|
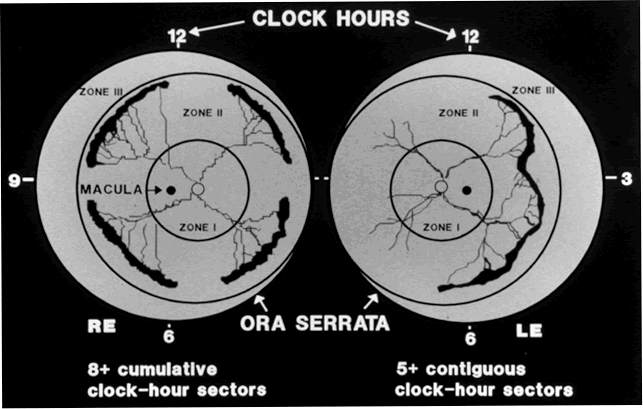
Although several screening protocols have been suggested, we have found that children whose birth weight was 1500 g or less should be screened initially at 4 to 6 weeks after birth, and then every 2 weeks until they reach retinal vascular maturity, which is when nasal vessels in the horizontal meridian have grown to within 1 disc diameter of the ora serrata. During that time, if a child shows threshold disease as defined by the Cryo-ROP Study, namely five clock-hours of contiguous or eight clock-hours of discontiguous neovascularization (stage 3 ROP with plus disease), it is recommended that the child have peripheral ablation with either cryotherapy or laser treatment.19 Newer studies have suggested that zone 1 children may require earlier peripheral ablation.20 The child who fails to respond to peripheral ablation may require further surgical intervention.
Two large series reported that scleral buckling for stages 4A and 4B retinal detachments resulted in a retinal reattachment rate of approximately 70%.21,22 These studies were both retrospective; however, they did show a strong trend toward a higher reattachment rate with scleral buckling than the natural history of these detachments, which have a 55% chance of progression of retinal detachment from stage 4 to stage 5 ROP.23
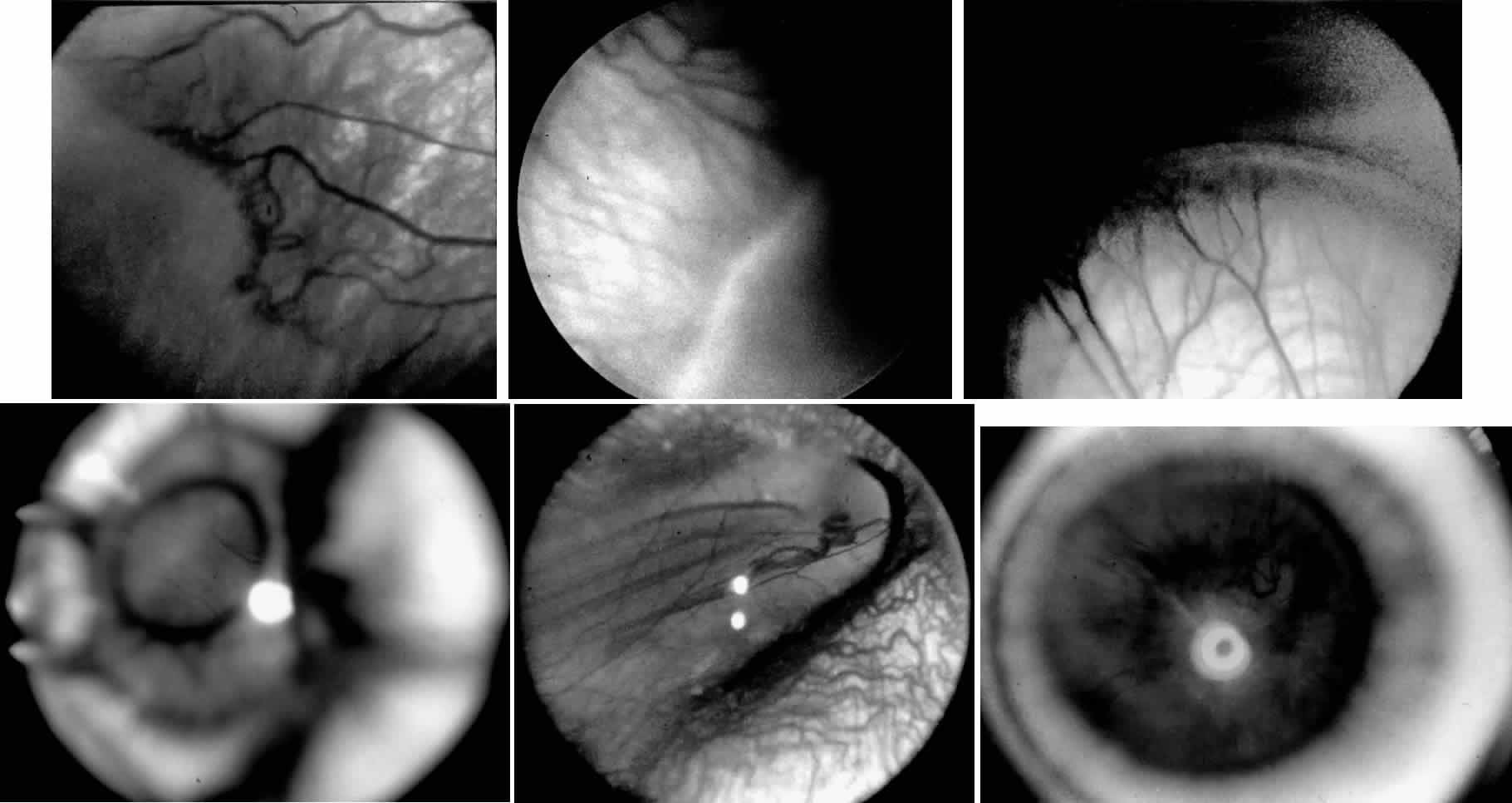
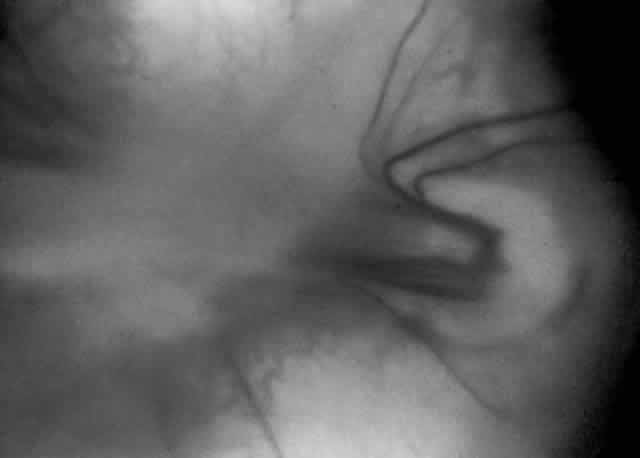
When first assessing a child's retinal detachment, one must judge the amount of effusive versus tractional detachment (Figs. 4 and 5). Scleral buckling would be considered for the child who shows a predominately effusive stage 4B detachment as opposed to a stage 4 predominately tractional detachment, for which lens-sparing vitrectomy may be recommended.24 For a child who has a great deal of retrolenticular touch, lensectomy/ vitrectomy and membrane peeling would be recommended. It appears that the “window” for lens-sparing vitrectomy may be rather brief. In one series, the postconceptual age of the lens-sparing vitrectomized eyes was 42.6 weeks, as opposed to 46.9 weeks for eyes that needed lensectomy/vitrectomy and membrane peeling. This small time difference in the postconceptual age shows the often rapid evolution of this detachment from one in which the lens is salvageable to one in which the lens is unsalvageable. This highlights the need for timely screening of eyes, identification of eyes with progressive disease, and rapid intervention.25
|
|
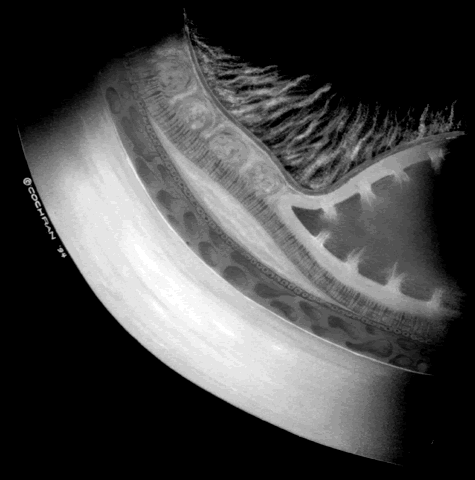
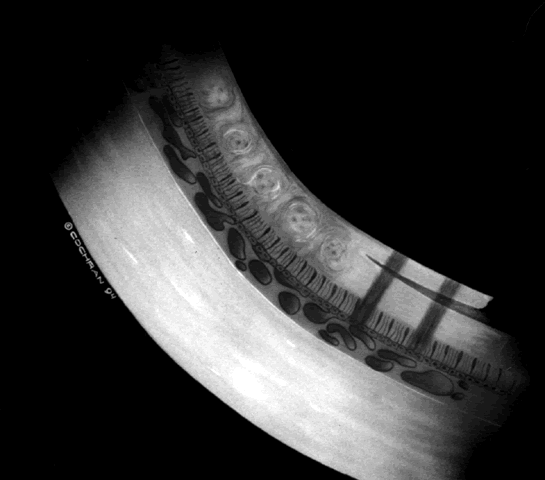
The techniques of vitreous surgery in children require an understanding of the anatomy of the pars plicata. A premature child does not have a well-defined pars plana, and therefore pars plicata entry is the only entry possible to avoid the lens upon entering the vitreous cavity. A term infant who is 8 months post term has a 2 mm pars plana.12 Infants who are premature at the time of vitreous surgery require entry immediately posterior to the iris root to avoid damage to the neurosensory retina and crystalline lens.
After vitreous surgery, particularly one that is lens-sparing, the final visual result depends on the child's central nervous system, refractive status, and competition with the fellow eye. With lens-sparing vitrectomy techniques, however, visual acuities can be made as good as 20/60, even in ROP cases.26,27 The child who has lensectomy/vitrectomy as well as membrane peeling with appropriate refractive correction can have a visual acuity as good as 20/200 to 20/400.28 The child's initial aphakic status can greatly affect his or her final visual outcome. This is why we believe strongly that prompt screening in order to time surgical intervention appropriately as well as reacting quickly to the child's surgical need are important means of optimizing final visual outcome. A child's refractive status is always difficult to deal with, especially if the child is aphakic or the red reflex is compromised. In children, we should not forget the need to assess near vision and the use of low vision aids.
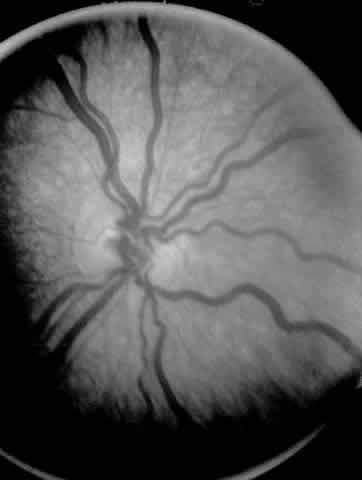
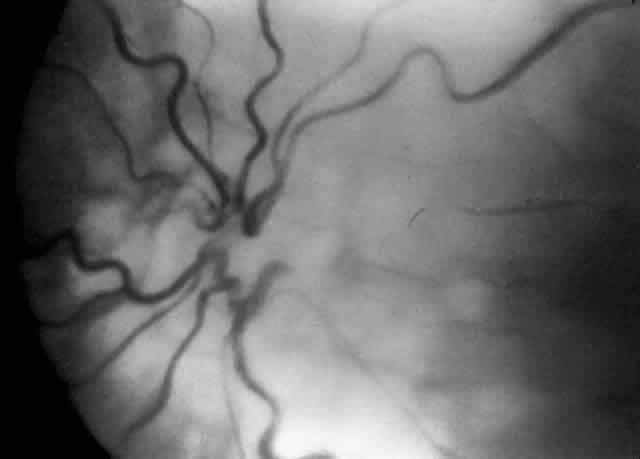
A child's retinal detachments progress at variable rates; thus the rate of detachment must be assessed on an individual basis. In children with RUSH disease, the eye has a very immature retina with much of the vascularized/avascular retinal juncture in zone 1. These eyes tend to progress to retinal detachment very quickly, often within 1 to 2 weeks. We have described another uncommon entity called very posterior zone 1 retinopathy of prematurity.29 In this disease, the macula is disorganized and not clearly visible. The posterior pole presents a syncytium of vessels all in zone 1. All of these eyes that we followed have gone on to have tractional retinal detachment. If there is to be any hope of vision in these patients, management requires a very rapid and broad peripheral ablative treatment followed by early vitreous surgery intervention.
The care of ROP patients requires a careful and rapidly performed screening examination, rapid intervention with peripheral ablation, scleral buckling, lens-sparing vitrectomy, or lensectomy/ vitrectomy and membrane peeling. Given a prompt intervention, we have come to believe that ROP can be managed with results comparable to those for patients with diabetes or proliferative vitreoretinopathy. To date, Droste and Trese30 are the only investigators to have reported on a consecutive series managed in that fashion, and this series showed improved visual results. Historically, visual results in ROP have been poor if retinal detachment intervention was delayed. With the advent of appropriate screening and rapid surgical intervention, however, improved visual results are possible.