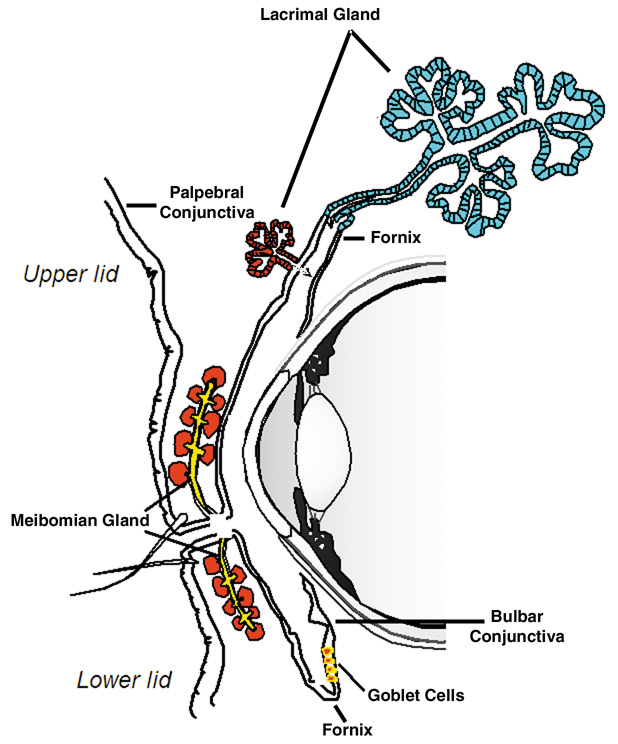
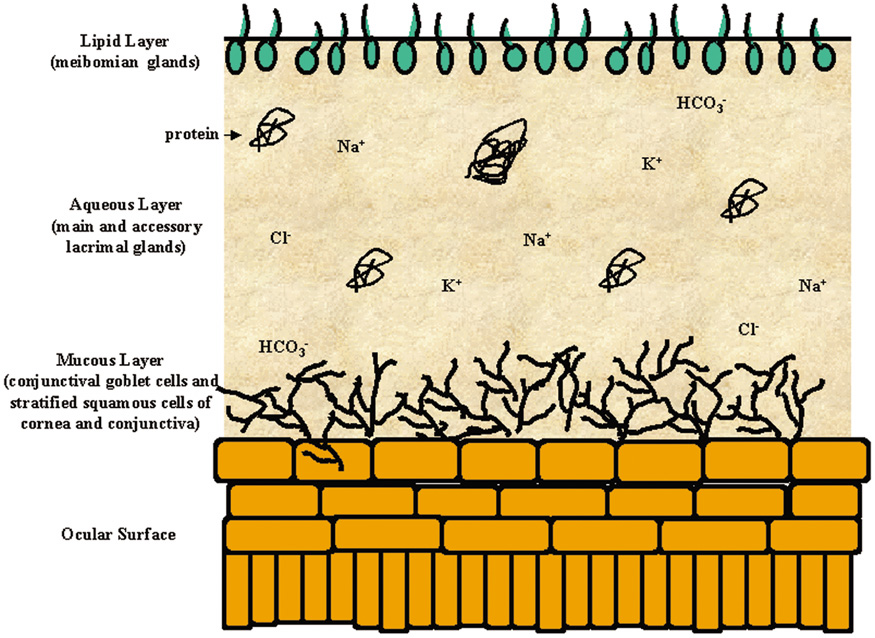
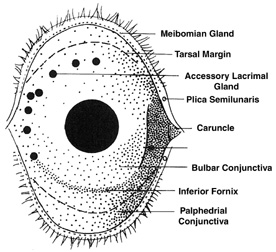
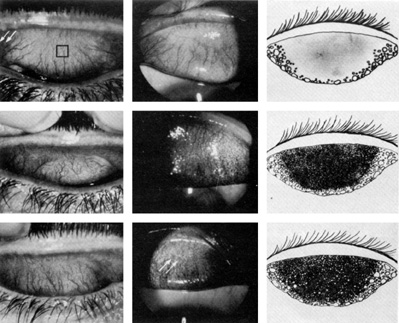
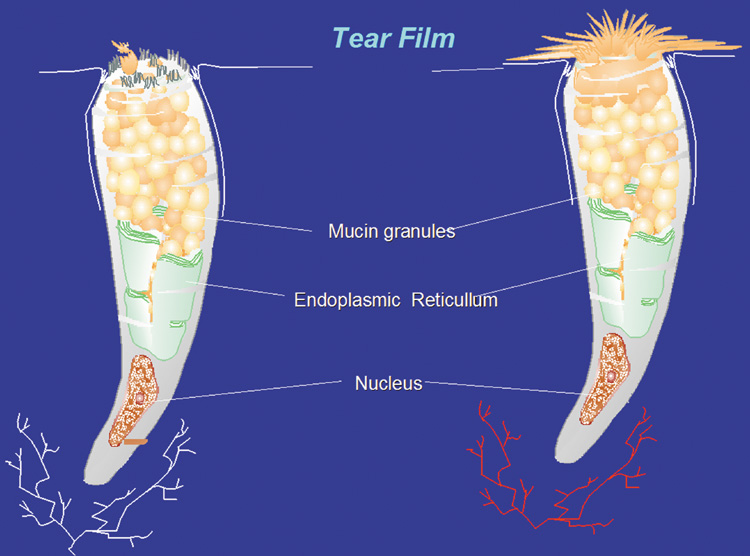
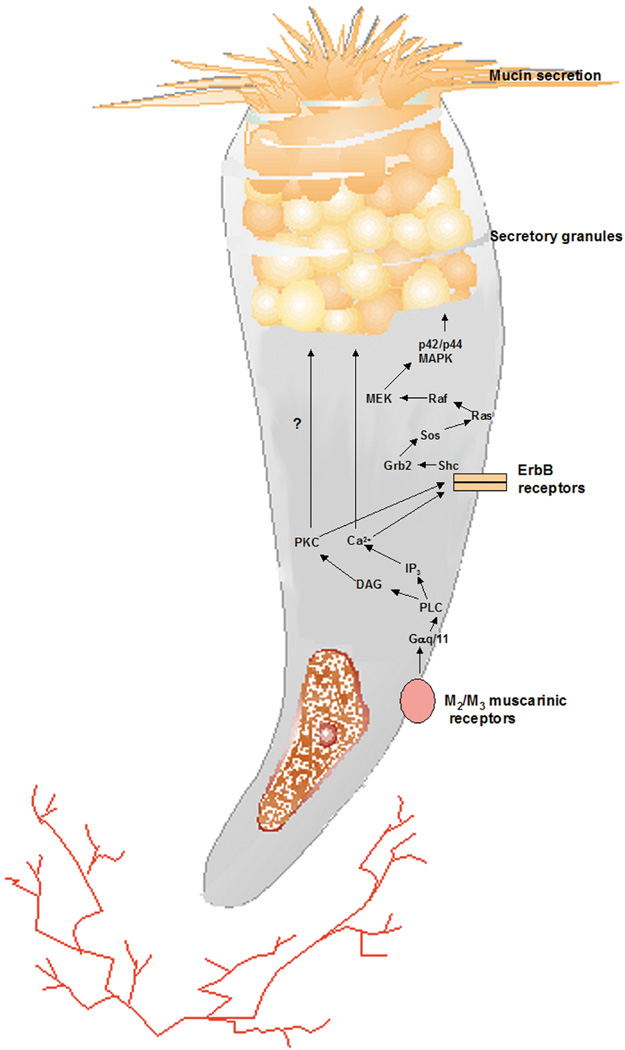
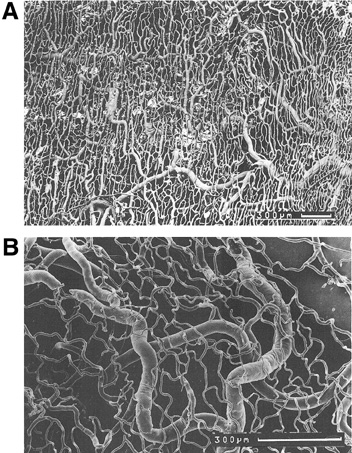
1. Lamberts DW: Physiology of the tear film. In: Smolin G, Thoft RA (eds). The Cornea. 3rd ed. Boston: Little, Brown and Company, 1994 2. Prydal JI, Artal P, Woon H, Campbell FW: Study of human precorneal tear film thickness and structure using laser
interferometry. Invest Ophthalmol Vis Sci 33:2006–2011, 1992 3. Nichols JJ, King-Smith PE: Thickness of the pre- and post-contact lens tear film measured in vivo
by interferometry. Invest Ophthalmol Vis Sci 44:68–77, 2003 4. King-Smith PE, Fink BA, Fogt N, et al: The thickness of the human precorneal tear film: Evidence from reflection
spectra. Invest Ophthalmol Vis Sci 41:3348–3359, 2000 5. McCulley JP, Shine WE: Meibomian gland and tear film lipids: structure, function and control. Adv Exp Med Biol 506(Pt A):373–378, 2002 6. Greiner JV, Allansmith MR: Effect of contact lens wear on the conjunctival mucous system. Ophthalmology 88:821–832, 1981 7. Kessing SV: Mucous gland system of the conjunctiva. A quantitative normal anatomical
study. Acta Ophthalmol (Copenh) Suppl 95:91+, 1968 8. Kruse FE, Chen JJ, Tsai RJ, Tseng SC: Conjunctival transdifferentiation is due to the incomplete removal of limbal
basal epithelium. Invest Ophthalmol Vis Sci 31:1903–1913, 1990 9. Wei ZG, Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells are preferentially located in fornical epithelium: Implications
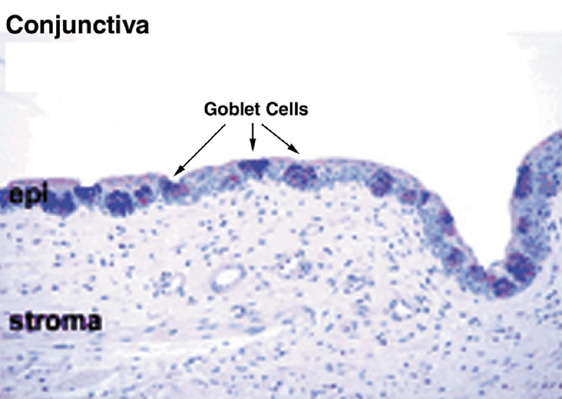
on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci 36:236–246, 1995 10. Nagasaki T, Zhao J: Uniform distribution of epithelial stem cells in the bulbar conjunctiva. Invest Ophthalmol Vis Sci 46:126–132, 2005 11. Gipson IK, Joyce N, Zieske J: The anatomy and cell biology of the human cornea, limbus, conjunctiva, and
adnexa. In: Foster C, Azar D, Dohlman C (eds). The Cornea. Philadelphia: Lippincott Williams & Wilkens, 2005:1–35 12. Pepperl JE, Ghuman T, Gill KS, et al: Conjunctiva. In: Jeager E (ed). Duane's Foundations of Clinical Ophthalmology. Philadelphia: Lippincott, Williams & Wilkins, 1996:1–30 13. Allansmith MR, Baird RS, Greiner JV: Density of goblet cells in vernal conjunctivitis and contact lens-associated
giant papillary conjunctivitis. Arch Ophthalmol 99:884–885, 1981 14. Lemp MA: The Dry Eye: A Comprehensive Guide. Heidelberg, Germany: Springer Verlag, 1992 15. Carroll JM, Kuwabara T: Ocular pemphigus. An electron microscopic study of the conjunctival and
corneal epithelium. Arch Ophthalmol 80:683–695, 1968 16. de Toledo C, Brunner A Jr: Superficial digitiform structures of the human conjunctiva studied on the
electron microscope. Rev Bras Oftalmol 26:283–287, 1967 17. Watsky MA, Jablonski MM, Edelhauser HF: Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res 7:483–486, 1988 18. Maurice DM: Electrical potential and ion transport across the conjunctiva. Exp Eye Res 15:527–532, 1973 19. Hosoya K, Horibe Y, Kim KJ, Lee VH: Na+-dependent L-arginine transport in the pigmented rabbit conjunctiva. Exp Eye Res 65:547–553, 1997 20. Yang JJ, Ueda H, Kim K, Lee VH: Meeting future challenges in topical ocular drug delivery: Development
of an air-interfaced primary culture of rabbit conjunctival epithelial
cells on a permeable support for drug transport studies. J Control Release 65:1–11, 2000 21. Horibe Y, Hosoya K, Kim KJ, Lee VH: Kinetic evidence for Na+-glucose co-transport in the pigmented rabbit conjunctiva. Curr Eye Res 16:1050–1055, 1997 22. Basu SK, Haworth IS, Bolger MB, Lee VH: Proton-driven dipeptide uptake in primary cultured rabbit conjunctival
epithelial cells. Invest Ophthalmol Vis Sci 39:2365–2373, 1998 23. Horibe Y, Hosoya K, Kim KJ, Lee VH: Carrier-mediated transport of monocarboxylate drugs in the pigmented rabbit
conjunctiva. Invest Ophthalmol Vis Sci 39:1436–1443, 1998 24. Gukasyan HJ, Kannan R, Lee VH, Kim KJ: Regulation of L-cystine transport and intracellular GSH level by a nitric
oxide donor in primary cultured rabbit conjunctival epithelial cell
layers. Invest Ophthalmol Vis Sci 44:1202–1210, 2003 25. Steuhl KP: Ultrastructure of the conjunctival epithelium. Dev Ophthalmol 191–104, 1989 26. Dartt DA: Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res 21:555–576, 2002 27. Chait R: Absorption of protein through the conjunctival mucus membrane. Arch Ophthalmol 43526, 1950 28. Hansen E: Conjunctivale auleiringer red bruk av adrenalin oyedraper. Tidsskr Nor Laegeforen 84:678, 1964 29. Belmonte C, Acosta MC, Gallar J: Neural basis of sensation in intact and injured corneas. Exp Eye Res 78:513–525, 2004 30. Acosta MC, Tan ME, Belmonte C, Gallar J: Sensations evoked by selective mechanical, chemical, and thermal stimulation
of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 42:2063–2067, 2001 31. Muller LJ, Marfurt CF, Kruse F, Tervo TM: Corneal nerves: Structure, contents and function. Exp Eye Res 76:521–542, 2003 32. Belmonte C, Garcia-Hirschfeld J, Gallar J: Neurobiology of ocular pain. Prog Retin Eye Res 16:117–156, 1997 33. Macintosh SR: The innervation of the conjunctiva in monkeys. An electron microscopic
and nerve degeneration study. Albrecht Graefes Arch Klin Exp Ophthalmol 192:105–116, 1974 34. Elsas T, Edvinsson L, Sundler F, Uddman R: Neuronal pathways to the rat conjunctiva revealed by retrograde tracing
and immunocytochemistry. Exp Eye Res 58:117–126, 1994 35. Acosta MC, Peral A, Luna C, et al: Tear secretion induced by selective stimulation of corneal and conjunctival
sensory nerve fibers. Invest Ophthalmol Vis Sci 45:2333–2336, 2004 36. Diebold Y, Rios JD, Hodges RR, et al: Presence of nerves and their receptors in mouse and human conjunctival
goblet cells. Invest Ophthalmol Vis Sci 42:2270–2282, 2001 37. Li Y, Kuang K, Yerxa B, et al: Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor
agonists stimulate Cl– and fluid secretion. Am J Physiol Cell Physiol 281:C595–C602, 2001 38. Shiue MH, Kulkarni AA, Gukasyan HJ, et al: Pharmacological modulation of fluid secretion in the pigmented rabbit conjunctiva. Life Sci 66:L105–111, 2000 39. Kompella UB, Kim KJ, Lee VH: Active chloride transport in the pigmented rabbit conjunctiva. Curr Eye Res 12:1041–1048, 1993 40. Shi XP, Candia OA: Active sodium and chloride transport across the isolated rabbit conjunctiva. Curr Eye Res 14:927–935, 1995 41. Turner HC, Alvarez LJ, Bildin VN, Candia OA: Immunolocalization of Na-K-ATPase, Na-K-Cl and Na-glucose cotransporters
in the conjunctival epithelium. Curr Eye Res 21:843–850, 2000 42. Turner HC, Alvarez LJ, Candia OA: Cyclic AMP-dependent stimulation of basolateral K+ conductance in the rabbit conjunctival epithelium. Exp Eye Res 70:295–305, 2000 43. Zwick E, Daub H, Aoki N, et al: Critical role of calcium-dependent epidermal growth factor receptor transactivation
in PC12 cell membrane depolarization and bradykinin signaling. J Biol Chem 272:24767–24770, 1997 44. Hamann S, Zeuthen T, La Cour M, et al: Aquaporins in complex tissues: distribution of aquaporins 1–5 in
human and rat eye. Am J Physiol 274(5 Pt 1):C1332–1345, 1998 45. Candia OA, Shi XP, Alvarez LJ: Reduction in water permeability of the rabbit conjunctival epithelium by
hypotonicity. Exp Eye Res 66:615–624, 1998 46. Hosoya K, Kompella UB, Kim KJ, Lee VH: Contribution of Na+-glucose cotransport to the short-circuit current
in the pigmented rabbit conjunctiva. Curr Eye Res 15:447–451, 1996 47. Kompella UB, Kim KJ, Shiue MH, Lee VH: Possible existence of Na+-coupled amino acid transport in the pigmented rabbit conjunctiva. Life Sci 57:1427–1431, 1995 48. Kompella UB, Kim KJ, Shiue MH, Lee VH: Cyclic AMP modulation of active ion transport in the pigmented rabbit conjunctiva. J Ocul Pharmacol Ther 12:281–287, 1996 49. Diebold Y, Rios JD, Hodges RR, et al: Presence of nerves and their receptors in mouse and human conjunctival
goblet cells. Invest Ophthalmol Vis Sci 42:2270–2282, 2001 50. Hosoya K, Ueda H, Kim KJ, Lee VH: Nucleotide stimulation of Cl– secretion in the pigmented rabbit conjunctiva. J Pharmacol Exp Ther 291:53–59, 1999 51. Murakami T, Fujihara T, Nakamura M, Nakata K: P2Y2 receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res 21:782–787, 2000 52. Alvarez LJ, Turner HC, Zamudio AC, Candia OA: Serotonin-elicited inhibition of Cl– secretion in the rabbit conjunctival epithelium. Am J Physiol Cell Physiol 280:C581–592, 2001 53. Shiue MH, Kim KJ, Lee VH: Modulation of chloride secretion across the pigmented rabbit conjunctiva. Exp Eye Res 66:275–282, 1998 54. Alvarez LJ, Candia OA, Turner HC, Zamudio AC: Phorbol ester modulation of active ion transport across the rabbit conjunctival
epithelium. Exp Eye Res 69:33–44, 1999 55. Komatsu M, Arango ME, Carraway KL: Synthesis and secretion of Muc4/sialomucin complex: Implication of
intracellular proteolysis. Biochem J 368(Pt 1):41–48, 2002 56. Argueso P, Gipson IK: Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res 73:281–289, 2001 57. Jumblatt MM, McKenzie RW, Steele PS, et al: MUC7 expression in the human lacrimal gland and conjunctiva. Cornea 22:41–45, 2003 58. McKenzie RW, Jumblatt JE, Jumblatt MM: Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci 41:703–708, 2000 59. Argueso P, Spurr-Michaud S, Russo CL, et al: MUC16 mucin is expressed by the human ocular surface epithelia and carries
the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci 44:2487–2495, 2003 60. Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK: Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci 36:1818–1827, 1995 61. Inatomi T, Spurr-Michaud S, Tisdale AS, et al: Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci 37:1684–1692, 1996 62. Gamache DA, Wei ZY, Weimer LK, et al: Corneal protection by the ocular mucin secretagogue 15(S)-HETE
in a rabbit model of desiccation-induced corneal defect. J Ocul Pharmacol Ther 18:349–361, 2002 63. Jumblatt JE, Cunningham LT, Li Y, Jumblatt MM: Characterization of human ocular mucin secretion mediated by 15(S)-HETE. Cornea 21:818–824, 2002 64. Dartt DA, McCarthy DM, Mercer HJ, et al: Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res 14:993–1000, 1995 65. Rios JD, Forde K, Diebold Y, et al: Development of conjunctival goblet cells and their neuroreceptor subtype
expression. Invest Ophthalmol Vis Sci 41:2127–2137, 2000 66. Kessler TL, Mercer HJ, Zieske JD, et al: Stimulation of goblet cell mucous secretion by activation of nerves in
rat conjunctiva. Curr Eye Res 14:985–992, 1995 67. Rios JD, Zoukhri D, Rawe IM, et al: Immunolocalization of muscarinic and VIP receptor subtypes and their role
in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci 40:1102–1111, 1999 68. Dartt DA, Kessler TL, Chung EH, Zieske JD: Vasoactive intestinal peptide-stimulated glycoconjugate secretion from
conjunctival goblet cells. Exp Eye Res 63:27–34, 1996 69. Jumblatt JE, Jumblatt MM: Detection and quantification of conjunctival mucins. Adv Exp Med Biol 438:239–246, 1998 70. Jumblatt JE, Jumblatt MM: Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit
and human conjunctiva. Exp Eye Res 67:341–346, 1998 71. Dartt DA, Rios JR, Kanno H, et al: Regulation of conjunctival goblet cell secretion by Ca2+ and protein
kinase C. Exp Eye Res 71:619–628, 2000 72. Kanno H, Horikawa Y, Hodges RR, et al: Cholinergic agonists transactivate the EGFR and stimulate MAPK to induce
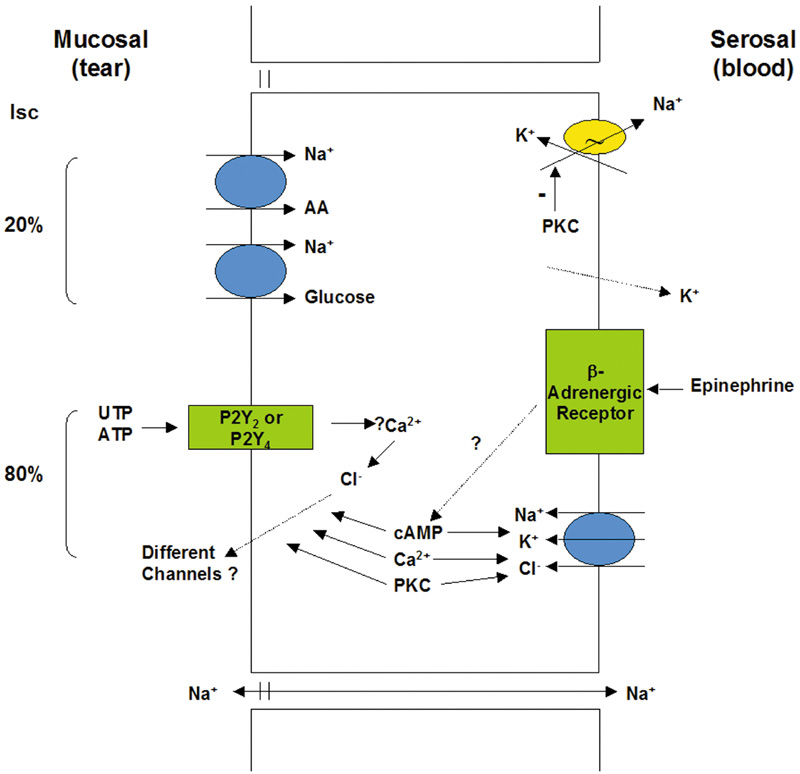
goblet cell secretion. Am J Physiol Cell Physiol 284:C988, 2003 73. Shatos MA, Rios JD, Horikawa Y, et al: Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci 44:2477–2486, 2003 74. Narawane MA, Lee VH: IGF-I and EGF receptors in the pigmented rabbit bulbar conjunctiva. Curr Eye Res 14:905–910, 1995 75. Horikawa Y, Shatos MA, Hodges RR, et al: Activation of mitogen-activated protein kinase by cholinergic agonists
and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci 44:2535–2544, 2003 76. Sacks EH, Wieczovek R, Jakobiec FA, Knowles DM: Lymphocytic subpopulations in the normal human conjunctiva: A monoclonal
antibody study. Ophthalmology 93:1276, 1986 77. Knop N, Knop E: Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci 41:1270–1279, 2000 78. Spyratos S: Étude de la vascularisation superficielle de l'oeil. Ann Ocul 199:754, 1966 79. Oduntan AO: Organization of capillaries in the primate conjunctiva. Ophthalmic Res 24:40–44, 1992 80. Holden BA, Sweeney DF: The oxygen tension and temperature of the superior palpebral conjunctiva. Acta Ophthalmol (Copenh) 63:100–103, 1985 81. Graffin A, Coddry E: A note on peripheral blood vascular beds in the bulbar conjunctiva of man. Bull Johns Hopkins Hosp 92:423, 1953 82. Graffin A, Coddry E: Studies of peripheral blood vascular beds in the bulbar conjunctiva man. Bull Johns Hopkins Hosp 93:275, 1953 83. Scarpelli P, Pellegrini M, Brancato R: L'ultrastruttura die capillari della conjiuntiva umana. Ann Ottal 92:977, 1966 84. Tamura T: Ultrastructure of human conjunctival capillaries. Acta Soc Ophthalmol Jpn 71:109, 1967 85. Deodati F: L'angiographie fluoresceinique du segment anterieu: son interet ses
possibilities. Bull Soc Ophthalmol 70:33–52, 1970 86. Lockard I, Debacker H: Conjunctival circulation in relation to circulatory disorders. J South Carolina Med Assoc 63:201, 1967 87. Lee R: Anatomical and physiologic aspects of the capillary bed in the bulbar conjunctiva
of man in health and disease. Angiology 63:69, 1955 88. Romani J: Manifestations angiopathiques chez les diabetiques et chez les obeses. Presse Med 77:1969, 1969 89. Francois J, Neetens A: Importance clinique de l'angioscopie conjonctivale. Acta Cardiol Angiol 16:109, 1967 90. Agarwal L, Chabra H, Batta R: Conjunctival vessels in diabetes mellitus. Orient Arch Ophthalmol 41:41, 1966 91. Hood J, Hodges RE: Ocular lesions in scurvy. Am J Clin Nutr 22:559–567, 1969 92. Wolf S, Arend O, Schulte K, et al: Quantification of retinal capillary density and flow velocity in patients
with essential hypertension. Hypertension 23:464–467, 1994 93. Landesman R, Douglas RG, Dreishpoon G, Holze E: The vascular bed of the bulbar conjunctiva in the normal menstrual cycle. Am J Obstet Gynecol 66:988–998, 1953 94. Sugar HS, Riazi A, Schaffner R: The bulbar conjunctival lymphatics and their clinical significance. Trans Am Acad Ophthalmol Otolaryngol 61:212–223, 1957 95. Takayama T: Studies in histological changes of bulbar conjunctiva with special regard
to the arterioles. Acta Soc Ophthalmol Jpn 64:1962, 1960 96. Kojo T: Changes on bulbar conjunctiva with age with special regard to the nature
to be split and extensibility. Acta Soc Ophthalmol Jpn 64:2895, 1960 97. Frayser R, Knisely WH, Barnes R, Satterwhite WM Jr: In vivo observations on the conjunctival circulation in elderly subjects. J Gerontol 19:494–500, 1964 98. Ivanor V: On the resistance of the bulbar conjunctival vessels. Vestn Oftalmol 55:6, 1970 99. Schwab IR, Linberg JV, Gioia VM, et al: Foreshortening of the inferior conjunctival fornix associated with chronic
glaucoma medications. Ophthalmology 99:197–202, 1992 100. Holly FJ: Formation and rupture of the tear film. Exp Eye Res 15:515–525, 1973 101. Jester JV, Nicolaides N, Smith RE: Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci 20:537–547, 1981 102. Seifert P, Spitznas M: Immunocytochemical and ultrastructural evaluation of the distribution of
nervous tissue and neuropeptides in the meibomian gland. Graefes Arch Clin Exp Ophthalmol 234:648–656, 1996 103. Olami Y, Zajicek G, Cogan M, et al: Turnover and migration of meibomian gland cells in rats' eyelids. Ophthalmic Res 33:170–175, 2001 104. LeDoux MS, Zhou Q, Murphy RB, et al: Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci 42:2434–2441, 2001 105. Seifert P, Spitznas M: Vasoactive intestinal polypeptide (VIP) innervation of the human
eyelid glands. Exp Eye Res 68:685–692, 1999 106. Chung CW, Tigges M, Stone RA: Peptidergic innervation of the primate meibomian gland. Invest Ophthalmol Vis Sci 37:238–245, 1996 107. Sullivan DA, Yamagami H, Liu M, et al: Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol 506(Pt A):389–399, 2002 108. Wickham LA, Gao J, Toda I, et al: Identification of androgen, estrogen and progesterone receptor mRNAs in
the eye. Acta Ophthalmol Scand 78:146–153, 2000 109. Esmaeli B, Harvey JT, Hewlett B: Immunohistochemical evidence for estrogen receptors in meibomian glands. Ophthalmology 107:180–184, 2000 110. Suzuki T, Sullivan BD, Liu M, et al: Estrogen and progesterone effects on the morphology of the mouse meibomian
gland. Adv Exp Med Biol 506(Pt A):483–488, 2002 111. Tiffany JM: Physiological properties of the meibomian glands. Prog Retin Eye Res 14:47–74, 1995 112. Hodges RR, Dartt DA: Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol 231:129–196, 2003 113. Greiner JV, Covington HI, Allansmith MR: Surface morphology of the human upper tarsal conjunctiva. Am J Ophthalmol 83:892–905, 1977 114. Shatos MA, Rios JD, Tepavcevic V, et al: Isolation, characterization, and propagation of rat conjunctival goblet
cells in vitro. Invest Ophthalmol Vis Sci 42:1455–1464, 2001 |