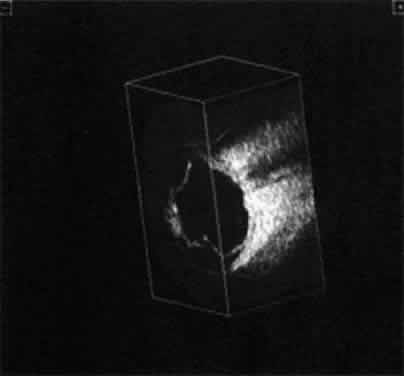
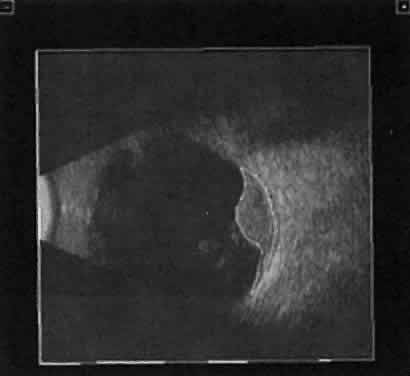
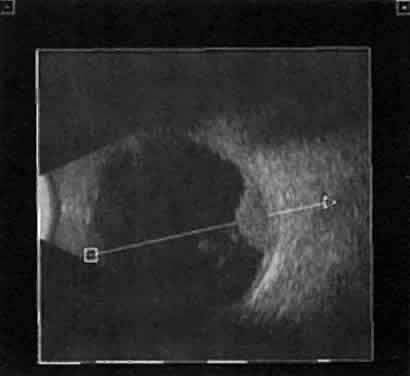
| We prefer simultaneous B-scan and A-scan imaging, relying on both B-scan
gray scale and A-scan amplitude interpretation for ultrasound tissue
characterization. Tumor height is usually calculated from A-scan images; this
is an important measurement for initial and follow-up examination.10 TUMORS A-scan tumor characterization is often extremely helpful to the clinician. A
thorough understanding of ophthalmic pathology is critical to interpretation
and often predictive of typical A-scan tumor patterns. Choroidal malignant melanoma, perhaps the most widely studied intraocular
mass lesion, has the most reproducible and reliable A-scan pattern. Usually, the
initial echo seen in A-scan is a high-amplitude spike secondary
to the strong vitreoretinal surface echo overlying the tumor mass. Once
the examining sonic beam has passed into the tumor tissue, a
rapidly declining amplitude cadence is noted, a consequence of increasing
ultrasonic tissue homogeneity. Clinical knowledge of the typical
microscopic tumor pattern of tightly packed, homogeneous small cells makes
anticipation of relatively low reflectivity possible (Fig. 10). This same low-amplitude reflectivity in B-scan imaging produces a picture
that makes the melanoma mass appear hollow. Often, tumor-infiltrated
choroid also appears dark (Fig. 11). This change in the normally highly reflective choroidal tissue is widely
but inaccurately called choroidal excavation. The terms “hollowing” and “choroidal excavation” are misleading because
these tumors are not hollow and the choroid is not excavated. Nevertheless, these
terms have been used so frequently in past literature
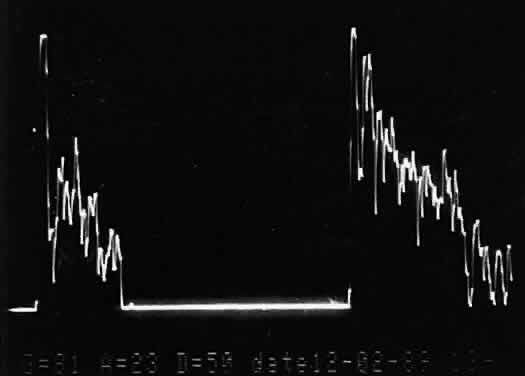
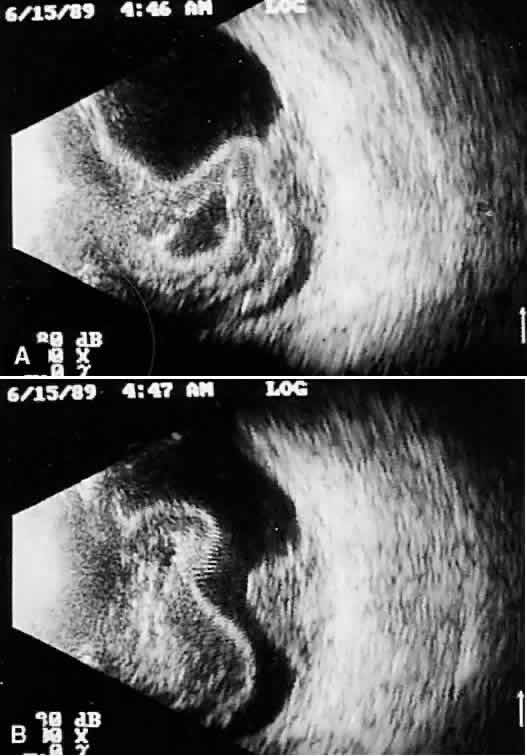
that any change in vocabulary is unlikely. 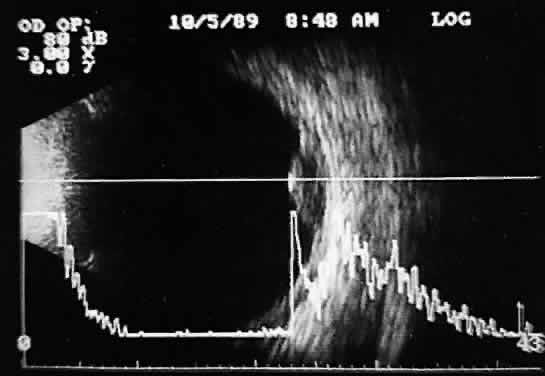 Fig. 10. Contact B-scan and simultaneous A-scan: choroidal malignant melanoma. Note
the strong initial echo from the overlying retinal tissue, followed
by a rapid decline in A-scan echo amplitude within the deeper tumor
tissue, a consequence of increasingly homogeneous tissue. High reflectivity
is again seen at the level of the sclera and orbit. Fig. 10. Contact B-scan and simultaneous A-scan: choroidal malignant melanoma. Note
the strong initial echo from the overlying retinal tissue, followed
by a rapid decline in A-scan echo amplitude within the deeper tumor
tissue, a consequence of increasingly homogeneous tissue. High reflectivity
is again seen at the level of the sclera and orbit.
|
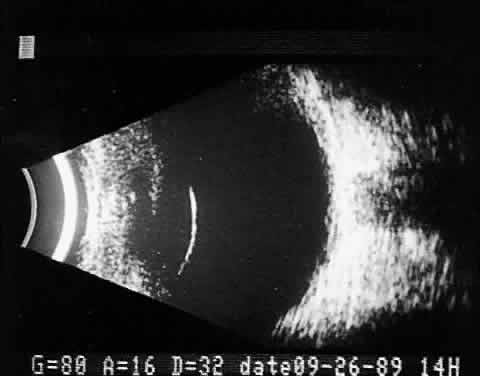
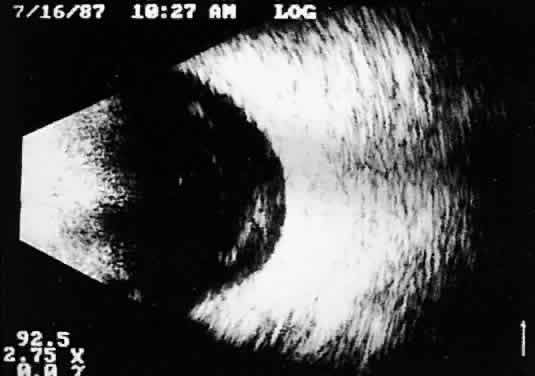
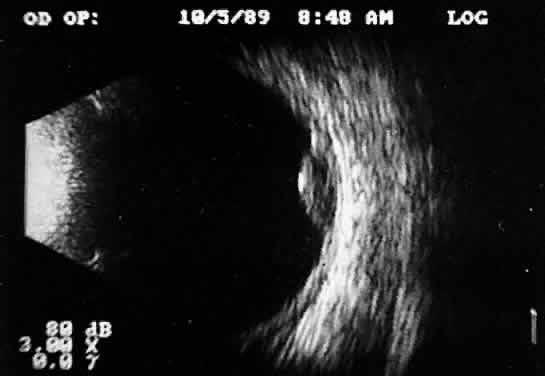 Fig. 11. Contact B-scan: malignant melanoma, demonstrating hollowing and choroidal
excavation. Fig. 11. Contact B-scan: malignant melanoma, demonstrating hollowing and choroidal
excavation.
|
Tumors with great acoustic heterogeneity, such as choroidal hemangiomas, where
adjoining cell and tissue layers have marked differences in acoustic
impedance, create large echo amplitudes at each interface. These
tumor types have typical high internal reflections at each major interface. These
high internal reflections make the lesions appear solid
white in B-scan displays and produce highamplitude spikes during A-scan
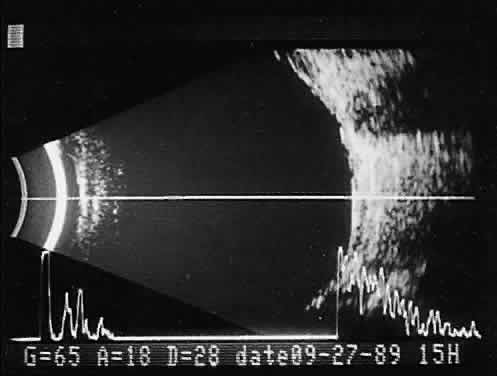
imaging (Fig. 12). 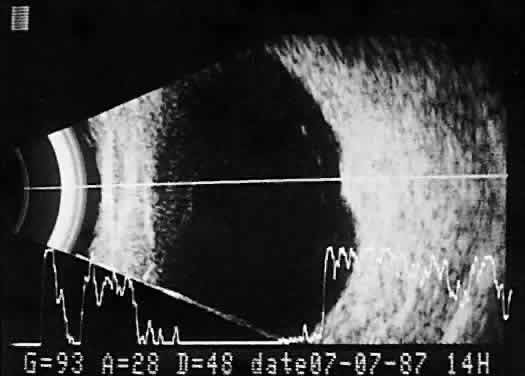 Fig. 12. Contact B-scan and simultaneous A-scan: choroidal hemangioma. Ultrasonically
heterogeneous tissue shows strong reflectivity at all levels, appearing
white in B-scan gray scale. Fig. 12. Contact B-scan and simultaneous A-scan: choroidal hemangioma. Ultrasonically
heterogeneous tissue shows strong reflectivity at all levels, appearing
white in B-scan gray scale.
|
Calcification in any type of tumor tissue creates a strong acoustic interface, resulting
in high-amplitude A-scan patterns as well as white echoes
in B-scan imaging. Behind the area of calcification, there is usually
partial or complete shadowing of the sclera and orbital fat. Bony
tumors of the choroid, some retinoblastomas, and drusen of the optic
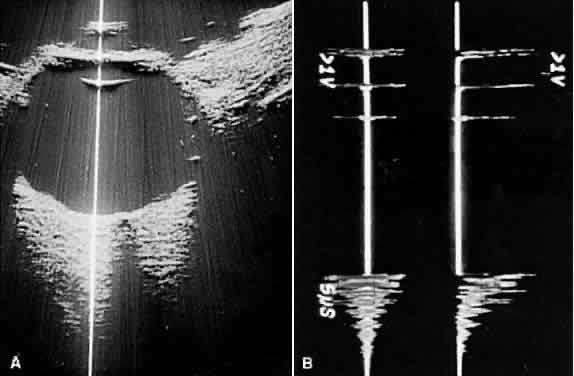
nerve head are typical examples where calcification may be found (Fig. 13). 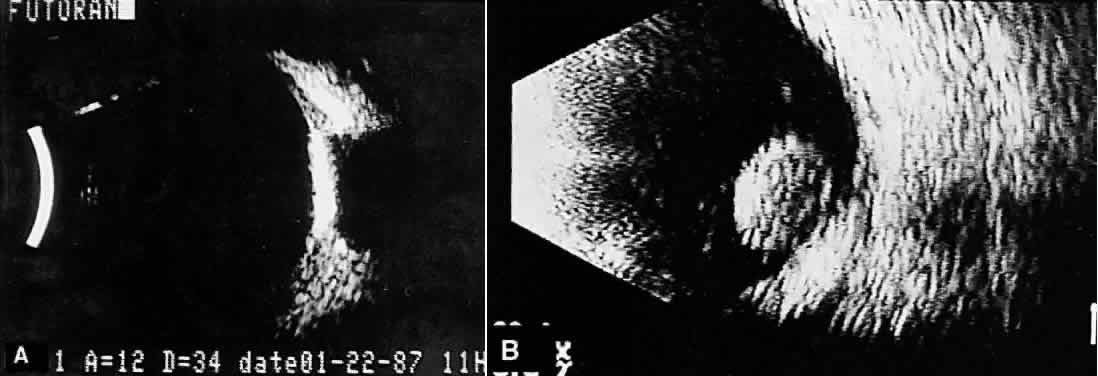 Fig. 13. Contact B-scans. A. Choroidal osteoma, demonstrating orbital tissue shadowing. B. Dislocated lens. Note shadowing of orbital tissues directly behind the
highly reflective lens. Fig. 13. Contact B-scans. A. Choroidal osteoma, demonstrating orbital tissue shadowing. B. Dislocated lens. Note shadowing of orbital tissues directly behind the
highly reflective lens.
|
Unfortunately, there are far more tumor types than there are different
ultrasound patterns, and many mass lesions, both benign and malignant, have
similar ultrasound appearances. Further, the echo patterns change
from one area to another within the same mass lesion. Although much
rhetoric swirls in the ophthalmic literature concerning the quality of
a variety of instruments, the techniques used, and the expertise of the
examiner, the ultrasonic patterns of many tumors simply do not demonstrate
differentiating features. In such situations, the ultrasound examiner
must hedge the diagnosis, compiling a list of possibilities rather
than one. On occasion, interpretation is simply not possible with
any degree of reliability. Some ultrasonographers prefer to use A-scan alone without simultaneous
B-scan imaging for their tissue pattern interpretation.11 Separate A-scan units, some of which are packaged as an additional module
housed in a simultaneous B-scan and A-scan instrument, are available. The
separate units use modified amplifiers and are standardized against
special test phantoms to permit comparison of results from one device
to another.12,13 TRAUMA AND FOREIGN BODY EVALUATIONS Ultrasound examinations for ocular trauma and intraocular foreign bodies
are among the most difficult, for a variety of reasons (Figs. 14 and 15). The examiner is often presented with recently injured, unstable, or
open globes with multiple complex injuries. Extreme care is necessary
to avoid undue pressure during ultrasound evaluation, and concerns for
contamination are significant. Understandably, probe contact is often
minimized, and unless additional sterile methylcellulose solution is
used to improve signal transfer, less-than-optimal images result. These
poor-quality images are difficult or impossible to interpret, especially
in opaque media situations with multiple abnormalities. Further, patient
noncompliance and the examiner's inexperience frequently
lead to incomplete examination, limiting 3D analysis. 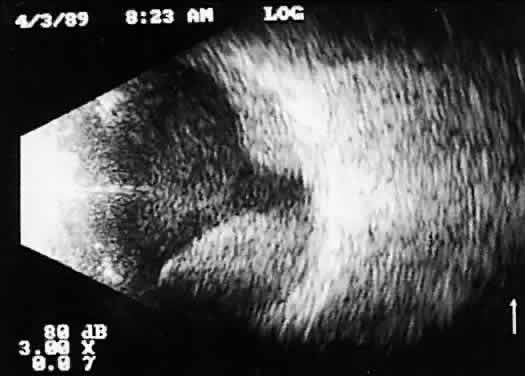 Fig. 14. Contact B-scan: choroidal hemorrhage/vitreous hemorrhage secondary to blunt
trauma. Fig. 14. Contact B-scan: choroidal hemorrhage/vitreous hemorrhage secondary to blunt
trauma.
|
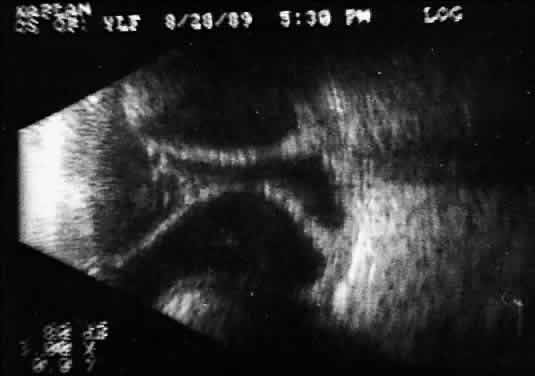 Fig. 15. Contact B-scan: large choroids (anteroposterior view) and portion of scleral
implant (at bottom of display screen). Fig. 15. Contact B-scan: large choroids (anteroposterior view) and portion of scleral
implant (at bottom of display screen).
|
Despite the difficulties, there are several helpful concepts that aid in
the examination of trauma cases: - Always examine the globe visually (by slit-lamp technique preferably before
ultrasonography) to determine if ocular integrity has been disrupted
anteriorly. The possibility of a posterior nonvisible ocular wall
rupture must also be considered.
- Clean the examining probe with soap, water, and alcohol, and use skin contact
rather than scleral contact whenever possible.
- Ultrasonically examine the normal globe first to gain patient confidence.
- Use sterile methylcellulose solution to permit good signal transfer with
less probe contact pressure.
- Take your time and be extremely gentle.
- Consider every trauma patient a potential foreign body patient.
- Radiography for foreign bodies should be part of every evaluation of ocular
trauma resulting in opaque media and preferably should be done before
ultrasonography.
Ocular and orbital foreign body patients require the most careful ultrasonic
evaluation, as well as multiple ancillary tests, to provide sufficient
diagnostic information. Like ocular trauma, these injuries usually
occur unexpectedly and involve high-velocity projectiles. Patient
histories are understandably poor or intentionally misleading. Because
most foreign bodies are metallic and visible radiographically, appropriate
head position for anteroposterior and lateral x-rays of the involved
eye and orbit is critical. Routine radiographic imaging quickly supplies
information concerning the number, size, and shape of metallic
foreign bodies, as well as the presence of any intraocular air. Small
bubbles that enter the globe at the time of injury and remain within
the vitreous can be confused ultrasonically with small metallic foreign
bodies. The large acoustic impedance mismatch between vitreous and air
is similar to that between vitreous and metal. Computed tomography scans are extremely helpful in foreign body localization, although
it is often difficult to pinpoint the exact intraocular
or extraocular position of metallic foreign bodies close to the ocular
wall. Metal detection devices add information about the magnetic or
nonmagnetic properties of intraocular metallic foreign bodies. Until
the magnetic qualities of any potential intraocular foreign bodies are
determined, magnetic resonance imaging should not be performed. Ultrasound evaluation in patients with intraocular foreign bodies provides
extremely useful additional information concerning associated ocular
injuries and another method for localization, especially with nonmetallic
intraocular foreign bodies not visible using x-ray techniques. Ultrasonically, foreign
bodies have great reflectivity once the examining
beam is placed perpendicular to a reflective surface of the foreign
body. These abnormalities remain visible even with extreme attenuation
of the examining signal (Fig. 16). Many metallic foreign bodies, especially those that are round or spherical, demonstrate “ringing,” a string of reflections that
extend posterior to the foreign body in the form of a cometlike tail. Ringing
is an ultrasound artifact produced by multiple “ping-pong” reflections
of sound pulses within the foreign body before
they return to the examining probe. The string of returning echoes produces
an unusual display image. 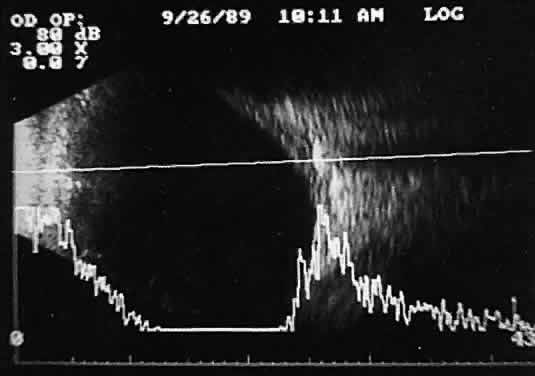 Fig. 16. Contact B-scan and simultaneous A-scan: “buried” choroidal
metallic foreign body. Note strong B-scan gray-scale image and high A-scan
echo amplitude. Fig. 16. Contact B-scan and simultaneous A-scan: “buried” choroidal
metallic foreign body. Note strong B-scan gray-scale image and high A-scan
echo amplitude.
|
When combined with radiographic information and metal localization technique, ultrasonically
derived information aids enormously in the clinical
evaluation of the patient with an intraocular foreign object. However, ultrasound
alone is not sufficient to rule out a foreign object. PHTHISIS Once the globe has become small and shrunken, ultrasound evaluation becomes
nearly impossible. Often, calcifications of a variety of ocular tissues
make signal transfer difficult. General landmarks become obscure, and
interpretation is unreliable. Small tumor identification in such
situations is usually beyond the scope of reason. ORBITAL ULTRASONOGRAPHY Orbital ultrasonography is far more complex than ocular evaluation for
a number of reasons. The shape and depth of the orbit make access for
diagnostic ultrasound techniques more difficult. Ocular examinations are
usually performed with 10-megacycle probes or higher frequencies. Lower
ultrasound frequencies (5 to 8 megacycles) are often used to penetrate
to orbital tissue depths. Lower-frequency probes have less resolution
capability. Further, recognition of abnormalities within orbital
tissues is more difficult because orbital tissues are highly reflective, making
the appearance of subtle gray-scale changes in B-scan or amplitude
changes in A-scan more difficult to appreciate against highly
reflective tissues. Nevertheless, a vast quantity of literature is available
concerning orbital technique and tissue characterization.8,9,14,15 In general, orbital ultrasonography is most useful in the evaluation of
patients with exophthalmos related to a number of diseases, including
primary and secondary tumors of the orbit, inflammatory diseases, and
changes secondary to thyroid disease. The displacement of orbital fat by more homogeneous tumor tissue is appreciated
more readily in B-scan imaging. Tissue differentiation is better
appreciated with simultaneous A-scan or standardized A-scan techniques. Two
caveats are important: orbital interpretation is not for the
novice, and examination of the deeper portions of the orbit, including
the orbital apex, is usually best performed by other techniques, such
as computed tomography or magnetic resonance imaging. Inflammatory conditions
such as pseudotumor can involve a variety of orbital tissues, causing
infiltration and thickening of the extraocular muscles, as well
as space-occupying lesions within the orbital fat. Frequently, inflammatory
exudate accumulates in the potential space between Tenon's
capsule and the sclera. Such accumulations are easily detected during
ultrasonography as dark, relatively echo-free spaces just outside
the strong scleral echo. Thyroid orbitopathy and exophthalmos are also frequently associated with
thickening of the extraocular muscles, as well as other changes of the
orbital contents. POSTERIOR VITREORETINAL INTERFACE EVALUATION Recent improvements in image quality and fused, real-time display have
made ultrasound image interpretation easier for every ultrasonographer. Visualization
of subtle changes such as movement and recognition of
the posterior formed vitreous hyaloid are now possible, even in clear
media situations. These clear vitreous structures, which are often exceedingly
difficult to appreciate optically, can be recognized ultrasonically
after a relatively short period of training (Fig. 17). Clinically, establishing the position of the posterior hyaloid is important
in evaluating a variety of vitreous retinal disorders, such as
macular holes, tractional detachments, and partial or complete vitreous
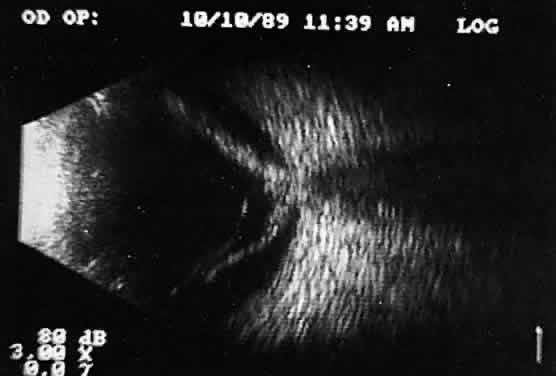
separations.16,17 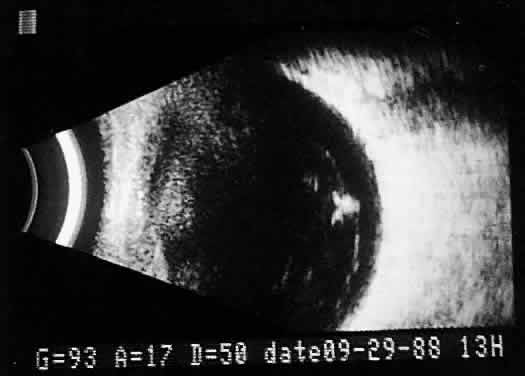 Fig. 17. Contact B-scan: posterior formed vitreous face separation, with prominent
Weiss ring evident. Fig. 17. Contact B-scan: posterior formed vitreous face separation, with prominent
Weiss ring evident.
|
|