital
plate of the maxilla at the anteromedial corner of the orbital floor
just lateral to the lacrimal excretory fossa. This muscle travels
in a course similar to that of the reflected superior oblique tendon. As
noted before, the fascia of the inferior rectus divides to encircle
the inferior oblique, and their joined fascia just anterior to the oblique
forms the suspensory ligament of the globe before continuing as
the capsulopalpebral fascia and lower lid retractor complex. The 37-mm
inferior oblique muscle remains muscular until its insertion on
the globe, where a tendon several millimeters in length or the muscle
fibers themselves may enter into the sclera. The insertion is 2.2 mm
inferior and lateral to the macula and may be found 9.5 mm posterior
to the lateral rectus insertion. The nerve enters the middle of the muscle
at the lateral border of the inferior rectus muscle. Blood supply
for the extraocular muscles is from the medial and lateral muscular branches
of the ophthalmic artery, the lacrimal artery, and the infraorbital
artery. Except for the lateral rectus, each muscle receives two
anterior ciliary arteries that communicate with the major arteriole circle
of the ciliary body. The lateral rectus is supplied by a single vessel
derived from the lacrimal artery.32 Levator Palpebrae Superioris Arising from the lesser wing of the sphenoid above Zinn's annulus, the
levator origin is lateral to the superior oblique muscle and above
the superior rectus muscle (see Fig. 6). The levator extends arteriorly in the superior orbit with a thin
layer of fat, the supraorbital artery, frontal nerve, and the trochlear
nerve separating it from the orbital roof. The levator rests upon
the superior rectus, and these muscles are attached by a fascial sheath
along their medial borders (see Fig. 11). Both muscles are innervated by the superior division of the oculomotor
nerve, which enters at the posterior one-third of the muscles
from the inferior surface. The muscle sheath of the levator is thin, like the other extraocular muscle
sheaths, except on the medial edge, where it joins with the superior
rectus. The muscular portion of the levator is approximately 40 mm
in length, in contrast to its aponeurosis, which is 14 to 20 mm from
Whitnall's ligament to the anterior inferior tarsus border.33 Immediately behind the superior orbital rim, a transverse fibrous condensation
attaches superiorly to the widening levator, termed the superior transverse Whitnall's ligament (see Fig. 11).19 Whitnall's ligament is a thick condensation of elastic fibers of
the anterior sheath of the levator, located at the transition from fleshy
levator muscle to fibrous aponeurosis. Whitnall's ligament acts
as a suspensory ligament for the upper lid as well as a fulcrum for
the levator muscle to change vector force from an anterior-posterior
direction to a superior-inferior direction.34 The ligament terminates medially in the fascia surrounding the trochlea. Laterally, Whitnall's ligament forms septa through the lacrimal
gland before attaching to the inner lateral orbital wall, up to 10 mm
superior to the lateral orbital tubercle. In the older person, Whitnall's
ligament or the levator aponeurosis becomes attenuated, leading
to upper eyelid ptosis. External repair of aponeurogenic blepharoptosis
involves incising the septum to reach the levator, dissecting superiorly
towards the musculoaponeurotic junction, releasing the inferior
aspect of the levator aponeurosis from the tarsus and underlying Muller's
muscle, and then suturing tarsus to a higher position on
the levator to achieve the desired lid height. The numerous techniques
of levator repair include posterior approaches and small-incision
repairs.35 Ptosis of the medial eyelid has been suggested to result from medial disinsertion
of the ligament.36 As the aponeurosis approaches tarsus, it splits into an anterior layer
that inserts into the pretarsal orbicularis bundles and skin, and a posterior
layer that inserts onto the inferior half of the anterior tarsus. In
his description of the levator aponeurosis,37 Whitnall gives a length of 7 mm from the aponeurosis origin to the orbicularis
and cutaneous insertions (see Fig. 8). The upper lid crease is created by these anterior insertions of
the aponeurosis. A light and electron microscopic study by Stasior38 revealed an elastic attachment system for the levator palpebrae superioris
muscle complex that forms an intricate insertion into the upper eyelid. As
the levator aponeurosis approaches the mid-tarsal level, approximately
two-thirds of the aponeurotic elastic fibers
radiated away from the tarsus to fuse onto the pretarsal orbicularis muscle
bundles. The remaining one-third of the aponeurotic elastic
fibers is inserted onto the anterior surface of the inferior tarsus. It
is this complex elastic fiber network that degenerates with age, rather
than the aponeurosis itself. In addition to the palpebral insertions, the levator aponeurosis expands
into a broad, fibrous sheath to insert into the orbital rims behind
the medial and lateral commissures of the eye as medial and lateral “horns” of
the levator. Confusion between the lateral horns
below and the ends of the superior transverse suspensory ligament above
should be avoided. The lateral horn is a strong, fibrous band incompletely
dividing the lacrimal gland into two lobes and continuing inferiorly
to insert on the lateral orbital tubercle and the lateral canthal
tendon. The medial horn, in contrast, becomes filmy as it passes over
the reflected superior oblique tendon to insert onto the posterior medial
canthal tendon and posterior lacrimal crest (see Fig. 11). Histologic sections studying lateral canthal anatomy demonstrated that
the lateral canthal ligament is formed by fibrous extensions of the upper
and lower tarsal plates and orbicularis muscle that unite into a common
ligament 1 mm in thickness and 3 mm wide.39 As the lateral canthal ligament approaches the orbital rim, it widens
to 6 to 7 mm as the lateral horn of the levator aponeurosis, the check
ligament of the lateral rectus muscle, and Lockwood's ligament fuse
with it before its bony insertion into the lateral orbital tubercle
of Whitnall located 5 mm inside the orbital rim. Knowledge of lateral
canthal ligament anatomy is important when reconstructing the lateral
canthal angle and taking a periosteal bite inside the orbital rim to
simulate the normal anatomic insertion. Elevating a short periosteal
flap based inside the lateral orbital wall, to which the lateral lid tissues
are secured, may provide a more correct and secure anatomic reapposition
of the lax lid well inside the lateral wall. This periosteal
flap technique may be performed through small incisions without lateral
canthotomy and cantholysis and has been suggested for ectropion repair
and as lateral canthal advancement in repair of exophthalmic lid retraction.39–41 Müller's Muscle Arising from the underside of the striated levator muscle approximately 15 mm
above the superior tarsal border is the smooth superior tarsal
muscle of Müller. It is firmly attached to the levator only at its
origin and may be easily separated from the latter below to form the
postaponeurotic space described by Jones. The superior tarsal muscle
inserts at the upper border of the tarsus, where the peripheral arterial
arcade is found between the overlying levator aponeurosis and Müller's
muscle (see Fig. 8). In Horner's syndrome, sympathetic denervation results in 2 mm
of upper lid ptosis. The analog of Müller's smooth muscle
in the lower lid is inferred in Horner's syndrome from the way
the lower lid rides up on the cornea, suggesting atonia secondary to loss
of sympathetic innervation. This inferior tarsal muscle is less well
developed but found posterior to the capsulopalpebral fascia and firmly
adherent to the underlying conjunctiva. The exact sympathetic nerve
course to these smooth muscles is unknown.42 An inverse Horner's syndrome refers to an irritative instead of ablative
effect on normal sympathetic innervation in which one sees lid
retraction; a lung tumor, for example, can irritate sympathetic fibers
destined for Müller's muscle. Müller's muscle infiltration
and scarring occurs invariably in thyroid eye retraction and, therefore, this
muscle may be excised or recessed in conjunction with
levator aponeurosis recession.43 The Globe The globe is located in the anterior orbit situated slightly superiorly
and laterally. The superior, medial, and inferior orbital rims extend
anteriorly to be on about the same frontal plane as the front of the
eye. The lateral rim is recessed 12 to 18 mm behind the cornea as measured
by exophthalmometry. Attached to the eye are the six extraocular
muscles, the optic nerve, the long and short posterior ciliary nerves, the
anterior and posterior ciliary arteries, and the vortex veins (Fig. 17). The globe is covered behind the corneal limbus by Tenon's
fascia and is supported in the orbit by Lockwood's ligament. The
average volume of the eye is about 6.5 cc compared to the orbital volume, which
is about 29.7 cc.2 The shape is not truly spheric; rather it is formed by the union of two
spheres, being that of the cornea and the sclera, with radius of curvatures
equal to 8 and 12 mm, respectively. 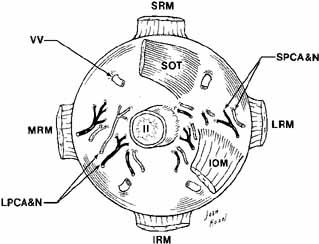 Fig. 17 Posterior view of the right globe after enucleation. (SRM, superior rectus muscle; VV, vortex veins; SOT, superior oblique tendon; II, cranial nerve II; LRM, lateral rectus muscle; SPCA & N, short posterior ciliary artery and nerve; LPCA & N, long posterior ciliary artery and nerve; MRM, medial rectus muscle; IRM, inferior rectus muscle; IOM, inferior oblique muscle) Fig. 17 Posterior view of the right globe after enucleation. (SRM, superior rectus muscle; VV, vortex veins; SOT, superior oblique tendon; II, cranial nerve II; LRM, lateral rectus muscle; SPCA & N, short posterior ciliary artery and nerve; LPCA & N, long posterior ciliary artery and nerve; MRM, medial rectus muscle; IRM, inferior rectus muscle; IOM, inferior oblique muscle)
|
The average adult and newborn infant globe dimensions are given in Table 2.
TABLE 2. Average Globe Dimensions
| Adult | |
| Anterior-posterior | 24 mm |
| Vertical | 23 mm |
| Horizontal | 23.5 mm |
| Newborn Infant | |
| Anterior-posterior | 16.4 mm |
| Vertical | 16 mm |
| Horizontal | 15.4 mm | Orbital Nerves Entering the orbit are the optic (cranial nerve II), the oculomotor (cranial
nerve III), the trochlear (cranial nerve
IV), the abducens (cranial nerve VI), the first and second
divisions of the trigeminal (cranial nerve V), the sympathetics, and
the parasympathetics of the third and fifth cranial nerves. The
nerves crowd together along with the ophthalmic artery to enter
the orbit at its apex, whereas the orbital venous blood drains via
the superior and inferior ophthalmic veins into the cavernous sinus (see Fig. 6). Obviously, single lesions in this crowded area can result in multiple
deficits often termed orbital apex syndromes. The intraorbital courses of the nerves are discussed in the order in which
they are mentioned previously. Optic Nerve (II) The optic nerve represents peripherally extended nerve tracts of the brain. Unlike
other cranial nerves, they contain supporting neuroglial cells
and are bathed by cerebrospinal fluid within investing layers continuous
with brain coverings. The course and lengths of the visual fibers
are intraocular (1 mm), intraorbital (25 mm), intracanalicular (5 to 9 mm), intracranial (16 mm), chiasmatic, optic
tract, ganglionic, optic radiation, and occipital
cortex. The axons of the optic nerve arise from the ganglion cell layer of the
retina and course through the scleral lamina cribrosa to join in forming
the massive optic nerve. The nerve is 1.5 mm in diameter within the
eye but expands to 3 to 4 mm at the back of the eye because of an increase
in supporting neuroglial cells and the onset of myelination.44 Its exit is about 3 mm medial and 1 mm below the posterior pole of the
eye. The intraorbital optic nerve is surrounded and cushioned by large lobules
of intraconal fat, which allow freedom of movement to the structure. The
intraorbital portion runs a sinusoidal course because it is longer
than the 18 mm from the posterior globe to the optic canal, which allows
for some leeway in proptosis before nerve compromise. The nerve
is covered by dura that thickens near the optic canal, where it becomes
continuous with the posterior periosteum. Cerebrospinal fluid within
the subarachnoid space around the nerve communicates freely with the
fluid bathing the midbrain, explaining instances of sudden respiratory
arrest following retrobulbar injection. Oculomotor Nerve (III) Within the anterior cavernous sinus, several millimeters behind the annulus
of Zinn, cranial nerve III divides into a superior and inferior division. The
branches are separated by the nasociliary nerve. The superior
branch rises within the muscle cone to reach the superior rectus
on its inferior side 15 mm from the orbital apex. Fibers then terminate
above in the levator palpebrae superioris by passing medial to the superior
rectus (90%) or through it (10%) (Fig. 18). 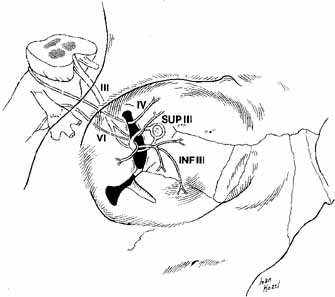 Fig. 18 Nerves to the extraocular muscles. The superior and inferior divisions
of the oculomotor nerve are separated by the nasociliary nerve within
the superior orbital fissure. The superior division supplies the superior
rectus and the levator palpebrae superioris muscles. The inferior
division supplies the inferior and the medial rectus muscles and the inferior
oblique muscle. The trochlear nerve supplies the superior oblique
muscle, whereas the abducens nerve innervates the lateral rectus muscle. (III, cranial nerve III; IV, cranial nerve IV; VI, cranial nerve VI; SUPIII, superior division of cranial nerve III; INFIII, inferior division of cranial nerve III) Fig. 18 Nerves to the extraocular muscles. The superior and inferior divisions
of the oculomotor nerve are separated by the nasociliary nerve within
the superior orbital fissure. The superior division supplies the superior
rectus and the levator palpebrae superioris muscles. The inferior
division supplies the inferior and the medial rectus muscles and the inferior
oblique muscle. The trochlear nerve supplies the superior oblique
muscle, whereas the abducens nerve innervates the lateral rectus muscle. (III, cranial nerve III; IV, cranial nerve IV; VI, cranial nerve VI; SUPIII, superior division of cranial nerve III; INFIII, inferior division of cranial nerve III)
|
The inferior branch of the oculomotor nerve travels underneath the optic
nerve to innervate the medial and inferior rectus muscles. Its large
terminal branch to the inferior oblique muscle continues anteriorly, intimately
associated with the lateral border of the inferior rectus. This
inferior oblique branch gives off a vertical parasympathetic twig
to the ciliary ganglion above, to eventually innervate the ciliary body
and iris sphincter. Trochlear Nerve (IV) At the superior orbital fissure, the thin trochlear nerve crosses over
the third nerve to enter the orbit temporal to Zinn's annulus and
medial to the frontal nerve. Its course is outside the muscle cone, thus
the superior oblique may continue to function after a retrobulbar
block (see Fig. 6). The nerve travels anteriorly from lateral to medial orbit to insert
into the lateral border of the superior oblique muscle at the posterior
one-third of the muscle belly. Abducens Nerve (VI) The abducens nerve enters the orbit through the intraconal section of the
superior orbital fissure to lie between the optic nerve and the lateral
rectus muscle. It travels along the lateral rectus muscle belly before
inserting into the inner surface of the muscle, where the posterior
third meets the anterior two-thirds. Trigeminal Nerve (V) The ophthalmic and maxillary divisions of the sensory trigeminal nerve
enter the orbit and pass through to supply the superior two-thirds
of the face (Figs. 19 and 20) . The ophthalmic division enters the orbit through the superior
orbital fissure as three branches: the lacrimal, frontal, and nasociliary. The
lacrimal nerve is the smallest branch, and it passes into the
orbit through the lateral end of the extraconal superior orbital fissure (see Figs. 6 and 19). It joins the lacrimal artery to reach the posterior aspect of the
lacrimal gland. Here, it forms superior and inferior branches; the
former supplies the gland, conjunctiva, and the lateral upper eyelid. The
inferior branch anastomoses with the zygomaticotemporal branch of
the maxillary trigeminal nerve, where it picks up parasympathetic secretory
fibers to the gland. The frontal branch passes just beneath the
periorbita, where it divides anteriorly in the orbit to form the supratrochlear
and larger supraorbital branch, which supply sensation to the
medial canthus, upper lid, and brow areas (see Fig. 19). The supraorbital nerve should be identified and spared during dissection
of the supraorbital rim, transcoronal forehead orbital approaches, or
during forehead lifts. The nasociliary branch of the ophthalmic
division is the only one to pass through Zinn's annulus. It passes
over the optic nerve with the ophthalmic artery to lie between the
superior oblique and medial rectus muscles. The nasociliary nerve gives
off a sensory route to the ciliary ganglion, two or three long ciliary
nerves to the globe, the anterior and posterior ethmoidal nerves
to supply the nasal mucosa, and the terminal infratrochlear branch to
supply the tip of the nose (Fig. 21). Involvement of this terminal infratrochlear branch of the nasociliary
nerve in herpes zoster ophthalmicus is termed Hutchinson's sign. 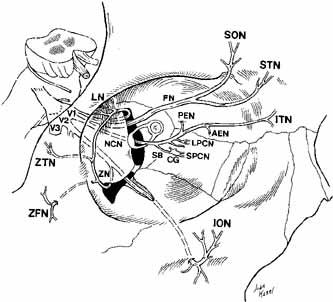 Fig. 19 Schematic drawing of the trigeminal nerve course in the orbit. (V1, Vl nerve; V2, V2 nerve; V3, V3 nerve; FN, frontal nerve; SON, supraorbital nerve; STN, supratrochlear nerve; LN, lacrimal nerve; ZTN, zygomaticotemporal nerve; ZFN, zygomaticofacial nerve; ZN, zygomatic nerve; NCN, nasociliary nerve; SB, sensory branch to the ciliary ganglion; CG, ciliary ganglion; SPCN, short posterior ciliary nerves; LPCN, long posterior ciliary nerves; PEN, posterior ethmoidal nerve; AEN, anterior ethmoidal nerve; ITN, infratrochlear nerve; ION, infraorbital nerve) Fig. 19 Schematic drawing of the trigeminal nerve course in the orbit. (V1, Vl nerve; V2, V2 nerve; V3, V3 nerve; FN, frontal nerve; SON, supraorbital nerve; STN, supratrochlear nerve; LN, lacrimal nerve; ZTN, zygomaticotemporal nerve; ZFN, zygomaticofacial nerve; ZN, zygomatic nerve; NCN, nasociliary nerve; SB, sensory branch to the ciliary ganglion; CG, ciliary ganglion; SPCN, short posterior ciliary nerves; LPCN, long posterior ciliary nerves; PEN, posterior ethmoidal nerve; AEN, anterior ethmoidal nerve; ITN, infratrochlear nerve; ION, infraorbital nerve)
|
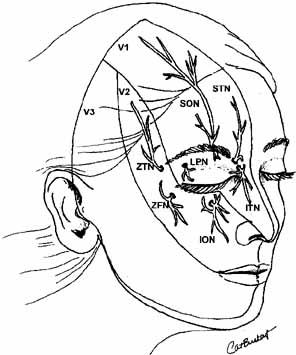 Fig. 20 Cutaneous distribution of V1 and V2 nerves. (V1, V1 nerve; V2, V2 nerve; SON, supraorbital nerve; STN, supratrochlear nerve: ITN, infratrochlear nerve; ION, infraorbital nerve; LPN, lateral palpebral nerve; ZFN, zygomaticofacial nerve; ZTN, zygomaticotemporal nerve) Fig. 20 Cutaneous distribution of V1 and V2 nerves. (V1, V1 nerve; V2, V2 nerve; SON, supraorbital nerve; STN, supratrochlear nerve: ITN, infratrochlear nerve; ION, infraorbital nerve; LPN, lateral palpebral nerve; ZFN, zygomaticofacial nerve; ZTN, zygomaticotemporal nerve)
|
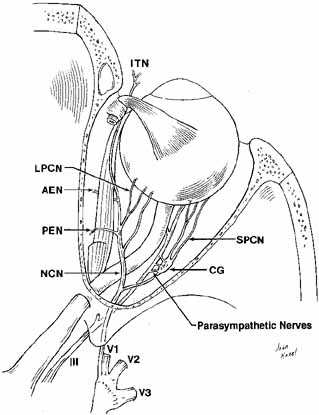 Fig. 21 Dissection to show the intraorbital nasociliary nerve course. The parasympathetic
nerve contribution to the ciliary ganglion from the inferior
division of III nerve is also shown. (V1, Vl nerve; V2, V2 nerve; V3, V3 nerve; NCN, nasociliary nerve; CG, ciliary ganglion; SPCN, short posterior ciliary nerves; LPCN, long posterior ciliary nerves; PEN, posterior ethmoidal nerve; AEN, anterior ethmoidal nerve; ITN, infratrochlear nerve) Fig. 21 Dissection to show the intraorbital nasociliary nerve course. The parasympathetic
nerve contribution to the ciliary ganglion from the inferior
division of III nerve is also shown. (V1, Vl nerve; V2, V2 nerve; V3, V3 nerve; NCN, nasociliary nerve; CG, ciliary ganglion; SPCN, short posterior ciliary nerves; LPCN, long posterior ciliary nerves; PEN, posterior ethmoidal nerve; AEN, anterior ethmoidal nerve; ITN, infratrochlear nerve)
|
The maxillary division of the trigeminal nerve exits the foramen rotundum
and crosses the pterygopalatine fossa before entering the orbit through
the inferior orbital fissure. The main component of the second division
of the trigeminal nerve is the infraorbital nerve that courses
anteriorly to enter the infraorbital groove 2.5 to 3 cm posterior to the
orbital rim, traverses the infraorbital canal, and then emerges from
the infraorbital foramen to provide sensation to the lower eyelid, cheek, and
upper lip (see Figs. 19 and 20). Sphenopalatine and posterior superior alveolar branches are formed
in the sphenomaxillary fossa to provide sensation to the nasal mucosa, gingiva, teeth, and
upper lip; middle and anterior superior alveolar
branches arise in the infraorbital canal. The zygomatic branch of
the maxillary nerve enters the inferior orbital fissure and divides into
the zygomaticotemporal and zygomaticofacial nerves, with the former
carrying parasympathetic secretory fibers from the sphenopalatine ganglion
to the lacrimal gland. Sympathetic Nerves The sympathetic nerve supply to the orbit controls pupillary dilatation, function
of the smooth muscles of the eyelids, and vasoconstriction. However, the
exact pathway of the sympathetic fibers to and through the
orbit is not clearly defined.45 Whitnall2 describes a separate sympathetic branch to the ciliary ganglion that arises
from the plexus traveling along the intracavernous carotid artery. In
the past it has been felt that the sympathetic fibers pass through
the optic foramen in humans2; however, studies in primate animal models by Lyon et al.45 suggest that the sympathetics travel through the superior orbital fissure
exclusively. Parasympathetic Nerves The parasympathetic supply to the lacrimal gland is discussed in the previous
section on the lacrimal gland. The parasympathetics to the eye
are carried to the ciliary ganglion by a branch from the inferior oblique
nerve; they synapse in the ganglion and pass to the globe via the
short ciliary nerves. The ciliary ganglion is situated 10 mm from the
orbital apex and 15 mm behind the globe and is frequently adherent to
the lateral aspect of the apical optic nerve. The parasympathetic motor
fibers to the ciliary body and iris sphincter muscles, that originate
in the Edinger-Westphal nucleus of the oculomotor nerve, synapse
in the ganglion, in contrast to the sympathetic and trigeminal sensory
fibers that traverse the ganglion without synapse. The sensory route
from the nasociliary nerve can be found reliably, but the less well-defined
sympathetic supply may arrive from a direct branch from
the sympathetic plexus, from a twig from the ophthalmic artery, or
both.46 Five or six short posterior ciliary nerves carry fibers from the ganglion
to enter the eye around the optic nerve. The majority enter lateral
to the nerve with one or two usually crossing to enter medially. Orbital Vessels The orbital arteries are independent of the fibrous septa and tend to remain
compartmentalized within each adipose space. In contrast, the veins
are embedded within the septa, with the degree of septal support related
to the caliber of the vessel.47 The presence of smooth muscle cells in the septa raises speculation that
the venous caliber may be related to the sympathetic tone. The orbital arteries are primarily branches of the ophthalmic artery with
small contributions from the internal maxillary artery (Fig. 22). The internal and external carotid systems have several areas of
anastomoses for collateral circulation. 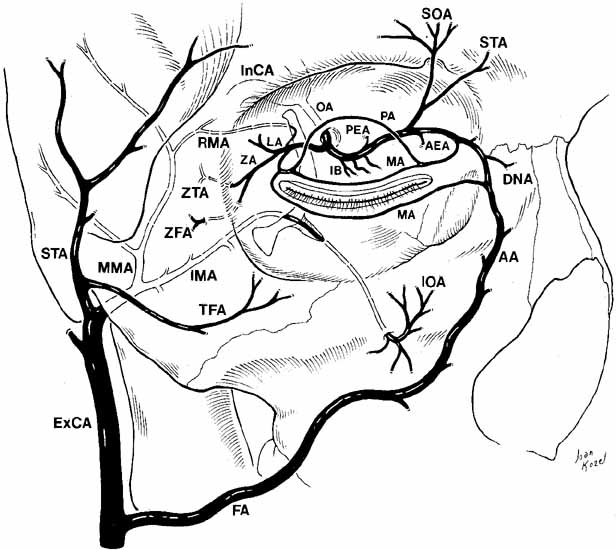 Fig. 22 Oblique view of the relationship of the internal and external carotid arterial
systems to the orbit. (ExCA, external carotid artery; FA, facial artery; AA, angular artery; IMA, internal maxillary artery; MMA, middle meningeal artery; TFA, transverse facial artery; STA, superficial temporal artery; InCA, internal carotid artery; OA, ophthalmic artery; LA, lacrimal artery; IB, intraconal branches; PEA, posterior ethmoidal artery; AEA, anterior ethmoidal artery; SOA, supraorbital artery; STA, supratrochlear artery; PA, peripheral arcade; MA, marginal arcade; ZA, zygomatic artery; ZTA, zygomaticotemporal artery; ZFA, zygomaticofacial artery; IOA, infraorbital artery; DNA, dorsal nasal artery; RMA, recurrent middle meningeal artery) Fig. 22 Oblique view of the relationship of the internal and external carotid arterial
systems to the orbit. (ExCA, external carotid artery; FA, facial artery; AA, angular artery; IMA, internal maxillary artery; MMA, middle meningeal artery; TFA, transverse facial artery; STA, superficial temporal artery; InCA, internal carotid artery; OA, ophthalmic artery; LA, lacrimal artery; IB, intraconal branches; PEA, posterior ethmoidal artery; AEA, anterior ethmoidal artery; SOA, supraorbital artery; STA, supratrochlear artery; PA, peripheral arcade; MA, marginal arcade; ZA, zygomatic artery; ZTA, zygomaticotemporal artery; ZFA, zygomaticofacial artery; IOA, infraorbital artery; DNA, dorsal nasal artery; RMA, recurrent middle meningeal artery)
|
The ophthalmic artery is the first large branch off the internal carotid
artery just as it emerges from the cavernous sinus in the area posterior
to the anterior clinoid process. The optic nerve is tightly fixed
to the dura within the canal and is supplied by pial branches of the
ophthalmic artery. The ophthalmic artery enters the orbit on the inferolateral
aspect of the nerve and soon crosses the orbit to pursue a medial
course.48 In 75% to 89% of orbits, the ophthalmic artery crosses above
the optic nerve. Approximately 10 mm posterior to the globe, the
ophthalmic artery provides the central retinal artery branch, which enters
the ventral surface of the optic nerve. Other branches include two
or three long posterior ciliary arteries to supply the choroid, the
muscular arteries, and the lacrimal, supraorbital, and ethmoidal arteries. After
supplying the extraocular muscles, the muscular arteries enter
the globe at the tendinous insertions to continue as anterior ciliary
arteries that anastomose with the long posterior ciliary arteries
in a network supplying the anterior ocular structures. The lateral rectus
supplies one anterior ciliary artery to the anterior ocular circulation, whereas
the other rectus muscles supply two each. The terminal
ophthalmic artery exits the orbit as the supratrochlear, dorsal nasal, and
medial palpebral arteries. Because of the variability in the order
in which the ophthalmic artery gives origin to its branch arteries, the
most frequent pattern is shown in Figure 23. 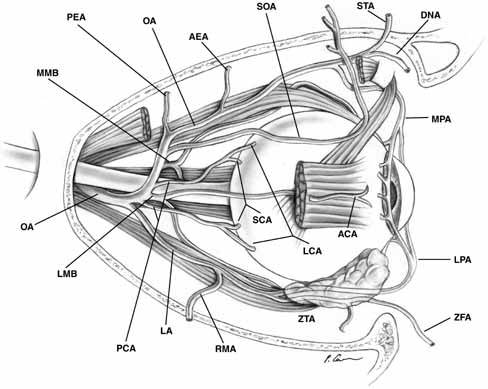 Fig. 23 Most common branching pattern of the ophthalmic artery in the orbit. (AEA, anterior ethmoidal artery; ANA, anterior ciliary artery; DNA, dorsal nasal artery; LA, lacrimal artery; LCA, long ciliary artery; LMB, lateral muscular branch; LPA, lateral palpebral artery; MMB, medial muscular branch; MPA, medial palpebral artery; OA, ophthalmic artery; PCA, posterior ciliary arteries; PEA, posterior ethmoida1 artery; RMA, recurrent middle meningeal artery; SCA, short ciliary artery; SOA, supraorbital artery; STA, supratrochlear artery; ZFA, zygomaticofacial artery; ZTA, zygomaticotemporal artery) Fig. 23 Most common branching pattern of the ophthalmic artery in the orbit. (AEA, anterior ethmoidal artery; ANA, anterior ciliary artery; DNA, dorsal nasal artery; LA, lacrimal artery; LCA, long ciliary artery; LMB, lateral muscular branch; LPA, lateral palpebral artery; MMB, medial muscular branch; MPA, medial palpebral artery; OA, ophthalmic artery; PCA, posterior ciliary arteries; PEA, posterior ethmoida1 artery; RMA, recurrent middle meningeal artery; SCA, short ciliary artery; SOA, supraorbital artery; STA, supratrochlear artery; ZFA, zygomaticofacial artery; ZTA, zygomaticotemporal artery)
|
Orbital Lymphatic Drainage The human orbit is traditionally thought to be devoid of any lymphatic
vessels or lymph nodes.49 Thus, the presence of lymphoid aggregates or fully formed lymph nodes
in this area is presumed to be pathologic.50,51 The function of the lymphatic vessels is to return large protein molecules
and excess tissue fluid to the vascular system from which they are
constantly being lost. The pathway by which large protein molecules
and fluid are removed from the orbit has been the subject of many animal
studies.51,52 Early studies involving the surgical ligation of lymphatic tissue close
to the orbit suggest that the lymphatic system plays a role in the removal
of fluid from the eye. One study found that surgical blockage of
the cervical lymph channels in dogs results in edema of the optic nerve
and retina.52 Lymphoscintigraphy, in which radioactive-labeled colloids are injected
into the body and collected in regional lymph nodes, was performed
extensively with different lymphotropic tracers injected into the
retrobulbar space of rabbits.53 Although 90% of the tracer remained in the orbit for over 1 week, there
was a definite amount of tracer material in the regional lymph
nodes, including the bilateral deep cervical lymph nodes and the ipsilateral
superficial cervical and submandibular nodes. Significant activity
was also seen near both optic nerves. Thus, these studies also suggested
the presence of slow lymph drainage from the orbit. The Rhesus and, more recently, the Cynomolgus monkey have been described
as an excellent animal model for orbital research.54,55 McGetrick et al.51 studied the lymphatic drainage from the Cynomolgus monkey orbit using
retrobulbar injections of 99mtechnetium sulfur colloid, a lymphotropic tracer, and india ink. Injections
outside the muscles were removed by the conjunctival and eyelid lymphatics. Colloids
injected into the orbit spread along connective tissue
septa and did not reach lymph nodes over a 24-hour period. A
small amount of india ink left the posterior orbit and was demonstrated
entering the contralateral orbit. Studies by Sherman et al.56 and Gausas et al.57 distinguished orbital lymphatic channels from blood capillaries histochemically
by light microscopy using a 5'-nucleotidase and
alkaline phosphatase double staining method on human surgical specimens. Lymphatic
vessels, which stained brown with 5'-nucleotidase
and met strict morphologic criteria for lymphatic vessels were identified
in the lacrimal gland and dura mater of the optic nerve. Lymphatics
were not identified in the extraocular muscles or orbital fat using
this technique. Conversely, blood vessels contained higher alkaline
phosphatase activity than lymphatic vessels.
Cook et al.58,59 used
this same staining method and morphologic criteria to identify analogous
lymphatic systems in the eyelids of humans and the Cynomolgus monkey.
Both a superficial or preorbicularis muscle plexus and a deep or pretarsal
(postorbicularis muscle) lymphatic plexus were identified with 5'-nucleotidase
in the upper and lower lids. No lymphatics were seen traversing the orbicularis,
suggesting that the two drainage systems may work independently of each
other. Using injections of 99mtechnetium sulfur colloid at
specific sites in monkey eyelids, lymphoscintigraphy revealed lymphatic
drainage of the medial and central lower lid along the facial vein to
the submandibular lymph nodes, while the entire upper lid, medial canthus,
and lateral lower lid drain into the preauricular parotid lymph nodes.
The central upper lid also had dual drainage into the submandibular nodes.59
This study parallels traditional depictions of lymphatic drainage in humans,
except that the medial upper lid and medial canthus has been thought to
drain into the submandibular rather than into the preauricular nodes (see
Fig. 24).25
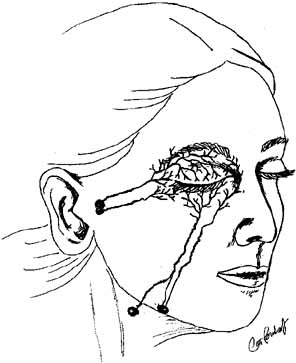 Fig. 24 Currently accepted pattern of lymphatic drainage in human eyelids. Fig. 24 Currently accepted pattern of lymphatic drainage in human eyelids.
|
Further elucidation of a lymphatic drainage system in the human orbit would
increase the understanding of many disease processes, including orbital
metastases, thyroid eye disease, orbital lymphomas and lymphangiomas, and
cell-mediated immunity within the orbit. |