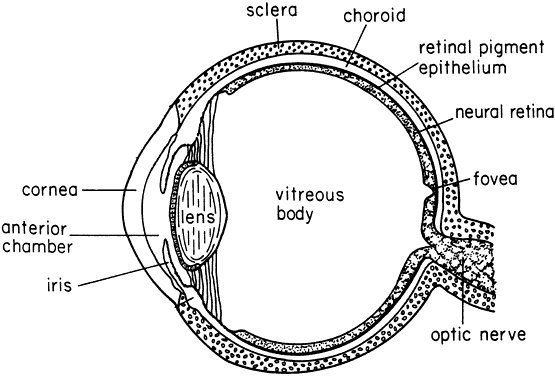
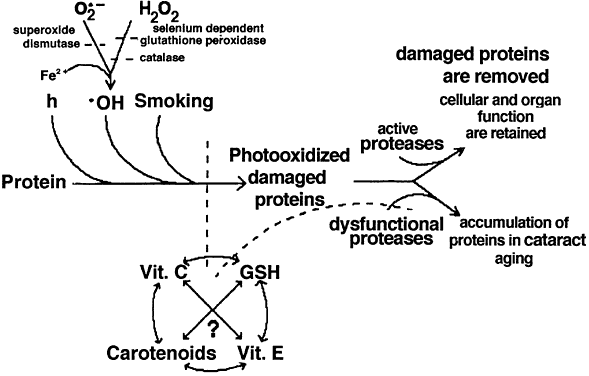
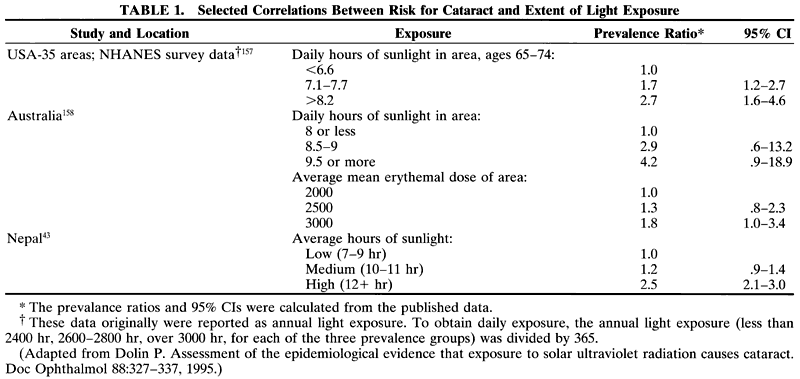
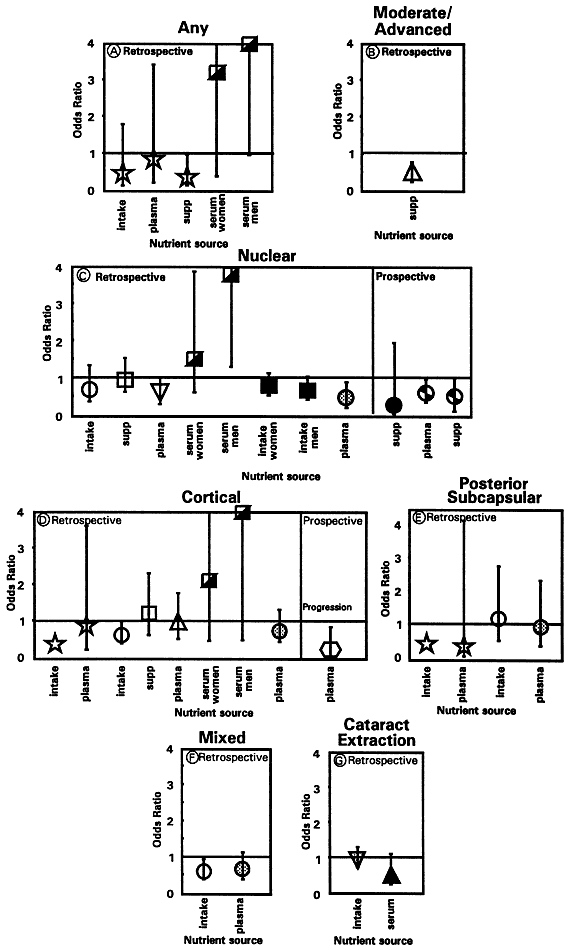
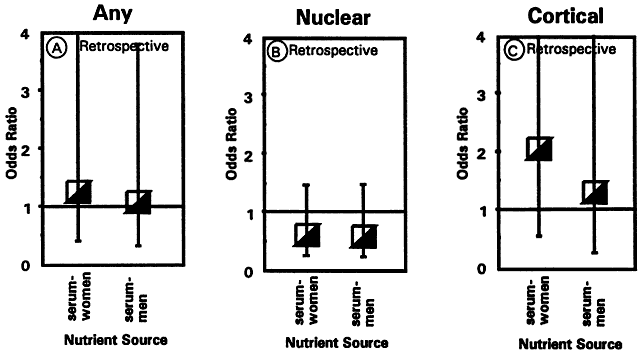
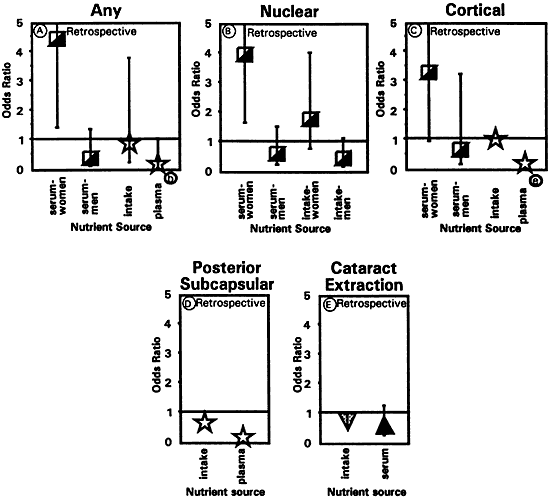
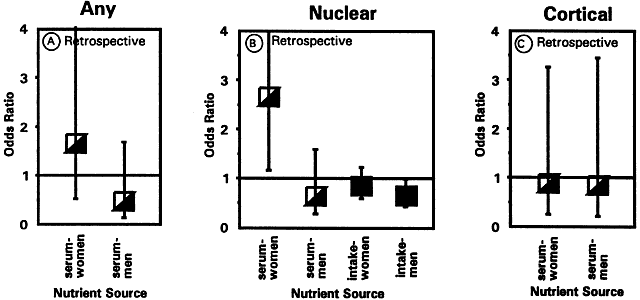
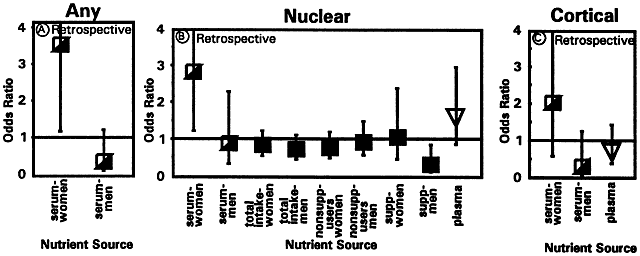
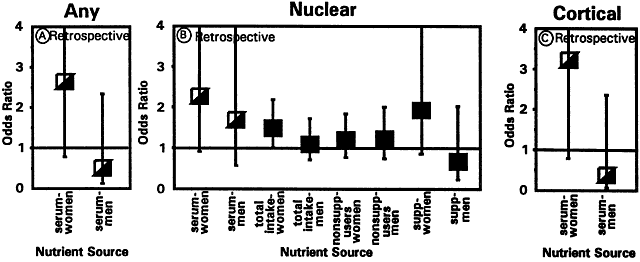
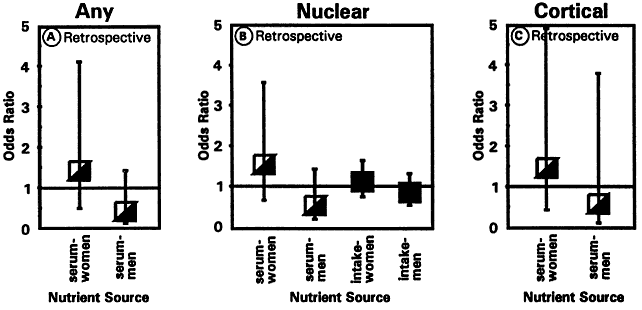
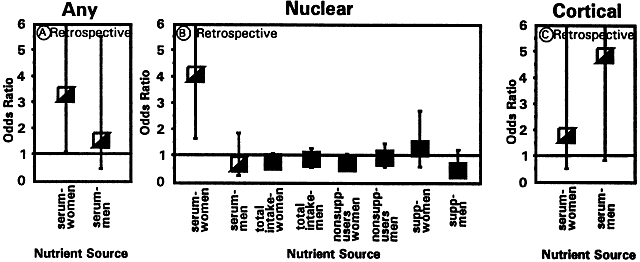
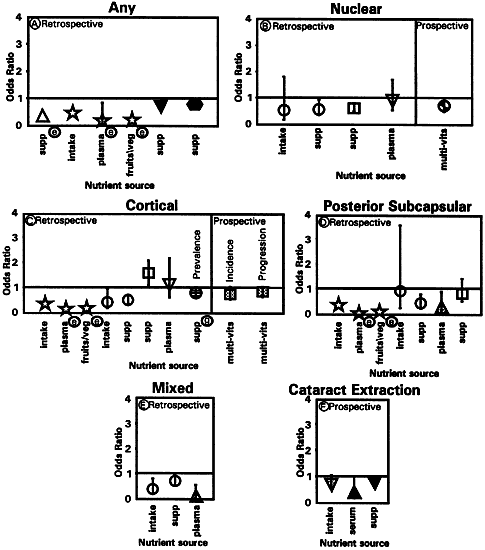
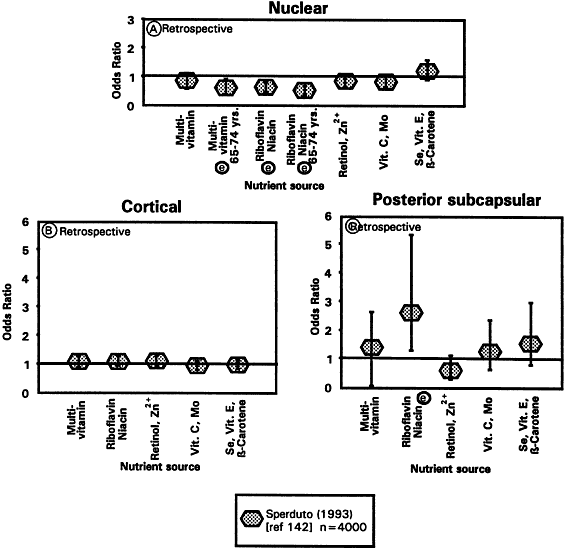
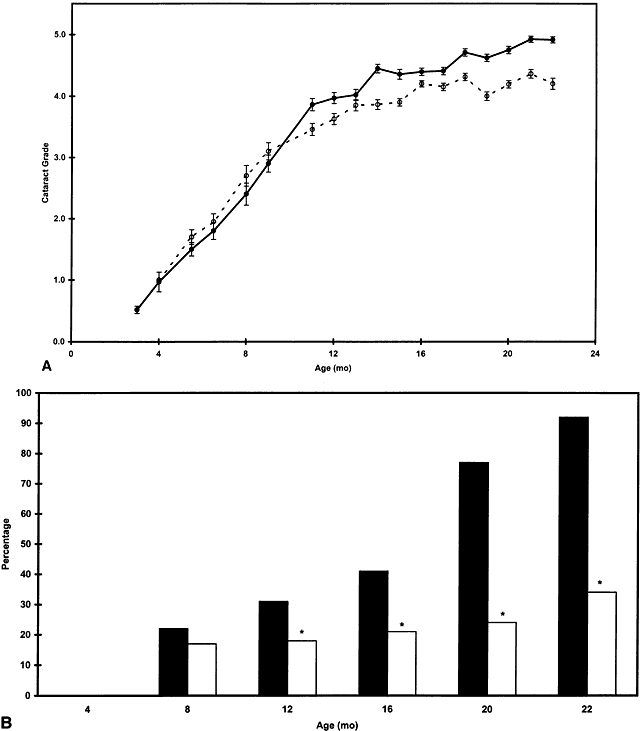
1. Muller HK, Buschke W: Vitamin C in Linse, Kammerwasser and Blut normalem and pathologischem Linsentstoffwech. Arch F Augenh 108:368–390, 1934 2. Taylor A, Jacques PF, Dorey CK: Oxidation and aging: Impact on vision. J Toxicol Indust Health 9:349-71, 1993 3. Bunce GE, Kinoshita J, Horwitz J: Nutritional factors in cataract. Annu Rev Nutr 10:233–254, 1990 4. Jacques PF, Chylack LT Jr, Taylor A: Relationships between natural antioxidants
and cataract formation. In Frei B (ed): Natural Antioxidants
Human Health and Disease. Orlando, FL: Academic Press 5. Taylor A: Vitamin C. In Hartz SC, Russell RM, Rosenberg IH (eds): Nutrition
in the Elderly: The Boston Nutritional Status Survey. London: Smith
Gordon Limited, 1992:147–150 6. Taylor A: Cataract: Relationships between nutrition and oxidation. J Am Coll Nutr 12:138–146, 1993 7. Blondin J, Taylor A: Measures of leucine aminopeptidase can be used to anticipate UV-induced
age-related damage to lens proteins: Ascorbate can delay this damage. Mech Ageing Dev 41:39–46, 1987 8. Blondin J, Baragi VJ, Schwartz E et al: Delay of UV-induced eye lens protein damage in guinea pigs by dietary ascorbate. Free Radiol Biol Med 2:275–281, 1986 9. Taylor A, Jacques PF: Relationships between aging, antioxidant status, and cataract. Am J Clinical Nutr 62:1439S–1447S, 1995 10. Taylor A: Oxidative stress and antioxidant function in relation to risk
for cataract. In Sies H (ed): Antioxidants in Disease Mechanisms and
Therapeutic Strategies (A Volume of Advances in Pharmacology Series). San
Diego: Academic Press, 1997:515–536 11. Taylor A, Jacques P: Antioxidant status and risk for cataract. In Bendich
A, Deckelbaum RJ (eds): Preventive Nutrition: The Guide for Health
Professionals. Totawa, NJ: Humana Press, 1997:267–283 12. Kupfer C: The conquest of cataract: A global challenge. Trans Ophthal Soc UK 104:1–10, 1984 13. Schwab L: Cataract blindness in developing nations. Internat Ophthalmol Clin 30:16–18, 1990 14. World Health Organization: Use of intraocular lenses in cataract surgery
in developing countries. Bull WHO 69:657–666, 1991 15. Klein BEK, Klein R, Linton KLP: Prevalence of age-related lens opacities in a population: The Beaver Dam
Eye Study. Ophthalmology 99:546–552, 1992 16. Klein R, Klein BE, Linton KL et al: The Beaver Dam Eye Study: The relation of age-related maculopathy to smoking. Am J Epidemiol 37:190–200, 1993 17. Leibowitz H, Krueger D, Maunder C et al: The Framingham Eye Study Monograph. Surv Ophthalmol (Suppl) 24:335–610, 1980 18. Chatterjee A, Milton RC, Thyle S: Prevalence and etiology of cataract in Punjab. Br J Ophthalmol 66:35–42, 1982 19. Wang G-M, Spector A, Luo C-Q et al: Prevalence of age-related cataract in Ganzi and Shanghai: The Epidemiological
Study Group. Chinese Med J 103:945–951, 1990 20. Whitfield R, Schwab L, Ross-Degnan D et al: Blindness and eye disease in Kenya: Ocular status survey results from the
Kenya Rural Blindness Prevention Project. Br J Ophthalmol 74:333–340, 1990 21. Chan CW, Billson FA: Visual disability and major causes of blindness in NSW: A study of people
aged 50 and over attending the Royal Blind Society 1984 to 1989. Aust N Zealand J Ophthalmol 19:321–325, 1991 22. Dana MR, Tielsch JM, Enger C et al: Visual impairment in a rural Appalachian community: Prevalence and causes. JAMA 264:2400–2405, 1990 23. Salive ME, Guralnik J, Christian W et al: Functional blindness and visual impairment in older adults from three communities. Ophthalmology 99:1840–1847, 1992 24. Wormald RPL, Wright LA, Courtney P et al: Visual problems in the elderly population and implications for services. Br Med J 304:1226–1229, 1992 25. Seddon JM, Christen WG, Manson JE et al: The use of vitamin supplements and the risk of cataract among US male physicians. Am J Public Health 84:788–792, 1994 26. Young RW: Optometry and the preservation of visual health. Optom Vis Sci 70:255–262, 1992 27. Taylor A, Tisdell FE, Carpenter FH: Leucine aminopeptidase (bovine lens): Synthesis and kinetic properties
of ortho, meta, and para substituted leucyl-anilides. Arch Biochem Biophys 210:90–97, 1981 28. Jacques PF, Taylor A: Micronutrients and age-related cataracts. In Bendich
A, Butterworth CE (eds): Micronutrients in Health and in Disease Prevention. New
York: Marcel Dekker, 1991:359–379 29. Chylack LT Jr, Wolfe JK, Singer DM et al: The lens opaci-ties classification system III. Arch Ophthalmol 111:831–836, 1993 30. Chylack LT Jr, Wolfe JK, Friend J et al: Nuclear cataract: Relative contributions
to vision loss of opalescence and brunescence. Invest Ophthalmol
Vis Sci 35:42632, 1994 (abstract) 31. Wolfe JK, Chylack LT Jr, Leske MC et al: Lens nuclear color and visual
function. Invest Ophthalmol Vis Sci 34:2550, 1993 (abstract) 32. Taylor HR, West SK, Rosenthal FS et al: Effect of ultraviolet radiation on cataract formation. N Engl J Med 319:1429–1433, 1988 33. Zigman S, Datiles M, Torczynski E: Sunlight and human cataract. Invest Ophthalmol Vis Sci 18:462–467, 1979 34. Wong L, Ho SC, Coggon D et al: Sunlight exposure, antioxidant status, and cataract in Hong Kong fishermen. J Epidemiol Community Health 47:46–49, 1993 35. Hirvela H, Luukinen H, Laatikainen L: Prevalence and risk factors of lens opacities in the elderly in Finland: A
population-based study. Ophthalmology 102:108–117, 1995 36. Cruickshanks KJ, Klein BE, Klein R: Ultraviolet light exposure and lens opacities: The Beaver Dam Eye Study. Am J Public Health 82:1658–1662, 1992 37. The Italian-American Cataract Study Group: Risk factors for age related
cortical, nuclear, and posterior subcapsular cataracts. Am J Epidemiol 133:541–553, 1991 38. Wang G-M, Spector A, Luo C-Q et al: Prevalence of age-related cataract in Ganzi and Shanghai. Chinese Med J 103:945–951, 1990 39. Klein BE, Cruickshanks KJ, Klein R: Leisure time, sunlight exposure and cataracts. Doc Ophthalmol 88:295–305, 1994-1995 40. Javitt JC, Taylor HR: Cataract and latitude. Doc Ophthalmol 88:307–325, 1995 41. Dolin P: Assessment of the epidemiological evidence that exposure to solar ultraviolet
radiation causes cataract. Doc Ophthalmol 88:327–337, 1995 42. Zigman S: Effects of near ultraviolet radiation on the lens and retina. Doc Ophthalmol 55:375–391, 1983 43. Brilliant LB, Grasset NC, Pokhrel RP et al: Associations among cataract prevalence, sunlight hours, and altitude in
the Himalayas. Am J Epidemiol 118:250–264, 1983 44. Minassian DC, Baasanhu J, Johnson GJ et al: The relationship between cataract and climatic droplet keratopathy in Mongolia. Acta Ophthalmol 72:490–495, 1994 45. Wolff SP: Cataract and UV radiation. Documenta Ophthalmol 88:201–204, 1995 46. Zigman S, Paxhia T, McDaniel T et al: Effect of chronic near-ultraviolet radiation on the gray squirrel lens
in vivo. Invest Ophthalmol Vis Sci 32:1723–1732, 1991 47. Zigler JS, Goosey JD: Singlet oxygen as a possible factor in human senile nuclear cataract development. Curr Eye Res 3:59–65, 1984 48. Varma SD, Chand O, Sharma YR et al: Oxidative stress on lens and cataract formation. Role of light and oxygen. Curr Eye Res 3:35–57, 1984 49. Taylor A, Jahngen-Hodge J, Huang LL et al: Aging in the eye lens: Roles for proteolysis and nutrition in formation
of cataract. AGE 14:65–71, 1991 50. Rao CM, Qin C, Robison WG Jr et al: Effect of smoke condensate on the physiological integrity and morphology
of organ cultured rat lenses. Curr Eye Res 14:295–301, 1995 51. Shalini VK, Luthra M, Srinivas L et al: Oxidative damage to the eye lens caused by cigarette smoke and fuel smoke
condensates. Ind J Biochem Biophys 31:261–266, 1994 52. Zigman S, McDaniel T, Schultz JB et al: Damage to cultured lens epithelial cells of squirrels and rabbits by UV-A (99.9%) plus
UV-B (0.1%) radiation and alpha tocopherol protection. Mol Cell Biochem 143:35–46, 1995 53. Smith D, Palmer V, Kehyias J et al: Induction of cataracts by X-ray exposure of guinea pig eyes. Lab Animal 22:34–39, 1993 54. Calissendorff BM, Lonnqvist B, el Azazi M: Cataract development in adult bone marrow transplant recipients. Acta Ophthalmol Scand 73:52–154, 1995 55. Palmquist BM, Phillipson B, Barr PO: Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol 60:113–117, 1984 56. Schocket SS, Esterson J, Bradford B et al: Induction of cataracts in mice by exposure to oxygen. Israel J Med 8:1596–1601, 1972 57. Giblin FJ, Schrimscher L, Chakrapani B et al: Exposure of rabbit lens to hyperbaric oxygen in vitro: Regional effects
on GSH level. Invest Ophthalmol Vis Sci 29:1312–1319, 1988 58. Giblin FJ, Padgaonkar VA, Leverenz VR et al: Nuclear light scattering, disulfide formation and membrane damage in lenses
of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res 60:219–235, 1995 59. Berman ER: Biochemistry of the Eye. New York: Plenum Press, 1991:210–308 60. Schectman G, Byrd JC, Gruchow HW: The influence of smoking on vitamin C status in adults. Am J Health 79:158–162, 1989 61. Russell-Briefel R, Bates MW, Kuller LH: The relationship of plasma carotenoids to health and biochemical factors
in middle-aged men. Am J Epidemiol 22:741–749, 1985 62. Giraud DW, Martin HD, Driskell JA: Plasma and dietary vitamin C and E levels of tobacco chewers, smokers, and
nonusers. J Am Diet Assoc 95:798–800, 1995 63. Chow CK, Thacker RR, Changchit C et al: Lower levels of vitamin C and carotenes in plasma of cigarette smokers. J Am Coll Nutr 5:305–312, 1986 64. Mezzetti A, Lapenna D, Pierdomenico SD et al: Vitamins E, C, and lipid peroxidation in plasma and arterial tissue of
smokers and non-smokers. Atherosclerosis 112:91–99, 1995 65. Bolton-Smith C, Casey CE, Gey KF et al: Antioxidant intakes assessed using a food-frequency questionnaire: Correlation
with biochemical status in smokers and non-smokers. Br J Nutr 65:337–346, 1991 66. Flaye DE, Sullivan KN, Cullinan TR et al: Cataracts and cigarette smoking: The City Eye Study. Eye 3:379–384, 1989 67. West SK, Munoz B, Emmett EA et al: Cigarette smoking and risk of nuclear cataracts. Arch Ophthalmol 107:1166–1169, 1989 68. West S: Does smoke get in your eyes? JAMA 268:1025–1026, 1992 69. Hankinson SE, Willett WC, Colditz GA et al: A prospective study of cigarette smoking and risk of cataract surgery in
women. JAMA 268:994–998, 1992 70. Mares-Perlman JA, Brady WE, Klein BEK et al: Serum carotenoids and tocopherols and severity of nuclear and cortical
opacities. Invest Ophthalmol Vis Sci 36:276–288, 1995 71. Taylor A, Davies KJA: Protein oxidation and loss of protease activity may lead to cataract formation
in the aged lens. Free Radic Biol Med 3:371–377, 1987 72. Taylor A, Jacques PF, Nadler D et al: Relationship in humans between ascorbic acid consumption and levels of
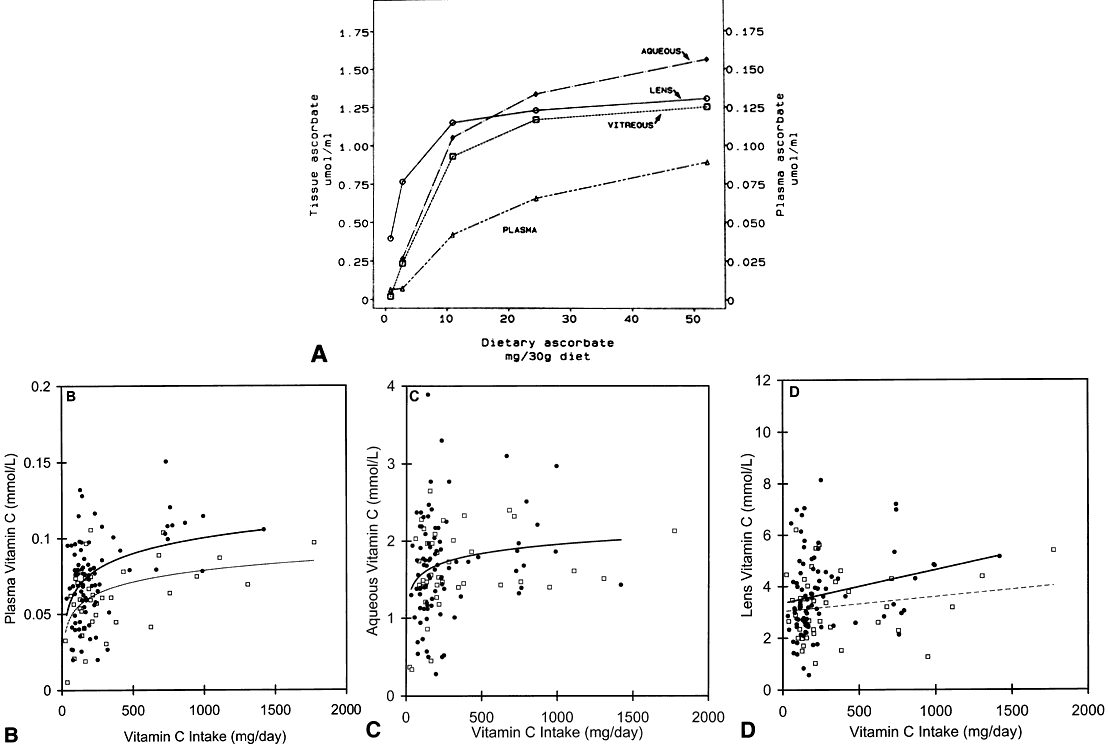
total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr Eye Res 10:751–759, 1991 73. Taylor A, Jacques P, Nowell T Jr et al: Vitamin C in human and guinea pig aqueous, lens, and plasma in relation
to intake. Curr Eye Res 16:857–864, 1997 74. Reddy VN: Glutathione and its function in the lens—anoverview. Exp Eye Res 150:771–778, 1990 75. Mune M, Meydani M, Jahngen-Hodge J et al: Effect of calorie restriction on liver and kidney glutathione in aging
Emory mice. Age 18:49, 1995 76. Sastre J, Meydani M, Martin A et al: Effect of glutathione monoethyl ester administration on galactose-induced
cataract in the rat. Life Chem Rep 12:89–95, 1994 77. Taylor A, Jahngen-Hodge J, Smith D et al: Dietary restriction delays cataract and reduces ascorbate levels in Emory
mice. Exp Eye Res 61:55–62, 1995 78. Taylor A, Lipman RD, Jahngen-Hodge J et al: Dietary calorie restriction in the Emory mouse: Effects on lifespan, eye
lens cataract prevalence and progression, levels of ascorbate, glutathione, glucose, and
glycohemoglobin, tail collagen breaktime, DNA and
RNA oxidation, skin integrity, fecundity and cancer. Mech Ageing Dev 79:33–57, 1995 79. Levine M: New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med 314:892–902, 1986 80. Frei B, Stocker R, Ames BN: Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Nat Acad Sci USA 85:9748–9752, 1988 81. Berger J, Shepard D, Morrow F et al: Relationship between dietary intake and tissue levels of reduced and total
vitamin C in the guinea pig. J Nutr 119:1–7, 1989 82. Berger J, Shepard D, Morrow F et al: Reduced and total ascorbate in guinea pig eye tissues in response to dietary
intake. Curr Eye Res 7:681–686, 1988 83. Nakamura B, Nakamura O: Ufer das vitamin C in der Linse and dem Kammerwasser der menschlichen Katarakte. Graefes Arch Clin Exp Ophthalmol 134:197–200, 1935 84. Wilczek M, Zygulska-Machowa H: Zawartosc Witaminy C W: Roznych typackzaem. J Klin Oczna 38:477–480, 1968 85. Kosegarten DC, Mayer TJ: Use of guinea pigs as model to study galactose-induced cataract formation. J Pharm Sci 67:1478–1479, 1978 86. Vinson JA, Possanza CJ, Drack AV: The effect of ascorbic acid on galactose-induced cataracts. Nutr Rep Int 33:665–668, 1986 87. Devamanoharan PS, Henein M, Morris S et al: Prevention of selenite cataract by vitamin C. Exp Eye Res 52:563–568, 1991 88. Nishigori H, Lee JW, Yamauchi Y et al: The alteration of lipid peroxide in glucocorticoid-induced cataract of
developing chick embryos and the effect of ascorbic acid. Curr Eye Res 5:37–40, 1986 89. Blondin J, Baragi VJ, Schwartz E et al: Dietary vitamin C delays UV-induced age-related eye lens protein damage. Ann NY Acad Sci 498:460–463, 1987 90. Garland DD: Ascorbic acid and the eye. Am J Clin Nutr 54:1198S–1202S, 1991 91. Naraj RM, Monnier VM: Isolation and characterization of a blue fluorophore from human eye lens
crystallins: In vitro formation from Maillard action with ascorbate
and ribose. Biochim Biophys Acta 1116:34–42, 1992 92. Bensch KG, Fleming EE, Lohmann W: The role of ascorbic acid in senile cataract. Proc Nat Acad Sci USA 82:7193–7196, 1985 93. Vasan S, Zhang X, Zhang X et al: An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 382:275–278, 1996 94. Rathbun WB, Holleschau AM, Cohen JF et al: Prevention of acetaminophen- and naphthalene-induced cataract and glutathione
loss by CySSME. Invest Ophthalmol Vis Sci 37:923–929, 1996 95. Martenssen J, Steinhertz R, Jain A et al: Glutathione ester prevents buthionine sulfoximine-induced cataracts and
lens epithelial cell damage. Biochemistry 86:8727–8731, 1989 96. Rathbun WB, Killen CE, Holleschau AM et al: Maintenance of hepatic glutathione homeostasis and prevention of acetaminophen
induced cataract in mice by L-cysteine prodrugs. Biochem Pharmacol 51:1111–1116, 1996 97. Vina J, Perez C, Furukawa T et al: Effect of oral glutathione on hepatic glutathione levels on rats and mice. Br J Nutr 62:683–691, 1989 98. Clark JI, Livesey JC, Steele JE: Delay or inhibition of rat lens opacification using pantethine and WR-77913. Exp Eye Res 62:75–84, 1996 99. Congdon NG, Duncan DD, Fisher D et al: UV light and lenticular opacities in the Emory mouse. Invest Ophthalmol Vis Sci 38:S10–20, 1997 100. Srivastava S, Ansari NH: Prevention of sugar induced cataractogenesis in rats by butylated hydroxytoluene. Diabetes 37:1505–1508, 1988 101. Leske MC, Wu SY, Hyman L et al: Biochemical factors in the lens opacities. Case-control study. The Lens
Opacities Case-Control Study Group. Arch Ophthalmol 113:1113–1119, 1995 102. Schalch W, Weber P: Vitamins and carotenoids: A promising approach to reducing the risk of
coronary heart disease, cancer and eye diseases. Adv Exp Med Biol 366:335–350, 1994 103. Machlin LJ, Bendich A: Free radical tissue damage: Protective role of antioxidants. FASEB J 1:441–445, 1987 104. Costagliola C, Iuliano G, Menzione M et al: Effect of vitamin E on glutathione content in red blood cells, aqueous
humor and lens of humans and other species. Exp Eye Res 43:905–914, 1986 105. Yeum K-J, Taylor A, Tang G et al: Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci 36:2756–2761, 1995 106. Stevens RJ, Negi DS, Short SM et al: Vitamin E distribution in ocular tissues following long-term dietary depletion
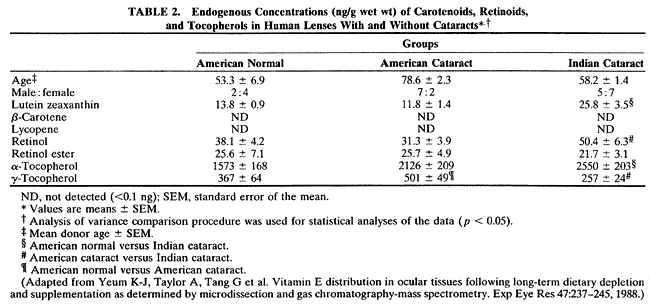
and supplementation as determined by microdissection and gas chromatography-mass
spectrometry. Exp Eye Res 47:237–245, 1988 107. Creighton MO, Ross WM, Stewart-DeHaan PJ et al: Modeling cortical cataractogenesis. VII: Effects of vitamin E treatment
on galactose induced cataracts. Exp Eye Res 40:213–222, 1985 108. Bhuyan DK, Podos SM, Machlin LT et al: Antioxidant in therapy of cataract. II: Effect of all roc-alpha-tocopherol (vitamin
E) in sugar-induced cataract in rabbits. Invest Ophthalmol Vis Sci 24:74, 1983 109. Bhuyan KC, Bhuyan DK: Molecular mechanism of cataractogenesis. III: Toxic metabolites of oxygen
as initiators of lipid peroxidation and cataract. Curr Eye Res 3:67–81, 1984 110. Hammond BR, Wooten BR, Snodderly DM: The density of the human crystalline lens is related to the macular pigment
carotenoids, lutein and zeaxanthin. Optom Vis Sci 74:499–504, 1997 111. Fridovich I: Oxygen: Aspects of its toxicity and elements of defense. Curr Eye Res 3:1–2, 1984 112. Giblin FJ, McReady JP, Reddy VN: The role of glutathione metabolism in detoxification of H2O2 in rabbit lens. Invest Ophthalmol Vis Sci 22:330–335, 1992 113. Eisenhauer DA, Berger JJ, Peltier CZ et al: Protease activities in cultured beef lens epithelial cells peak and then
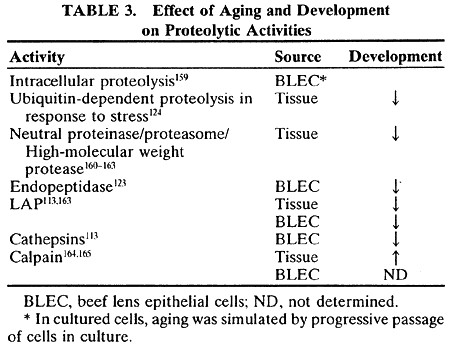
decline upon progressive passage. Exp Eye Res 46:579–590, 1988 114. Jahngen-Hodge J, Laxman E, Zuliani A et al: Evidence for ATP ubiquitin-dependent degradation of proteins in cultured
bovine lens epithelial cells. Exp Eye Res 52:341–347, 1991 115. Shang F, Taylor A: Oxidative stress and recovery from oxidative stress are associated with
altered ubiquitin conjugating and proteolytic activities in bovine lens
epithelial cells. Biochem J 307:297–303, 1995 116. Huang LL, Jahngen-Hodge J, Taylor A: Bovine lens epithelial cells have a ubiquitin-dependent proteolysis system. Biochim Biophys Acta 1175:181–187,1993 117. Jahngen JH, Lipman RD, Eisenhauer DA et al: Aging and cellular maturation cause changes in ubiquitin-eye lens protein
conjugates. Arch Biochem Biophys 276:32–37, 1990 118. Jahngen-Hodge J, Cyr D, Laxman E et al: Ubiquitin and ubiquitin conjugates in human lens. Exp Eye Res 55:897–902, 1992 119. Obin MS, Nowell T, Taylor A: The photoreceptor G-protein transducin (G1) is a substrate for ubiquitin-dependent proteolysis. Biochem Biophys Res Comm 200:1169–1176, 1994 120. Jahngen JH, Haas AL, Ciechanover A et al: The eye lens has an active ubiquitin protein conjugation system. J Biol Chem 261:13760–13767, 1986 121. Obin MS, Jahngen-Hodge J, Nowell T et al: Ubiquitinylation and ubiquitin-dependent proteolysis invertebratephotoreceptors (rod
outer segments): Evidence for ubiquitinylation of G, and
rhodopsin. J Biol Chem 271:14473–14484, 1996 122. Jahngen-Hodge J, Obin MS, Nowell TR Jr et al: Regulation of ubiquitin conjugating enzymes by glutathione following oxidative
stress. J Biol Chem 272:28218–28226, 1997 123. Shang F, Gong X, Palmer H et al: Age-related decline in ubiquitin conjugation in responses to oxidative
stresses in the lens. Exp Eye Res 64:21–30, 1997 124. Shang F, Gong X, Taylor A: Activity of ubiquitin-dependent pathway in response to oxidative stress: Ubiquitin-activating
enzyme is transiently unregulated.J Biol Chem 272:23086–23093, 1997 125. Shang F, Gong X, Taylor A: Changes in ubiquitin conjugation activities in young and old lenses in
response to oxidative stress. Invest Ophthalmol Vis Sci 36:S528, 1995 126. Mares-Perlman JA, Klein BEK, Klein R et al: Relationship between lens opacities and vitamin and mineral supplement
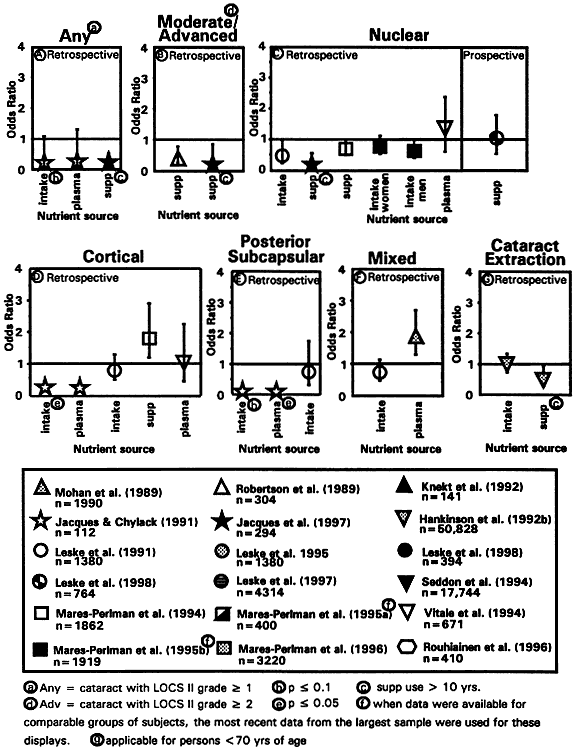
use. Ophthalmology 101:315–355, 1994 127. Robertson J McD, Donner AP, Trevithick JR: Vitamin E intake and risk for cataracts in humans. Ann N Y Acad Sci 570:372–382, 1989 128. Leske MC, Chylack LT Jr, Wu S: The lens opacities case-control study risk factors for cataract. Arch Ophthalmol 109:244–251, 1991 129. Mares-Perlman JA, Brady WE, Klein BEK et al: Diet and nuclear lens opacities. Am J Epidemiol 141:322–334, 1995b 130. Jacques PF, Chylack LT Jr: Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids
in cataract prevention. Am J Clin Nutr 53:3525-355S, 1991 131. Mohan M, Sperduto RD, Angra SK et al: India-US case-control study of age-related cataract. Arch Ophthalmol 107:670–676, 1989 132. Hankinson SE, Stampfer MJ, Seddon JM et al: Intake and cataract extraction in women: A prospective study. Br Med J 305:335–339, 1992 133. Vitale S, West S, Hallfrisch J et al: Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology 4:195–203, 1994 134. Jacques PF, Lahav M, Willett WC et al: Relationship between long-term vitamin
C intake and prevalence of cataract and macular degeneration. Exp
Eye Res 55(Suppl 1):5152, 1992 (abstract) 135. Knekt P, Heliovaara M, Rissanen A et al: Serum antioxidant vitamins and risk of cataract. Br Med J 305:1392–1394, 1992 136. Luthra R, Wa S-Y, Leske MC et al: Lens opacities and use of nutritional supplements: The Barbados study. Invest Ophthalmol Vis Sci 8:S450, 1997 137. Mares-Perlman JA, Brady WE, Klein BEK et al: Supplement use and 5-year progression of cortical opacities. Invest Ophthalmol Vis Sci 37:137, 1996 138. Rouhiainen P, Rouhiainen H, Salonen TJ: Association between low plasma vitamin E concentration and progression
of early cortical lens opacities. Am J Epidemiol 144:496–500, 1996 139. Leske MC, Chylack LT Jr, He Q et al: Antioxidant vitamins and nuclear opacities: The longitudinal study of cataract. Ophthalmology 105:831–836, 1998 140. Leske MC, Chylack LT Jr, He Q et al: Risk factors for a nuclear opalescence
in a longitudinal study. Am J Epidemiol Jan 1, 1998 141. Jacques PF, Taylor A, Hankinson SE et al: Long-term vitamin C supplement use and prevalence of early age-related
lens opacities. Am J Clin Nutr 66:911–916, 1997 142. Sperduto RD, Hu T-S, Milton RC et al: The Linxian Cataract Studies: Two nutrition intervention trials. Arch Ophthalmol 111:1246–1253, 1993 143. Libondi T, Menzione M, Auricchio G: In vitro effect of alpha-tocopherol on lysophosatiphatidylcholine-induced
lens damage. Exp Eye Res 40:661–666, 1985 144. Burton W, Ingold KU: Beta-carotene: An unusual type of lipid antioxidant. Science 224:569–573, 1984 145. Kwan M, Niinikoski J, Hunt TK: In vivo measurement of oxygen tension in the cornea, aqueous humor, and
the anterior lens of the open eye. Invest Ophthalmol Vis Sci 11:108–114, 1972 146. Erdman J: The physiologic chemistry of carotenes in man. Am J Clin Nutr 7:101–106, 1988 147. Di Mascio P, Murphy ME, Sies H: Antioxidant defense systems: The role of carotenoids, tocopherols and thiols. Am J Clin Nutr 53:194S–200S, 1991 148. Krinsky NI, Deneke SS: Interaction of oxygen and oxy-radicals with carotenoids. J Natl Cancer Inst 69:205–210, 1982 149. Micozzi MS, Beecher GR, Taylor HR et al: Carotenoid analyses of selected raw and cooked foods associated with a
lower risk for cancer. J Natl Cancer Inst 82:282–285, 1990 150. Daicker B, Schiedt K, Adnet JJ et al: Canthaxamin retinopathy. An investigation by light and electron microscopy
and physiochemical analyses. Graefes Arch Clin Exp Ophthalmol 225:189–197, 1987 151. Leske MC, Wu SY, Connell AMS et al: Lens opacities, demographic factors and nutritional supplements in the
Barbados Eye Study. Int J Epidemiol 26:1314–1322, 1997 152. Glynn RJ, Christen WG, Manson JAE et al: Body mass index. Arch Ophthalmol 113:1131–1137, 1995 153. Taylor A, Zuliani AM, Hopkins RE et al: Moderate caloric restriction delays cataract formation in the Emory mouse. FASEB J 3:1741–1746, 1989 154. Jacob RA, Otradovec CL, Russell RM et al: Vitamin C status and interactions in a healthy elderly population. Am J Clin Nutr 48:1436–1442, 1988 155. Harding JJ, van Heyningen R: Epidemiology and risk factors for cataract. Eye 1:537–541, 1987 156. McLaren DS: In: Nutritional Ophthalmology, 2nd ed. London: Academic Press, 1980 157. Hiller R, Giacometti L, Yuen K: Sunlight and cataract: An epidemiologic investigation. Am J Epidemiol 105:450–459, 1977 158. Taylor HR, West S, Munoz B et al: The long-term effects of visible light on the eye. Arch Ophthalmol 110:99–104, 1992 159. Taylor A, Berger J, Reddan J et al: Effects of aging in vitro on intracellular proteolysis in cultured rabbit
lens epithelial cells in the presence and absence of serum. In Vitro
Cell. Dev Biol 27A:287–292, 1991 160. Fleshman KR, Wagner BJ: Changes during aging in rats lens endopeptidase activity. Exp Eye Res 39:543–551, 1984 161. Ray K, Harris H: Purification of neutral lens endopeptidase: Close similarity to a neutral
proteinase in pituitary. Proc Natl Acad Sci USA 82:7545–7549, 1985 162. Murakami K, Jahngen JH, Lin S et al: Lens proteasome shows enhanced rates of degradation of hydroxyl radical
modified alpha-crystallin. Free Radic Biol Med 8:217–222, 1990 163. Taylor A, Brown MJ, Daims MA et al: Localization of leucine aminopeptidase in hog lenses using immunofluorescence
and activity assays. Invest Ophthalmol Vis Sci 24:1172–1181, 1983 164. Varnum MD, David LL, Shearer TR: Age-related changes in calpain II and calpastatin in rat lens. Exp Eye Res 49:1053–1065, 1989 165. Yoshida H, Yumoto N, Tsukahara I et al: The degradation of alpha-crystallin at its carboxyl-terminal portion by
calpain in bovine lens. Invest Ophthalmol Vis Sci 27:1269–1273, 1986 166. Wefers H, Sies H: The protection by ascorbate and glutathione against microsomal lipid peroxidation
is dependent on vitamin E. FEBS Lett 174:353–357, 1988 167. Burton GW, Wronska U, Stone L et al: Biokinetics of dietary RRR-alpha-tocopherol in the male guinea pig at three
dietary levels of vitamin C and two levels of vitamin E: Evidence
that vitamin C does not “spare” vitamin E in vivo. Lipids 25:199–210, 1990 168. Chen S: A protective role for glutathione-dependent reduction of dehydroascorbic
acid in lens epithelium. Invest Ophthalmol Vis Sci 36:1804, 1995 169. Sasaki H, Giblin FJ, Winkler BS et al: Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr 58:103–105, 1993 170. Bohm F, Edge R, Land EJ et al: Carotenoids enhance vitamin E antioxidant efficiency. J Am Chem Soc 119:621–622, 1997 171. Valgimigli L, Lucarini M, Pedulli GF et al: Does beta-carotene really protect vitamin E from oxidation? J Am Chem Soc 119:8095–8096, 1997 |