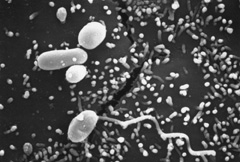
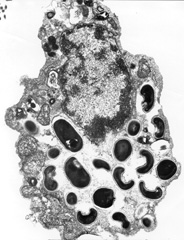
1. Wittner M, Weiss LM: The Microsporidia and Microsporidiosis, Washington, D.C.: ASM Press, 1999 2. Ashton N, Wirasinha PA: Encephalitozoonosis (Nosematosis) of the cornea. Br J Ophthalmol 57:669, 1973 3. Franzen C, Muller A: Microsporidiosis: human diseases and diagnosis. Microbes Infect. 3:389, 2001 4. Lewis NL, Francis IC, Hawkins GS, et al: Bilateral microsporidial keratoconjunctivitis in an immunocompetent non-contact
lens wearer. Cornea 22:374, 2003 5. Theng J, Chan C, Ling ML, et al: Microsporidial keratoconjunctivitis in a healthy contact lens wearer without
human immunodeficiency virus infection: Ophthalmology 108:976, 2001 6. Lowder CY, Meisler DM, McMahon JT, et al: Microsporidia infection of the cornea in a man seropositive for human immunodeficiency
virus. Am J Ophthalmol 109:242, 1990 7. Didier ES, Didier PJ, Friedburg DN, et al: Isolation and characterization of a new human microsporidian, Encephalitozoon
hellem, from three AIDS patients with keratoconjunctivitis. J Infect Dis 163:617, 1991 8. Friedberg DN, Stenson SM, Orenstein JM, et al: Microsporidial keratoconjunctivitis in acquired immunodeficiency syndrome. Arch Ophthalmol 108:504, 1990 9. Mietz H, Franzen C, Hoppe T, et al: Microsporidia-induced sclerouveitis with retinal detachment. Arch Ophthalmol 120:864, 2002 10. Keeling PJ, Fast NM: Microsporidia: Biology and evolution of highly reduced intracellular parasites. Annu Rev Microbiol 56:93, 2002 11. Katinka MD, Duprat S, Cornillot E, et al: Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450, 2001 12. Hirt RP, Logsdon JM, Healy B, et al: Microsporidia are related to fungi: Evidence from the largest subunit of
RNA polymerase II and other proteins. Proc Natl Acad Sci 96:580, 1999 13. Williams BAP, Hirt RP, Lucocq JM, et al: A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418:865, 2002 14. Cotte L, Rabodonirina M, Chapuis F, et al: Waterborne outbreak of intestinal microsporidiosis in persons with and
without human immunodeficiency virus infection. J Infect Dis 180:2003, 1999 15. Dowd SE, Gerba CP, Pepper IL: Confirmation of human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol 64:3332, 1998 16. Enriquez FJ, Taren D, Cruz-Lopez A, et al: Prevalence of intestinal Encephalitozoonosis in Mexico. Clin Infect Dis 26:1227, 1998 17. Dowd SE, John D, Eliopolus J, et al: Confirmed detection of Cyclospora cayetanesis, Encephalitozoon intestinalis, and Cryptosporidium parvum in water used for drinking. J Water Health 1:117, 2003 18. Weiss LM: …And now microsporidiosis. Ann Intern Med 123:955, 1995 19. Silverstein BE, Cunningham ET, Margolis TP, et al: Microsporidial keratoconjunctivitis in a patient without human immunodeficiency
virus infection. Am J Ophthalmol 124:395, 1997 20. Cali A, Meisler DM, Lowder CY, et al: Corneal microsporidioses: characterization and identification. J Protozool 38:215S, 1991 21. Moon SJ, Mann PM, Matoba AY: Microsporidial keratoconjunctivitis in a healthy patient with a history
of LASIK surgery. Cornea 22:271, 2003 22. Bigliardi E, Sacchi L: Cell biology and invasion of the microsporidia. Microbes Infect 3:373, 2001 23. Franzen C: Microsporidia: How can they invade other cells? Trends Parasitol. 20:275, 2004 24. Didier ES, Snowden KF, Shadduck JA: Biology of microsporidian species infecting mammals. Adv Parasitol 40:283, 1998 25. Snowden KF, Didier ES, Orenstein JM, et al: Animal models of human microsporidial infections. Lab Anim Sci. 48:589, 1998 26. Braunfuchsova P, Salat J, Kopecky J: Comparison of the significance of CD4+ and CD8+ T lymphocytes
in the protection of mice against Encephalitozoon cuniculi infection. J Parasitol 88:797, 2002 27. Orenstein JM: Diagnostic pathology of microsporidiosis. Ultrastruct Pathol 27:141, 2003 28. Garcia LS: Laboratory identification of the microsporidia. J Clin Microbiol 40:1892, 2002 29. Conners MS, Gibler TS, Van Gelder RN: Diagnosis of microsporidia keratitis by polymerase chain reaction. Arch Ophthalmol 122:283, 2004 30. Diesenhouse MC, Wilson LA, Corrent GF, et al: Treatment of microsporidial keratoconjunctivitis with topical fumagillin. Am J Ophthalmol 115:293, 1993 31. Rosberger DF, Serdarevic ON, Erlandson RA, et al: Successful treatment of microsporidial keratoconjunctivitis with topical
fumagillin in a patient with AIDS. Cornea 12:261, 1993 32. Gross U: Treatment of microsporidiosis including albendazole. Parasitol Res 90:S14, 2003 33. Molina JM, Goguel J, Sarfati C, et al: Trial of oral fumagillin for the treatment of intestinal microsporidiosis
in patients with HIV infection. AIDS 14:1341, 2000 34. Rossi P, Urbani C, Donelli G, et al: Resolution of microsporidial sinusitis and keratoconjunctivitis by itraconazole
treatment. Am J Ophthalmol 127:210, 1999 35. Visvesvara GS, Belloso M, Moura H, et al: Isolation of Nosema algerae from the cornea of an immunocompetent patient. J Eukaryot Microbiol 46:10S, 1999 36. Canning EU, Curry A, Vavra J, et al: Some ultrastructural data on Microsporidium ceylonensis, a cause of corneal microsporidiosis. Parasite 5:247, 1998 37. Rauz S, Tuft S, Dart JK, et al: Ultrastructural examination of two cases of stromal microsporidial keratitis. J Med Microbiol 53:775, 2004 |