CLASSIFICATION
The classic classification of intermittent exotropia is based on distance/near differences. In 1897, Duane6 first presented a classification, which was modified by Burian7–10 into the accepted standard that is used today. The four types are
Basic exotropia, in which the deviation at distance fixation (6 m) is the
same as near fixation (1/3 m)
Divergence excess, in which the deviation at distance is more than 10 PD
greater than at near and remains so even when the near deviation is
measured following one hour of monocular occlusion
Pseudodivergence excess, in which the distance deviation is more than 10 PD
greater than the near deviation, but the near deviation becomes close
to the distance deviation in magnitude when measured following 1 hour
of monocular occlusion
Convergence insufficiency, in which the near deviation is more than 10 PD
greater than the distance deviation
The method of identifying patients in the pseudodivergence or simulated divergence excess group has elicited controversy. Brown11 suggested that these patients could be identified if they showed an increase in their near deviation when measured through a + 2.50 or + 3.00 (D) lens placed in front of each eye and that this was the same as 1 hour occlusion. Helveston12 reported that the 1 hour monocular occlusion and the + 3.00 (D) lenses were affecting different convergence mechanisms and should not be considered interchangeable. Cooper and associates13 pointed out the need to eliminate tonic proximal fusion with 1 hour occlusion and then not let the patient refuse before obtaining near measurements with + 3.00 (D) lenses for the purpose of calculating the AC/A ratio. Thus, the proper method of identifying a patient with pseudo- or simulated divergence excess exotropia is to measure the near deviation following 1 hour of monocular occlusion and then measure the near deviation with + 3.00 (D) lenses to look for the presence of a high AC/A ratio. This subject is beautifully reviewed and discussed in an excellent paper by Kushner and Morton.14
Intermittent exotropia is also classified secondarily based on fusion. Does the deviation remain intermittent by fusion at both near and distance fixation, is the deviation phoric at one fixation distance and intermittent at the other, or has the deviation become constant at one of these fixation distances? Some authors and strasbismologists will declare an exodeviation intermittent only if the deviation is intermittent or phoric at both near and far fixation distances. If the ocular alignment seen is constantly exotropic at 6 m but intermittent at 1/3 m, some label this presentation intermittent exotropia, whereas others classify it as constant exotropia. In this chapter, the term intermittent exotropia includes those patients whose deviation is intermittent at both far and near fixation distances or intermittent at one and phoric at the other. If a constant deviation initially is present at one fixation distance but is made intermittent by occlusion therapy before surgery, then this patient is classified as having intermittent exotropia. If the patient has constant exotropia at one or both fixation distances even following occlusion therapy, then that patient is considered as having constant exotropia.
Patients with third cranial nerve palsy, bilateral and unilateral internuclear ophthalmoplegia, and exotropic Duane's syndrome are excluded from consideration in this discussion.
TREATMENT GOALS
Treatment goals for intermittent exotropia are the same as for all types of strabismus: to establish and maintain good and equal vision, fusion and stereopsis, full ductions and versions, relief of asthenopic symptoms and diplopia, functionally and cosmetically straight eyes, and elimination of any abnormal head position. Amblyopia is not a common finding in intermittent exotropia, and when it occurs, the difference in acuity between the eyes is usually small. When seen, it often is related to anisometropia. Fusion is present in all patients with intermittent exotropia, and excellent stereopsis is present in at least two thirds. The remaining one third has monofixation.15 Asthenopic symptoms are rarely expressed or determined to be present before patients reach their midteen years, and face turns or head postures are rare in intermittent exotropia.
The goals of treatment of intermittent exotropia may be achieved through nonsurgical management in some patients. If this treatment fails, surgery is recommended.
Hardesty and associates16,17 and Pratt-Johnson and coworkers18 presented the results of treatment and discussed the criteria or parameters to be considered in evaluating the treatment of intermittent exotropia and determining whether a functional cure has been obtained. Their results and the follow-up are excellent.
NONSURGICAL MANAGEMENT
Amblyopia
Significant amblyopia is not a common finding in intermittent exotropia, but when detected, it must be treated first. I prefer occlusion therapy to pharmacologic penalization in patients with intermittent exotropia. When occlusion is made, there is the effect of antisuppression as well as that of amblyopia therapy. Penalization probably does not have an antisuppression effect but may provide adequate amblyopia therapy.
Spectacles
Appropriate spectacles must be prescribed to allow good and equal vision and enhance fusion. Accommodative convergence may also be stimulated by altering the spectacle prescription. If the patient is myopic or has minimal hyperopia, up to -3.00 diopters (D) may be added to the cycloplegic refraction. This “overminusing” stimulates accommodation and accommodative convergence. The goal in such therapy is to reduce the amount and frequency of the intermittent exotropia. Kusher19 considered whether overcorrecting minus lens therapy in intermittent exotropia causes myopia and concluded that it does not.
If the patient is significantly hyperopic (greater than + 4.00), the value of prescribing glasses may be looked at in two ways. The amount of the hyperopia can be reduced to stimulate accommodation. This approach is similar to the rationale of overminusing to improve exodeviation. However, if the deviation is poorly controlled and surgery is indicated, wearing and measuring the deviation with the full plus cycloplegic refractive error may increase the deviation and provide a truer measure of the deviation as well as worsen the control. In some instances, however, giving the full plus actually improves the control. Iacobucci and colleagues20 reported resolution of exotropia after providing full or nearly full hyperopic correction in seven patients.
I use the following guidelines in prescribing spectacles for patients with intermittent exotropia. If the patient has myopia of 0.75 D or more, spectacles are ordered. The prescription is written with an additional -0.25 D over the cycloplegic refraction to ensure that the patient is not underminused. I overminus in the case of some postoperative patients who are drifting back to exotropia. The second situation for using overcorrecting minus lenses is the intermittent exotropia patient with a proven high AC/A ratio. This high AC/A ratio is identified with the use of + 3.00 D lenses following 1 hour of monocular occlusion. Prescribing over minus lenses helps provide better control and actual reduction of the distance deviation. If an esotropia is produced at near fixation, then a bifocal is also prescribed. If not detected preoperatively, these patients will usually develop a near deviation of esotropia postoperatively and require a bifocal. Kushner21 reports successful treatment of 16 high AC/A intermittent exotropia patients with overcorrecting minus lenses and a bifocal. These patients are described in the section on treatment of undercorrection.
If the patient has hyperopia of + 4.50 D or less, spectacles are not ordered. If the patient's hyperopia measures + 5.00 D or more on cycloplegic refraction, I prescribe spectacles and reduce the hyperopia by 2.50 D. The goal is to allow the patient to see clearly with maximum accommodative effort of + 2.50 D at 6 m, which is within the normal range of accommodation for individuals younger than 20 years old. If larger amounts of hyperopia are not corrected, the patient may not accommodate and simply may be content with blurred vision. Blurred vision is a negative factor for fusion. If reducing the hyperopic prescription by 2.50 D produces blurred vision, particularly in older patients, then more hyperopia closer to the cycloplegic refraction must be given.
Astigmatism of 1.25 D in patients 5 years and older and 2.00 D in younger patients requires spectacles.
Occlusion
In the absence of amblyopia, part-time occlusion is prescribed at the initial visit when intermittent exotropia is diagnosed. This occlusion is done for 2 hours daily and on an alternate basis if one eye is not markedly dominant. In the presence of marked dominance, the dominant eye is occluded for 2 hours daily. The patient is examined again in 6 to 8 weeks. If the exotropia is well controlled and especially if the control has improved with occlusion therapy, patching is continued for up to 6 months. If the control remains poor or deteriorates while occlusion is being performed, surgery is advised. The occlusion is continued up to the date of surgery to minimize suppression. These visits allow the physician to measure the deviation more than once, thereby increasing the accuracy of determining the true amount of deviation and surgical target. The visits also provide an opportunity for the patient, family, and physician to discuss the treatment and expected results of treatment of intermittent exotropia.
Prisms
The use of base-in fusing prisms has not been successful in the treatment of intermittent exotropia. They compensate for the deviation but do not result in improvement of control or reduction in the amount of deviation.
INDICATIONS FOR SURGERY
In intermittent exotropia, fusion is present in the phoric phase and absent in the tropic phase. The most important reason for surgical intervention is to allow binocular vision (fusion) to occur as much of the time as possible and to reduce or eliminate the need for abnormal sensory adaptation. Thus, when exotropia occurs almost 50% of the time, when the eyes diverge frequently and spontaneously at one or both (far or near) fixation distances, or when the eyes remain tropic and the individual is unaware of the divergence, surgery should be considered. Each patient must be considered individually. How much the patient, the teacher, and the family and friends are aware of the divergence is an important factor in the surgical decision. If the patient, family, and friends report frequent manifest exotropia, the decision to perform surgery is easily reached. If the patient is a child and the parents deny the child's exotropia, although spontaneous exotropia is seen in the physician's office, the process becomes more difficult. The exotropia should be demonstrated to the family, and they should be asked to watch for it. They may deny seeing exotropia because they want to avoid surgery, and the decision to perform surgery may come solely from the office examination findings.
It is difficult to state how frequently exotropia must occur for surgery to be warranted. Most ophthalmologists would be comfortable with an occurrence of 50%. I begin to consider a surgical recommendation when the occurrence of exotropia is estimated at 25%, if the family sees it daily and for more than a few moments.
The frequency and duration of the occurrence of exotropia are more important factors than the size of the deviation in determining whether to proceed with surgery. The amount of the deviation only determines how much surgery should be done. A patient with a poorly controlled deviation of 13 to 15 PD (present in the tropic phase 50% of the time) is more of a candidate for surgery than a patient with a well-controlled intermittent exodeviation that measures 30 PD.
Surgery will not improve the degree of fusion (i.e., change monofixation to bifixation or improve the level of stereopsis as measured by the Titmus stereo test at near); however, it will improve the amount of time the individual is fusing.15,22 In a report by O'Neal and colleagues, the distance stereo acuity as measured by the Mentor B-Vat II Video acuity tester and binocular system deteriorated as the control of the distance exotropia worsened and was improved following surgical alignment.23,24 On rare occasions, surgery is performed because a patient reports asthenopia or intermittent diplopia, even though little or no tropia is seen during the examination, and only exophoria is noted.
After 6 months of age, the timing of surgery is not age dependent. The decision to proceed with surgery should be based on the degree of control of the deviation and the amount of time that the patient demonstrates a tropia. If the eyes are frequently exotropic, surgery should not be delayed, particularly if the family also notes a decrease in control of ocular alignment. In general, most patients do not undergo surgical correction until they are 2 to 3 years of age or older. If part-time occlusion therapy helps maintain control and allows surgery to be delayed, it may be better to operate after the patient is 3 years old. More accurate measurements may be possible, affording a truer surgical target angle, and postoperative therapy may be applied more easily. A study by Pratt-Johnson and colleagues showed a better cure rate for patients who were operated on before age 4 than for those operated on later.18 Baker and colleagues did not show a better sensory outcome resulting from early surgery.25
If surgery is considered, the patient should be measured at least twice before surgery is performed. The amount of deviation measured in intermittent exotropia may vary from 1 day, or even 1 hour, to the next. Having a reproducible measurement of the exodeviation that serves as the surgical target angle is the first step in obtaining a good surgical result.
CONTRAINDICATIONS
The most important consideration in performing surgery is the patient's general medical condition. Most strabismus surgery, and all strabismus surgery that is performed on children, is done with the patient under general anesthesia. Therefore, the most significant contraindication to surgery for intermittent exotropia is a medical condition that would not allow the patient to undergo general anesthesia. It is also important that an anesthesiologist competent to deal with pediatric patients be available.
Surgery should be delayed if the physician cannot obtain an accurate measurement of the deviation, or if the deviation is variable and different measurements are obtained during different examinations. Stable, accurate, and reproducible measurements are necessary before surgery should be performed.
SURGICAL TREATMENT
Choosing the Operation
The surgical procedures used to treat intermittent exotropia are bilateral lateral rectus muscle recession (bilateral recession), unilateral lateral rectus recession and resection of the medial rectus on the same eye (recess-resect), or resection of both medial rectus muscles (bimedial resection). If the deviation is large (greater than 45 PD), operating on three or four horizontal rectus muscles is usually necessary.
The surgeon determines the muscles on which to operate based on the amount and the far and near characteristics of the deviation. Another factor to consider is previous surgery. In general, a bilateral recession is chosen when the distance deviation is greater than the near deviation. In selected cases in which the distance deviation is greater than the near and 20 PD or less, a unilateral lateral rectus recession of 7 to 9 mm may be considered.26–28 When the deviation is the same at distance and near (basic deviation), either a bilateral lateral rectus recession or a recess-resect procedure may be performed. Kushner has pointed out that patients with basic deviations seem to have better results following a recess-resect procedure. There may be a tethering effect from the medial rectus resection in these patients who lack tonic proximal fusion. Those with simulated divergence excess deviations with tonic proximal fusion as proposed by Kushner do well with bilateral lateral rectus recession procedures.29
In convergence insufficiency, in which near deviation is 10 or more PD greater than the distance deviation, bimedial resection is the surgery of choice. Usually, a significant period of postoperative diplopia occurs, and it may last for several weeks. Base-out Fresnel prisms frequently are used for postoperative diplopia in this group of patients. The consecutive esotropia resolves with time. In some patients, with enough time, near divergence may return. Orthoptic exercises should be the first and principal method of treatment in this type of intermittent exotropia, with surgery a last resort. Von Noorden30 reported a series of six patients with convergence insufficiency who also had reduced near point of accommodation. These patients were treated surgically with a bimedial resection after a prolonged history of blurring, headache, eyestrain, and intermittent diplopia associated with near work. Five of six patients had a good result from the surgery, with follow-up of 7 to 14 months.30 Kraft and colleagues report good results in convergence insufficiency patients using a recess-resect procedure if the deviation is at least 8 PD of exotropia in the distance. 31
When the distance deviation is 35 to 45 PD, the surgeon should consider a recess-resect procedure rather than a bilateral recession of the lateral rectus muscles. Bilateral recession of the lateral rectus muscles is an excellent procedure for deviations as great as 30 to 35 PD, but it is less effective than a recess-resect procedure for deviations greater than 35 PD. Additionally, if a second procedure is required because exotropia recurs, the ability to perform a recess-resect procedure on the other eye offers a distinct advantage. If the first operation was a bilateral recession, the second would be a rerecession of both lateral recti muscles, a rerecession of one lateral muscle, and resection of the ipsilateral medial muscle, or a resection of both medial rectus muscles. None of these procedures is as straightforward or as effective as a recess-resect procedure performed on the unoperated fellow eye.
The most difficult surgical decision for me is presented by the patient who has a distance deviation of 35 to 45 PD and a near deviation of 10 to 15 PD or less that cannot be enlarged by prolonged monocular occlusion followed by + 3.00 lenses. I worry about overcorrecting the near deviation with a standard recess-resect procedure and undercorrecting the distance deviation with a bilateral lateral rectus recession. In patients who have these findings, one approach is to perform a large recession of the lateral rectus muscle (e.g., 8.5 mm) and a small resection of the ipsilateral medial rectus muscle (e.g., 3.5 mm).
When the distance deviation is 45 PD or greater, the choice should be three horizontal rectus muscles. This procedure usually consists of recessing both lateral rectus muscles and resecting one medial rectus muscles. Some clinicians suggest that the lateral rectus muscle should be recessed 0.5 to 1 mm less on the eye in which the medial rectus muscle is being resected to preserve comitant rotation of the globes.
The amounts of surgery suggested by Parks are listed in Table 1.32 These numbers provide a good starting point, but each ophthalmologist will vary her or his surgical technique and may find more deviations in surgical outcome than might be predicted from the published tables. Published surgical tables should be used as guidelines, but all surgeons have the responsibility to reevaluate their surgical results continually and adjust future amounts of surgery accordingly. For example, I have found that I must perform 0.5 mm less recession on the lateral rectus muscle for a recess-resect procedure than for a bilateral lateral rectus recession to correct the same amount of deviation. In a prospective clinical trial, Kushner recommends measuring the distance deviation (the target angle for surgery) following 1 hour of monocular occlusion at 6 m and while fixing on an outdoor target. He then performs surgery for the largest angle measured.33
TABLE 1. Quantity of Surgery Suggested According to the Size of the Angle
of Exodeviation*
| Exotropia Angle (degrees) | Lateral Rectus Recession in Both Eyes (mm) | Medial Rectus Resection in Both Eyes (mm) |
| 15 | 4 | 3 |
| 20 | 5 | 4 |
| 25 | 6 | 5 |
| 30 | 7 | 6 |
*Quantity of surgery suggested by Parks for intermittent exotropia based on the size of the exotropia as measured in prism diopters.
(Parks MM, Mitchell PR: Concomitant Exodeviations, p 10. In Tasman W, Jaeger E [eds]: Clinical Ophthalmology. Philadelphia: JB Lippincott, 1990.)
Other Considerations
If hypertropia is present along with intermittent exotropia, the first consideration is whether the hypertropia is present at distance and near fixation and whether any dysfunction of the oblique muscles is present. If the deviation is present at both distance and near when the patient is dissociated into the exotropic phase, no oblique muscle dysfunction is seen, and the vertical deviation is a true vertical deviation as opposed to a dissociated vertical deviation, then surgical correction of the vertical deviation is indicated if the amount is 8 PD or more. A vertical deviation that is present only at distance and not at near is ignored in the surgical plan.34 In the rare instance when this far-near dissociated hypertropia is associated with a unilateral inferior oblique overaction, the inferior oblique muscle should be weakened along with the surgery on the horizontal muscles.
If an A or V pattern is present in conjunction with the intermittent exotropia, the function of the oblique muscles must be evaluated. If the inferior oblique muscles are overacting in the presence of a V pattern and the superior oblique muscles are overacting in the presence of an A pattern, this dysfunction must be considered in the surgical plan.
In V patterns, the overacting inferior oblique muscles should be recessed if the superior oblique muscles are normal or underacting. If the superior oblique muscles are also overacting, then careful consideration must be given to the effect of the oblique muscle dysfunction on the V pattern and the effect that recessing the overacting inferior obliques will have on the pattern. Pseudooveraction of the inferior oblique muscles should be suspected when tight lateral rectus muscles are associated with exotropia. In this situation, it is believed that the lateral rectus muscle of the exotropic eye becomes tight or contracted. When the eye is taken into adduction and slight upgaze, the tight lateral rectus muscle slides around the globe, causing the eye to elevate and to simulate overaction of the inferior oblique muscle. This concept was first suggested by Jampolsky.
In A pattern deviations, if the inferior oblique muscles are normal or underacting, weakening the overacting superior oblique muscles can help collapse the pattern. However, if the inferior oblique muscles are overacting, care must be taken if a tenotomy of the overacting superior oblique muscles is performed because the A pattern may be converted to a V pattern.
For both A and V patterns, when weakening the overacting oblique muscles is part of the surgical plan, surgery for the exodeviation should be done as measured in primary gaze position and without consideration for any oblique surgery that is done. Most strabismologists support this approach in patients who have a V pattern with overacting inferior oblique muscles. However, controversy exists when an A pattern is associated with overacting superior oblique muscles. Some authors report no change in the exodeviation in primary gaze position after surgical weakening of overacting superior oblique muscles is performed.35–38 Others report an inward shift of the eyes in primary gaze position after surgical weakening of overacting superior oblique muscles has been performed in patients with A pattern strabismus.39,40 In a series of eight patients with A pattern exotropia and overacting superior oblique muscles, Biglan noted a mean reduction of 8 PD (from 12 to 4) in the amount of exodeviation seen in primary gaze position after tenotomy of the superior oblique muscles.41 This difference in findings could be the result of different surgical techniques, such as performing the superior oblique tenotomy on the nasal versus the temporal side of the superior rectus muscle.
When the oblique muscles are judged to be functioning normally in the presence of an A or V pattern, vertical displacement of the recessed or resected horizontal rectus muscles is required to treat the pattern. The recessed lateral muscles are moved down for an A pattern and up for a V pattern, and the resected medial muscles are moved in the opposite direction. When a muscle is infraplaced or supraplaced, the question arises as to how the recessed or resected insertion should be placed. The surgeon must determine whether the insertion should be moved and placed parallel to the original insertion, parallel to the limbus, or at a location between the two. Another suggestion is that the displaced pole (inferior pole in infraplacement and superior pole in supraplacement) should be recessed further or placed further posterior than the other pole to create a greater effect and limit any torsional effect. I prefer to make the new insertion parallel to the original insertion.
Surgical Technique
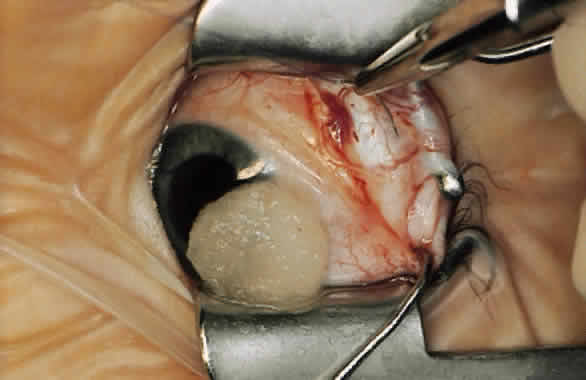
I favor a fornix incision more than a limbal incision unless extensive scarring from previous surgery makes the limbal approach a better option. A double-armed 6-0 Polyglactin 910 suture coated with polyglactin 370 (coated Vicryl) is used. This synthetic absorbable suture is 18 in long.
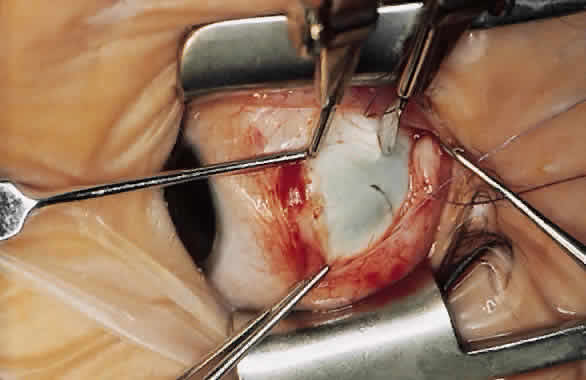
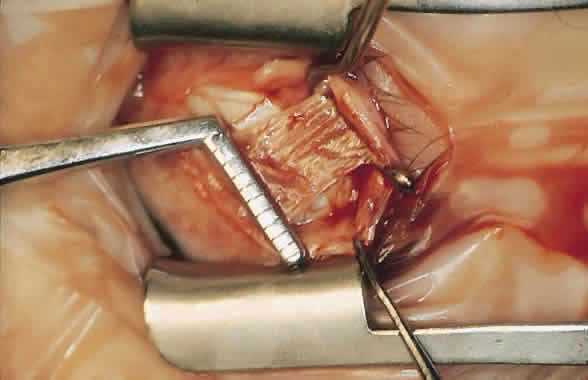
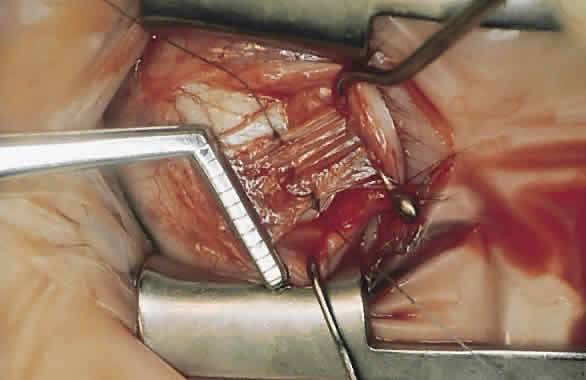
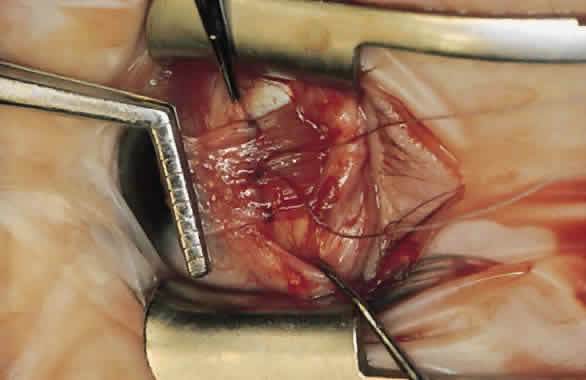
For a recession, the suture is placed in the muscle 1 mm from the scleral insertion. One end of the suture is started near the middle of the muscle (tendon) 1 mm back from the sclera and is passed into the belly of the muscle, exiting at the lateral margin (Figs. 1 and 2). The needle is passed back through the muscle, perpendicular to the plane of the muscle and 1.5 to 2 mm in from the muscle edge to create a lock bite (Fig. 3). The suture is double locked. The other end of the suture is passed similarly, from the middle of the muscle through the muscle belly, out the other side. A lock bite is taken. This suture placement is a modified von Pirquet technique that was popularized by Parks.42
|
After the sutures have been placed, the muscle is disinserted from the globe with Westcott scissors. The cut is made as nearly flush to the globe as possible, and a minimal stump from the original insertion is visible under the replaced conjunctiva postoperatively.
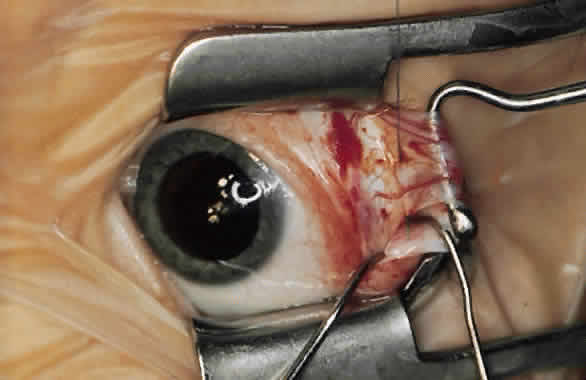
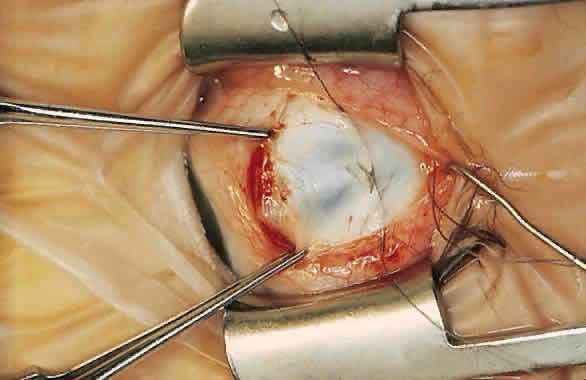
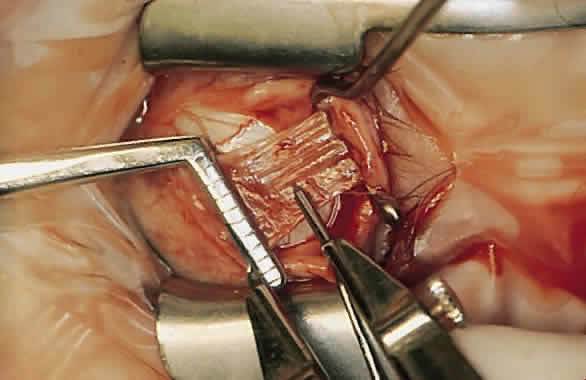
The advantage of this technique is that the suture that passes through the muscle between the lock bites at each edge maintains the normal width of the muscle when the muscle is removed from the insertion and resutured to the globe (Fig. 4). Placing the suture more than 1 mm from the insertion results in a resection as well as a recession and decreases the expected effect of the recession.
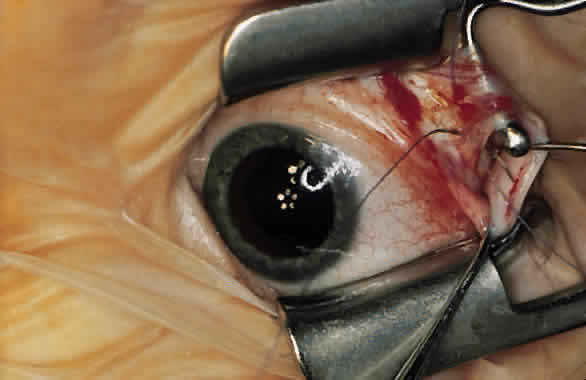
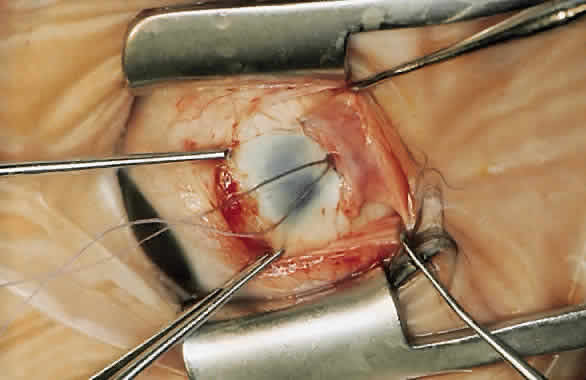
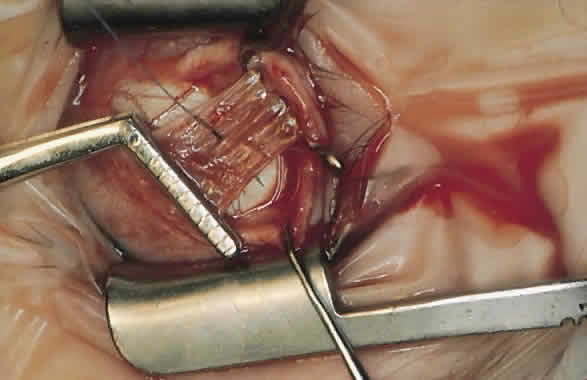
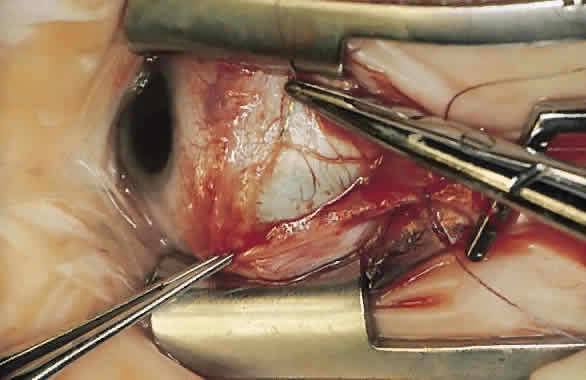
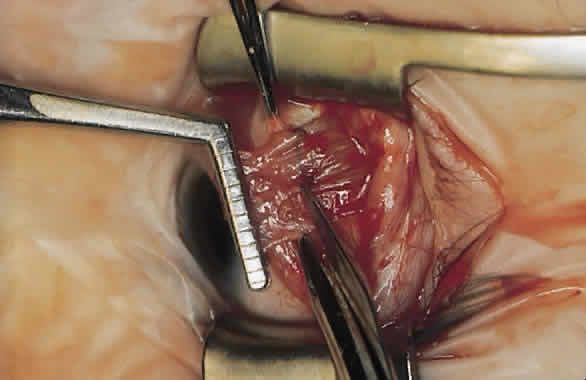
The caliper is set at a predetermined amount to mark the site of the new insertion. The sclera is marked for needle placement directly posterior to one pole of the original insertion. The mark and the start of the subsequent needle pass must be made perpendicular to the end of the insertion to eliminate unintentional supraplacement or infraplacement. The second mark is made perpendicular to the other end of the original insertion (Fig. 5). The needles are passed so that their tips cross (Fig. 6). This placement allows the knot to be tied on a spot rather than around the curve of the globe. Tying a knot around the curve of the globe is similar to tying a string around a ball. After the muscle is pulled up and the knot is tied, each end as well as the middle of the muscle is measured with the caliper from the original insertion (Figs. 7 and 8).
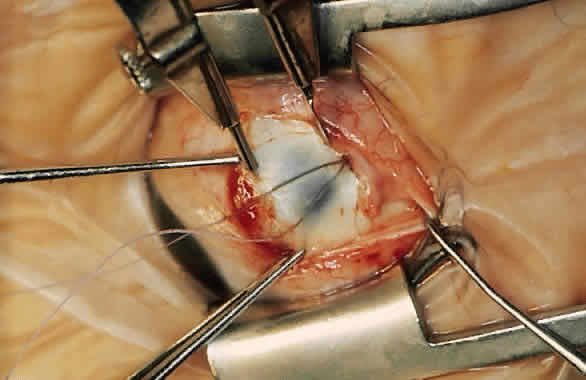
If the middle of the recessed muscle is sagging more than 0.5 mm, then one end of the suture is brought up through the middle of the muscle. The needle is passed just behind the area where the preplaced suture passes through the muscle. The middle of the muscle is then pulled up when the suture that is placed through the middle of the muscle is tied to the other end of the suture.
It is important to measure from the same place on the muscle insertion, either the anterior, middle, or posterior part. I find it easiest to measure from the posterior edge because it is usually the most distinct landmark after muscle disinsertion. The accuracy of recessions should be within 0.5 mm.
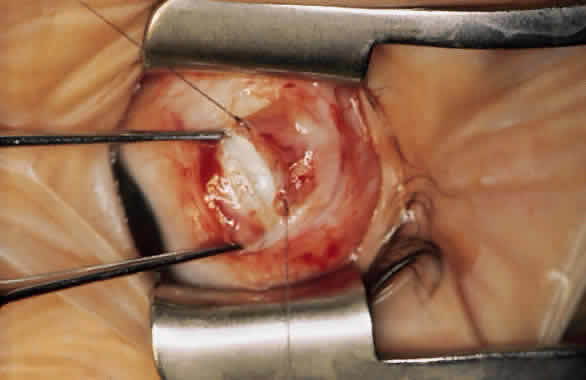
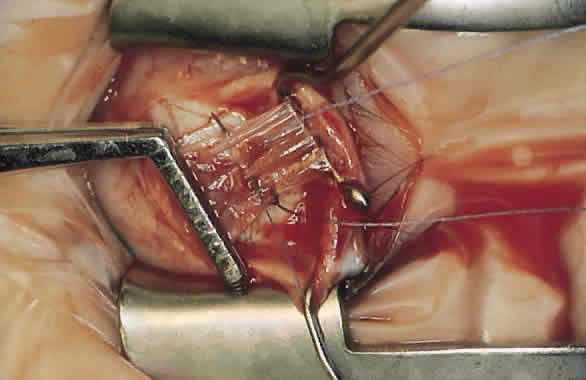
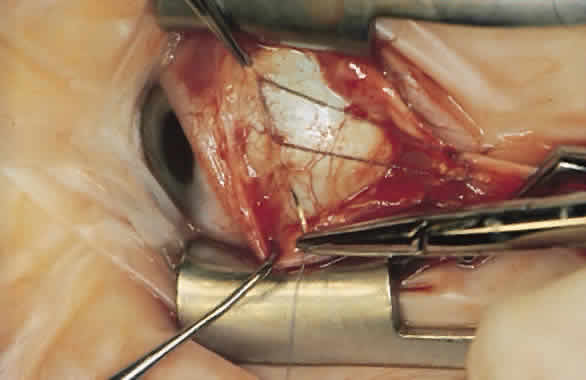
When a resection is performed, the medial rectus muscle is isolated. The muscle is cleaned of anterior Tenon's tissue and intermuscular septum back for 3 to 4 mm more than the amount of planned resection to allow for suture placement in the muscle.
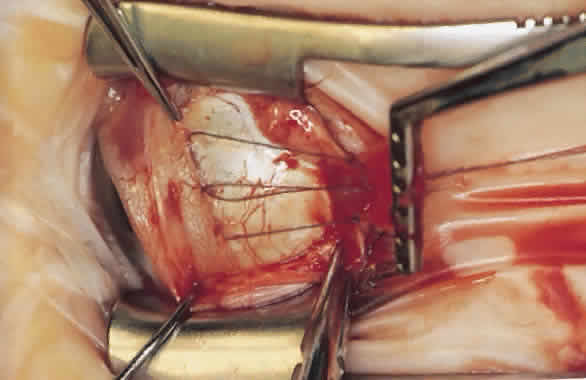
During a resection, a Jameson muscle clamp is placed around the muscle (tendon) as close to the insertion as possible (Fig. 9). With a caliper, the amount of muscle to be resected is measured back from the insertion on the belly of the muscle (Fig. 10). The double-armed 6-0 Polyglactin 910 suture is placed in the muscle at this point, following the procedure that is used for a recession. One end of the suture is locked at each margin of the muscle (Figs. 11, 12, and 13). The muscle is then disinserted. The preplaced sutures are positioned in the original scleral insertion. One is started at each end of the insertion, and both exit in the middle (Figs. 14 and 15). The sutures are passed up through the muscle belly, just behind the preplaced suture (Fig. 16). The Jameson clamp is used to position the muscle across the insertion at the level of the preplaced sutures, and the sutures are tied (Fig. 17). The muscle distal to the suture is then exercised (Fig. 18).
|
|
|
|
|
Postoperative Care
The first postoperative visit occurs within 1 to 3 days. Vision is measured, and ductions and versions are evaluated for the possibility of a slipped muscle. If a slipped muscle is suspected, operative exploration is indicated within 24 to 48 hours. A slipped muscle that is found and replaced at this time has no effect on motility. If surgical intervention is delayed, however, the muscle may be harder to find, and contractures may occur in both the slipped muscle and the antagonist muscle. At this point, more surgery than simple reattachment of the slipped muscle may be required to compensate for the contractures. I examined a patient with a slipped medial rectus muscle 2 days after surgery. The child had a difficult time standing and walking with the fellow eye occluded. This test may be used in addition to a careful evaluation of ductions and versions when a slipped muscle is suspected.
Infection is rarely evident less than 48 hours after the procedure. A topical antibiotic-steroid combination is used by some, but its penetration into an inflamed conjunctiva is questionable. Additionally, it may be difficult to place drops or ointment into the sore postoperative eyes of a child. I do not routinely use postoperative topical medications. Systemic antibiotics are used only when an infection is suspected. These eyes require close follow-up. If significant inflammation is present, particularly in an eye that has had several operations to correct strabismus, oral steroids (prednisone, 10 mg/day under age 5 years and 20 mg/day over age 5 years, for 5 days) will reduce the postoperative reaction and are more effective than topical steroids.
Esotropia is the desired alignment in the early postoperative period. If the eyes are straight or show a slight exodrift, a return of the intermittent exotropia is highly likely. The desired amount of esotropia varies with the age of the patient and the type of exotropia. For adults with intermittent exotropia, esotropia should be minimal: 6 to 8 PD. For children with intermittent exotropia, esotropia should be greater, especially if a bilateral lateral rectus recession was performed.43 In children who have undergone bilateral lateral rectus recession, my target is 12 to 20 PD of esotropia when examined on the second day after surgery. For a recess-resect procedure, my goal is 8 to 12 D. Esotropia of as much as 30 PD after a bilateral lateral rectus recession may result in a good postoperative result, but large amounts of esotropia after a recess-resect procedure may result in persistent overcorrection. For patients with sensory exotropia, the desired early postoperative position is up to 5 PD of esotropia.
If a strong fixation preference is seen or a difference in vision is detected, part-time patching is begun. When vision is equal but one eye is markedly dominant, the dominant eye is patched for 2 hours per day. If the patient is alternating and the esotropia is within the target range, no occlusion is done. I prefer to observe the patient every 1 to 2 weeks until the esotropia resolves. If the esotropia persists, patching, echothiophate iodide (Phospholine Iodide, Wyeth-Ayerst, Philadelphia, PA), hyperopic spectacles, and base-out Fresnel prisms to reestablish fusion are used in an attempt to achieve good alignment. When esotropia is present postoperatively, some surgeons do not schedule follow-up visits with patients for 6 weeks because the most frequent problem in intermittent exotropia treatment is the return of an exodeviation. However, I prefer to examine these patients at 7 to 14 day intervals until the esotropia resolves. Therapy is aimed at eliminating persistent secondary esotropia, the development of monofixation, and other complications.
For example, if a patient has esotropia of 20 PD on the first postoperative visit and shows no fixation preference, no treatment would be offered. The next examination would be in 2 weeks. If a marked reduction in esotropia is noted during this examination, the patient is rescheduled in 2 weeks. If the eyes are almost straight at that time, then the next visit would be 6 weeks later.
If the patient has esotropia of 20 PD at the 2 week visit, 4 hours daily patching of the dominant eye, or alternate patching if no marked dominance is present, should be started. A return appointment is scheduled for 1 week later. If the esotropia persists with little change, Phospholine Iodide (0.125% every day in both eyes) would be administered. The patient is reexamined after 1 week. If the esotropia persists, but some variability in the amount of esotropia is seen, Phospholine Iodide might be prescribed for only 4 days. Often, all that is needed is administration of Phospholine Iodide for 3 or 4 days to begin the eyes moving toward orthotropic alignment.
If esotropia persists at the next visit (5 to 6 weeks), patching is increased to half time and Phospholine Iodide administration is continued for 1 more week. A week later, if the esotropia persists, hyperopia of + 2.00 D or greater is prescribed or, if not hyperopic, plano glasses are lent the patient. In either case, press-on Fresnel prisms are placed to reestablish fusion. The amount of prism fitted is equal to the amount of the measured esotropic deviation at 6 m. The amount of prism is split between the two eyes. If the number to be applied is unequal, for example, 15 PD, 10 PD are placed in front of the preferred eye and 5 PD are placed in front of the nonpreferred eye. It is best if the prisms are fit in the ophthalmologist's office to avoid delay. If a supply of prisms is maintained in the office, they may be adjusted easily as changes occur in the deviation. Prisms may be reused and do not have to cover the entire lens. Frequently, after fusion is reestablished, there is an outward movement of the eyes. By reducing the amount of prism slowly over the next few visits, the eyes can be realigned, as described by Hardesty.44–46
If esotropia persists despite this treatment plan, the patient continues to wear the prisms to maintain fusion. Botulinum toxin A (Botox, Allergan, Irvine, CA) may be injected into one medial rectus muscle, or a second operation may be performed a few months later to correct the full amount of esotropia measured.
The few patients who continue to have esotropia will require a second operation, but most patients with intermittent exotropia who need a second operation do so because the exotropia returns. If exotropia returns and the first operation was a recess-resect procedure, the next operation will be a recess-resect procedure performed on the other eye. If the first operation was a bilateral lateral rectus muscle recession, the surgeon can either rerecess the lateral rectus muscle of both eyes or rerecess one lateral rectus muscle and resect the medial rectus muscle on the same eye. Usually, I prefer the latter approach because if subsequent surgery to correct an exodeviation is required, the other eye also may undergo a rerecess procedure on the lateral rectus muscle and a resection of the ipsilateral medial rectus muscle. I prefer not to resect both medial recti except when the patient has convergence insufficiency.
The decision to perform a second operation for return of intermittent exotropia should be made on the same basis and with the same considerations as the initial surgery. These considerations include an exodeviation greater than 12 PD, stable measurements of the angle of deviation, good general health, and frequency of the manifest deviation as noted by family, patient, and physician, with the divergence being seen on a daily basis from 25% to 50% or more of the time.
If an undercorrection is seen postoperatively, little nonsurgical therapy has proven successful. A spectacle correction can be prescribed to overminus the patient, forcing greater accommodation to align the eyes. This approach can be used only up to a certain age, and it has not been shown to have a lasting effect when the patient is returned to the normal spectacle refraction. Hardesty44 described the use of fusing base-in prisms for patients with undercorrection. The patients continue to use the prisms for several months or years. Hardesty44 reported cures in patients who otherwise would have required additional surgery.
Results
Most patients with intermittent exotropia show good alignment 6 weeks after surgery and then some have an exodrift with time. The biggest challenge is how to produce and maintain stability.
Hardesty and associates16 reported a functional cure rate of 78% in a series of 100 patients with intermittent exotropia. Of 100 patients, 27 underwent a second operation: 21 for undercorrection (return of the exodeviation) and 6 for persistent overcorrection. The patients had to meet the following criteria for cure: (1) no tropia, constant or intermittent, at either distance or near; (2) discontinuation of all supplementary therapy, such as prisms, orthoptics, and anticholinesterase medications; (3) no report by the patient or family of any manifest deviation; and (4) some degree of stereopsis as measured by the Titmus stereotest.16
Pratt-Johnson and associates18 reported a motor cure rate of 81% (with a manifest deviation of 10 PD or less 1 year after surgery) in a series of 100 patients who had divergence excess intermittent exotropia. If cure is defined as the presence of normal divergence and convergence amplitudes, with diplopia when these values are exceeded, and the presence of 40 seconds of near-stereo acuity, the cure rate was 41%.18 In this study, surgery performed in patients younger than 4 years old was a significant factor in obtaining a cure. Cure rates vary with the definition of cure.
In an attempt to determine the long-term stability of alignment in patients after surgery for intermittent exotropia, we reviewed the charts of 200 patients who underwent surgery for intermittent exotropia. The surgery was performed a minimum of 3 years earlier to allow at least a 3 year follow-up period, and all patients were observed for at least 6 months. Some were lost to follow-up after this 6 month period, and only 111 of 200 patients had at least a 3 year follow-up. This series shows the difficulty involved in obtaining long-term observations. A second operation was performed on 18 patients for return of intermittent exotropia and on 5 patients for persistent esotropia. Of 18 patients who underwent a second operation for exodeviation, 11 had the second operation more than 3 years after the first. Of the 11, 7 had good alignment and control 6 months postoperatively, and 4 of 11 continued to have good alignment 3 years after their initial surgery.
Although the percentage of patients who underwent a second operation was small in this series, another statistic may be more ominous. Of 111 patients who were followed for more than 3 years after surgery for intermittent exotropia and had no additional surgery, 41 had postoperative exodeviation measured at 6 m that was within 10 PD of their preoperative 6m exodeviation. The exodeviation usually was well controlled in this group of 41 patients. The exodeviation was not noted by the patients or families, so the patients were inclined to rate the surgery as a success. However, the surgery was not successful in producing an absolute reduction in the magnitude of the exodeviation, and some of these patients may need further surgery for exodeviation in the future.
Complications
Postoperative infection primarily may involve the conjunctiva, Tenon's space (including the operated extraocular muscles), or the intraocular space, such as occurs in endophthalmitis. Infective conjunctivitis is rare after eye muscle surgery, and an injected conjunctiva usually is not the result of infection, but a reaction to the surgery.
If postoperative infection involves an extraocular muscle, usually swelling and elevation of the conjunctiva overlying the muscle occur. The muscle usually has reduced function, and duction testing shows underaction of the involved muscle. The infection usually is treated with oral antibiotics. However, if no improvement is seen in 2 days, or if the situation seems to be worsening while the patient is receiving oral antibiotics, hospital admission, culture, and the use of intravenous antibiotics are indicated.
If the patient reports inappropriate pain, the eye is tender to touch, marked swelling and injection of the conjunctiva and lids are present, the situation seems to be worsening, and slit lamp examination shows intraocular inflammation, a diagnosis of endophthalmitis must be considered. Appropriate culture, consultation, and emergency basis treatment must be instituted as soon as possible.
Infections usually are manifest starting on the third day after surgery. A patient who was seen on the first or second day after surgery and reports a marked increase in pain, swelling, and redness after the third day must be examined to ensure that endophthalmitis is not the cause.
Penetration of the globe with a needle during extraocular muscle surgery is more common than postoperative infection. If a penetration or deep pass of the needle is suspected, the fundus must be examined. In these situations, I prefer to finish the operation and close the conjunctiva before examining the fundus. If penetration of the choroid or even the retinal pigment epithelium is seen, I do not use cryotherapy. If the penetration has gone through the retina, then cryotherapy is indicated. In either case, subconjunctival antibiotics are injected (cefazolin) and the patient is given oral antibiotics. The patient (if old enough to understand) and family are informed of the situation and told to call if the involved eye becomes more inflamed or painful than the other eye. Otherwise, follow-up is the same after the second postoperative day.
On the second postoperative day, in addition to looking for early signs of infection, the surgeon should pay attention to the version movements of the eyes and the duction movements of the operated muscles. If one of the operated muscles appears to be weak, with markedly decreased adduction or abduction of the involved eye, the possibility of a slipped muscle must be considered. If the limited function of the muscle does not seem to be related to swelling or postoperative reaction, and if the ocular alignment is significantly different from what was expected, the eye must be examined to determine whether a slipped muscle is present. If a slipped muscle is found, it must be reattached. It is important to reattach a slipped muscle within 24 to 48 hours of suspecting the diagnosis so that the muscle can be placed in its proper position. Because contracture of the slipped muscle and its antagonist will not have had time to occur, no other surgery should be needed.
One concern in performing surgery for intermittent exotropia is the fear of producing a monofixational small-angle esotropia. A patient who has intermittent exotropia and bifixation has been done a disservice if the surgical result is monofixation, or limited binocularity. Monofixation may occur in a small percentage of patients, and it is a good reason for ensuring that the exodeviation under surgical consideration is truly one of poor control and that surgery is necessary. However, the situation may not be as simple as it seems.
A study that investigated this concern of producing monofixation from bifixation concluded that approximately 30% of the patients with intermittent exotropia have monofixation rather than bifixation preoperatively in the phoric state.15 These patients have monofixation postoperatively and have not been poorly served by the surgery, particularly if after surgery, they have little or no measurable deviation and their condition is stable. Conversely, in this study, 70% of patients with intermittent exotropia had bifoveal fixation before and after surgery. Only one or two patients switched from bifixation to monofixation or the reverse, and at least one patient went each way. Aggressive postoperative therapy may be critical in preventing many new potential monofixations from occurring as a result of surgery. Careful postoperative care was exercised in this group of patients. Similar findings were reported by Haase and DeDecker.22 A discussion of this postoperative therapy is covered in the postoperative care section of this chapter.