
|
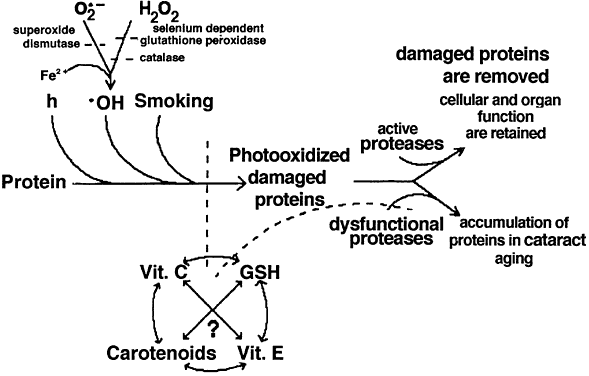
| Fig. 3. Proposed interaction between lens proteins, oxidants, light, smoking, antioxidants, antioxidant enzymes, and proteases. Lens proteins are extremely long lived. Lens proteins are subject to alteration by light and various forms of oxygen. They are protected indirectly by antioxidant enzymes: superoxide dismutase, catalase, and glutathione reductase/peroxidase. These enzymes convert active oxygen to less-damaging species. Direct protection is offered by antioxidants: glutathione (GSH), ascorbate (vita-min C), tocopherol (vitamin E), and carotinoids. Levels of reduced and oxidized forms of some, but perhaps not all (?), of these molecules are determined by interaction among the three and with the environment.166–171 Proteins that are damaged may accumulate and precipitate in cataract if there is insufficient proteolytic capability. When the proteolytic capability is sufficient, obsolete and damaged proteins may be reduced to their constituent amino acids. On aging, some of the eye antioxidant supplies are diminished, antioxidant enzymes inactivated, and proteases less active. This appears to be related to the accumulation, aggregation, and eventual precipitation in cataractous opacities of damaged proteins. (Taylor A, Dorey CK, Nowell T Jr. Oxidative stress and ascorbate in relation to risk for cataract and age-related maculopathy. In Packer L, Fuchs J [eds]: Vitamin C in Health and Disease. New York: Marcel Dekker, 1997.) |