1. Whitcup S: The double-edged ocular immune response: The Cogan lecture. Invest Ophthalmol Vis Sci 41:3243, 2000 2. Streilein J, Stein-Streilein J: Does innate immune privilege exist? J Leukoc Biol 67:479, 2000 3. Streilein J: Regional immunity and ocular immune privilege. Chem Immunol 73:11, 1999 4. Pleyer U, Baatz H: Antibacterial protection of the ocular surface. Ophthalmologica 211(Suppl 1):2, 1997 5. Klotz S, Penn C, Negvesky G et al: Fungal and parasitic infections of the eye. Clin Microbiol Rev 13:662, 2000 6. Nassif K: Ocular surface defense mechanisms. In Tabbara K, Hyndiuk R (eds). Infections
of the Eye. New York: Little, Brown and Company, 1996, p 35 7. Kernacki K, Barret R, Hazlett L: Evidence for TIMP-1 protection against P. aeruginosa-induced corneal ulceration and perforation. Invest Ophthalmol Vis Sci 40:3168, 1999 8. Thakur A, Kyd J, Xue M et al: Effector mechanisms of protection against Pseudomonas aeruginosa keratitis in immunized rats. Infect Immun 69:3295, 2001 9. Rhem M, Lech E, Patti J et al: The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect Immun 68:3776, 2000 10. Hein W: Organization of mucosal lymphoid tissue. In Kraehenbuhl J, Neutra
M (eds). Defense of Mucosal Surfaces: Pathogenesis, Immunity, and Vaccines. Berlin: Springer-Verlag, 1999, p 1 11. Kraehenbuhl J, Neutra M: Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev 72:853, 1992 12. Streilein J: Regional immunology of the eye. In Pepose J, Holland G, Wilhelmus
K (eds). Ocular Infection and Immunity. St Louis: Mosby, 1996, p 19 13. McClellan K: Mucosal defense of the outer eye. Surv Ophthalmol 42:233, 1997 14. Knop N, Knop E: Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci 41:1270, 2000 15. Chodosh J, Nordquist R, Kennedy R: Comparative anatomy of mammalian conjunctival lymphoid tissue: A putative
mucosal immune site. Dev Comp Immunol 22:621, 1998 16. Knop E, Knop N: Lacrimal drainage-associated lymphoid tissue (LDALT): A part of the human
mucosal immune system. Invest Ophthalmol Vis Sci 42:566, 2001 17. Saitoh-Inagawa W, Hiroi T, Yanagita M et al: Unique characteristics of lacrimal glands as a part of mucosal immune network: High
frequency of IgA-committed B-1 cells and NK1.1+ αβ T
cells. Invest Ophthalmol Vis Sci 41:138, 2000 18. Brinser J, Burd E: Principles of diagnostic ocular microbiology. In Tabbara
K, Hyndiuk R (eds). Infections of the Eye. New York: Little, Brown
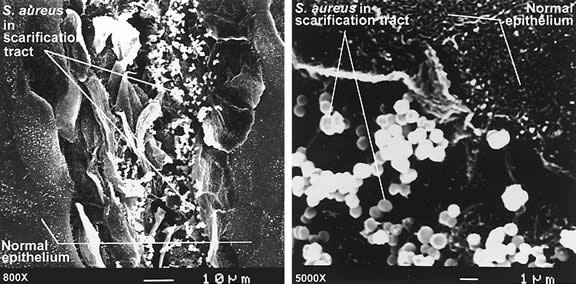
and Company, 1996, p 69 19. Armstrong R: The microbiology of the eye. Ophthalmic Physiol Opt 20:429, 2000 20. Argueso P, Gipson I: Epithelial mucins of the ocular surface: Structure, biosynthesis and function. Exp Eye Res 73:281, 2001 21. Strous G, Dekker J: Mucin-type glycoproteins. Crit Rev Biochem Mol Biol 27:57, 1992 22. Pflugfelder S, Liu Z, Monroy D et al: Detection of sialomucin complex (MUC4) in human ocular surface epithelium
and tear fluid. Invest Ophthalmol Vis Sci 41:1316, 2000 23. McKenzie R, Jumblatt J, Jumblatt M: Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci 41:703, 2000 24. Zhao H, Jumblatt J, Wood T et al: Quantification of MUC5AC protein in human tears. Cornea 20:873, 2001 25. Berry M, Ellingham R, Corfield A: Membrane-associated mucins in normal human conjunctiva. Invest Ophthalmol Vis Sci 41:398, 2000 26. Danjo Y, Watanabe H, Tisdale A et al: Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci 39:2602, 1998 27. Rolando M, Zierhut M: The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45(Suppl 2):S203, 2001 28. Kardon R, Price R, Julian J et al: Bacterial conjunctivitis in Muc1 null mice. Invest Ophthalmol Vis Sci 40:1328, 1999 29. Aho H, Saari K, Kallajoki M et al: Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci 37:1826, 1996 30. Qu X, Lehrer R: Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive
bacteria in human tears. Infect Immun 66:2791, 1998 31. Saari KM, Aho V, Paavilainen V et al: Group II PLA(2) content of tears in normal subjects. Invest Ophthalmol Vis Sci 42:318, 2001 32. Moreau J, Girgis D, Hume E et al: Phospholipase A2 in rabbit tears: A host defense against Staphylococcus aureus. Invest Ophthalmol Vis Sci 42:2347, 2001 33. Asensi V, Fierer J: Synergistic effect of human lysozyme plus ampicillin or beta-lysin on the
killing of Listeria monocytogenes. J Infect Dis 163:574, 1991 34. Janssen P, Muytjens H, Van Bijsterveld O: Non lysozyme antibacterial factor in human tears. Fact or fiction? Invest Ophthalmol Vis Sci 25:1156, 1984 35. Xiong Y, Yeaman M, Bayer A: In vitro antibacterial activities of platelet microbicidal protein and
neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother 43:1111, 1999 36. Paulsen F, Pufe T, Schaudig U et al: Detection of natural peptide antibiotics in human nasolacrimal ducts. Invest Ophthalmol Vis Sci 42:2157, 2001 37. Fujihara T, Hayashi K: Lactoferrin inhibits herpes simplex virus type-1 (HSV-1) infection to mouse
cornea. Arch Virol 140:1469, 1995 38. Comerie-Smith S, Nunez J, Hosmer M et al: Tear lactoferrin levels and ocular bacterial flora in HIV positive patients. Adv Exp Med Biol 350:339, 1994 39. Martin E, Ganz T, Lehrer R: Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol 58:128, 1995 40. Haynes R, Tighe P, Dua H: Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol 83:737, 1999 41. Hattenbach L, Gumbel H, Kippenberger S: Identification of beta-defensins in human conjunctiva. Antimicrob Agents Chemother 42:3332, 1998 42. Lehmann O, Hussain I, Watt P: Investigation of β-defensin gene expression in the ocular anterior
segment by semiquantitative RT-PCR. Br J Ophthalmol 84:523, 2000 43. Paulsen F, Pufe T, Schaudig U et al: Detection of natural peptide antibiotics in human nasolacrimal ducts. Invest Ophthalmol Vis Sci 42:2157, 2001 44. Chandler J: Ocular surface immunology. In Pepose J, Holland G, Wilhelmus
K (eds).Ocular Infection and Immunity. St Louis: Mosby, 1996, p 104 45. Rudner X, Kernacki K, Barrett R et al: Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear
neutrophil persistence, and corneal perforation. J Immunol 164:6576, 2000 46. Stumpf T, Shimeld C, Easty D et al: Cytokine production in a murine model of recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci 42:372, 2001 47. Hall L, Diaconu E, Patel R et al: CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is
essential for neutrophil recruitment to the cornea in helminth-mediated
keratitis (river blindness). J Immunol 166:4035, 2001 48. Waldrep J, Mondino B: Humoral immunity and the eye. In Pepose J, Holland
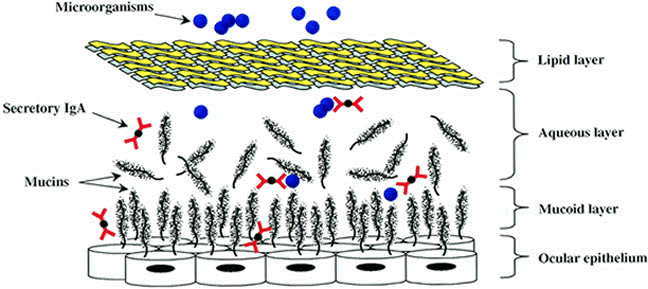
G, Wilhelmus K (eds). Ocular Infection and Immunity. St Louis: Mosby, 1996, p 33 49. Mondino B, Pleyer U: Host defense against bacterial and fungal disease. In
Tasman W, Jaeger E (eds.). Foundations of Clinical Ophthalmology, vol 2. Baltimore: Lippincott, Williams, & Wilkins, 1995, p 1 50. Willcox M, Morris C, Thakur A et al: Complement and complement regulatory proteins in human tears. Invest Ophthalmol Vis Sci 38:1, 1997 51. Mondino B, Chou H, Sumner H: Generation of complement membrane attack complex in normal human corneas. Invest Ophthalmol Vis Sci 37:1576, 1996 52. Sohn J, Kaplan H, Suk H et al: Complement regulatory activity of normal human intraocular fluid is mediated
by MCP, DAF, and CD59. Invest Ophthalmol Vis Sci 41:4195, 2000 53. Cocuzzi E, Szczotka L, Brodbeck W et al: Tears contain the complement regulator CD59 as well as decay-accelerating
factor (DAF). Clin Exp Immunol 123:188, 2001 54. Sohn J, Kaplan H, Suk H et al: Chronic low level complement activation within the eye is controlled by
intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci 41:3492, 2000 55. Bora N, Gobleman C, Atkinson J et al: Differential expression of the complement regulatory proteins in the human
eye. Invest Ophthalmol Vis Sci 34:3597, 1993 56. Cleveland R, Hazlett L, Leon M et al: Role of complement in murine corneal infection caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci 24:237, 1983 57. Hexham J, White K, Carayannopoulos L et al: A human immunoglobulin (Ig)A c-alpha-3 domain motif directs polymeric Ig
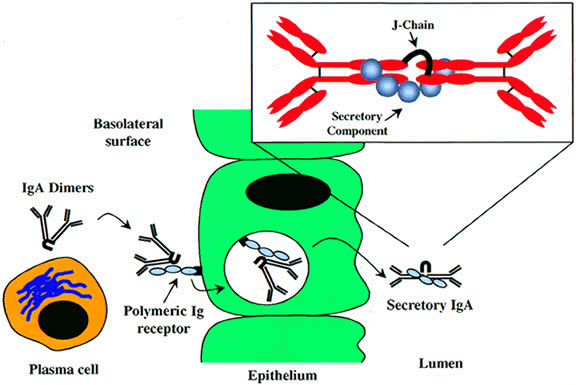
receptor-mediated secretion. J Exp Med 189:747, 1999 58. Leher H, Alizadeh H, Taylor W et al: Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 39:2666, 1998 59. Meek B, Klaren V, Van Haeringen N et al: IgA antibodies to Toxoplasma gondii in human tears. Invest Ophthalmol Vis Sci 41:2584, 2000 60. Hayashi K: Local immune responses in ocular virus infection and their implications
for future immunotherapy. Ophthalmologica 211(Suppl 1):45, 1997 61. Stahl J, Cook E, Graziano F et al: Human conjunctival mast cells: Expression of Fc epsilon RI, c-kit, ICAM-1, and
IgE. Arch Ophthalmol 117:493, 1999 62. Dekaris I, Zhu S, Dana M: TNF-alpha regulates corneal Langerhans cell migration. J Immunol 162:4235, 1999 63. Kaifi J, Hall L, Diaz C et al: Impaired eosinophil recruitment to the cornea in P-selectin-deficient mice
in Onchocerca volvulus keratitis (river blindness). Invest Ophthalmol Vis Sci 41:3856, 2000 64. Hingorani M, Metz D, Lightman S: Characterization of the normal conjunctival leukocyte population. Exp Eye Res 64:905, 1997 65. Cheng H, Tumpey T, Staats H et al: Role of macrophages in restricting herpes simplex virus type 1 growth after
ocular infection. Invest Ophthalmol Vis Sci 41:1402, 2000 66. Davidson H, Byrnes S, Montgomery P: The effect of immunization on rat serum and tear antibody responses to Chlamydia trachomatis. Reg Immunol 5:114, 1993 67. Pu Z, Silverstein A, Prendergast R: Conjunctival immunity: Compared effects of ocular or intestinal immunization
in rats. Invest Ophthalmol Vis Sci 24:1411, 1983 68. Engstrom R, Mondino B, Glasgow B et al: Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Invest Ophthalmol Vis Sci 32:1523, 1991 69. Liekfeld A, Schweig F, Jaeckel C et al: Intraocular antibody production in intraocular inflammation. Graefe's Arch Clin Exp Ophthalmol 238:222, 2000 70. Haynes R, McElveen J, Dua H et al: Expression of human beta-defensins in intraocular tissues. Invest Ophthalmol Vis Sci 41:3026, 2000 71. Aizuss D, Mondino B, Sumner H et al: The complement system and host defense against Pseudomonas endophthalmitis. Invest Ophthalmol Vis Sci 26:1262, 1985 72. Giese M, Mondino B, Glasgow B et al: Complement system and host defense against staphylococcal endophthalmitis. Invest Ophthalmol Vis Sci 35:1026, 1994 73. McMenamin P: The distribution of immune cells in the uveal tract of the normal eye. Eye 11:183, 1997 74. McMenamin P: Immunocompetent cells in the anterior segment. Prog Ret Eye Res 13:555, 1994 75. Tanigawa M, Bigger J, Kanter M et al: Natural killer cells prevent direct anterior-to-posterior spread of herpes
simplex virus type 1 in the eye. Invest Ophthalmol Vis Sci 41:132, 2000 76. Griffith T, Brunner T, Fletcher S et al: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189, 1995 |