| Although less common than contusion, penetrating injuries more often result
in severe visual impairment. Two types of injuries disrupt the continuity
of the corneoscleral layer of the globe: penetration by relatively
sharp objects, and rupture caused by massive blunt trauma. The visual
prognosis is favorable when the primary mechanical damage caused
by sharp penetration is limited to the anterior segment of the eye.9 Modern microsurgical techniques permit better wound closure and reconstruction
of the anterior ocular structures. Penetrating injuries involving
the posterior segment carry a less favorable prognosis. The primary
mechanical damage of vital structures by such injuries may be so great
that useful vision is instantly destroyed. In many cases, however, the
application of contemporary vitreoretinal microsurgical techniques
to prevent or treat secondary complications results in the preservation
of eyes that would otherwise be lost. Three current studies report
an incremental increase in the percent of penetrated eyes with a final
visual acuity of 0.1 from 29% in the 1930s to 67% in 1991.10–12 TERMINOLOGY To avoid confusion, penetration, in this chapter, is defined as a partial
cut, tear, or passage through a structure. The term perforation implies
complete cutting, tearing, or passage through the referenced structure. Therefore, a
corneal laceration is a perforation of the cornea
and a penetration of the globe. A foreign body that passes into and out
of the eye perforates the globe, but such injures are frequently called
double-penetrating, double-perforating, or through-and-through injuries. PATHOGENESIS The etiologies and, therefore, the mechanisms of damage of penetrating
injuries are highly variable. They cause a wide spectrum of acute structural
alterations and secondary complications that, consequently, call
for multiple methods of repair. Acute Effects of Ocular Contusion Penetration of the eye by relatively blunt objects causes compression of
the globe with resultant iridoparesis, iridodialysis, subluxation and
dislocation of the lens, traumatic cataract, choroidal rupture, and
retinal breaks at the vitreous base borders.13,14 Ruptures of the uvea produce anterior chamber, choroidal, subretinal, and
vitreous hemorrhage. Massive blunt trauma causes corneal and scleral
ruptures and may avulse the optic nerve. Acute Effects of Ocular Penetration Penetrating objects cause lacerations of the cornea, iris, lens, sclera, ciliary
body, choroid, retina, and optic nerve. Anterior chamber, choroidal, subretinal, and
vitreous hemorrhages result from lacerations
of uveal and retinal blood vessels. Perforation of the corneoscleral wall
permits prolapse and incarceration of the lens, uvea, retina, and
vitreous. Early Secondary Complications of Ocular Penetration The secondary complications of ocular penetration are important causes
of the visual impairment that results from such injuries. Examples of
early secondary complications include the toxic effects of intraocular
foreign material, such as copper, and the introduction of bacteria and
fungi with consequent infectious endophthalmitis. The chronic inflammation
caused by the presence of lens material, hemorrhage, and the incarceration
of vitreous and uvea, although less dramatic than infection
or violent toxicity, plays an important role in the stimulation of intraocular
fibrocellular proliferation.15–17 Intermediate Secondary Complications of Ocular Penetration Cleary and Ryan15 reported experimental evidence that blood and, to a lesser extent, lens
material in the presence of a large scleral wound caused fibrocellular
proliferation and membranes, the contraction of which produced tractional
retinal detachments (Fig. 4). The clinical consequences of fibrocellular proliferation and membrane
contraction are well established and include traction retinal detachments, retinal
breaks, rhegmatogenous retinal detachments, proliferative
vitreoretinopathy, cyclitic membranes, ciliary body detachments, hypotony, and
phthisis bulbi. 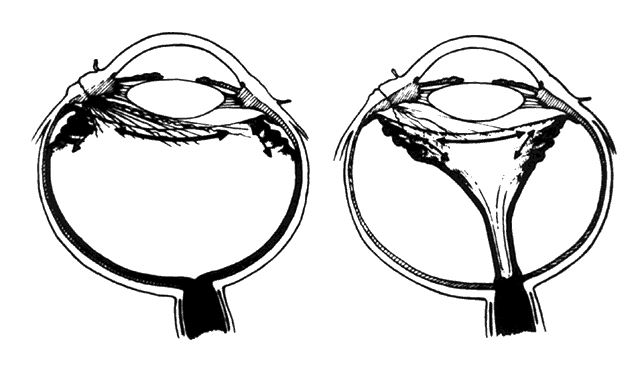 Fig. 4. Fibrocellular proliferation and membrane formation cause tractional retinal
detachment. (Cleary PE, Ryan SJ: Method of production and natural history of experimental
posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol 88:219, 1979. Published with permission from The American Journal
of Ophthalmology. Copyright by The Ophthalmic Publishing Company.) Fig. 4. Fibrocellular proliferation and membrane formation cause tractional retinal
detachment. (Cleary PE, Ryan SJ: Method of production and natural history of experimental
posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol 88:219, 1979. Published with permission from The American Journal
of Ophthalmology. Copyright by The Ophthalmic Publishing Company.)
|
Late Secondary Complications of Ocular Penetration Recurrent fibrocellular proliferation causing tractional and rhegmatogenous
retinal detachments and proliferative vitreoretinopathy is a common
late complication of penetrating injuries. Macular pucker is a similar
complication of lesser magnitude. Fungal endophthalmitis and sympathetic
ophthalmia are infrequent late complications of ocular penetration
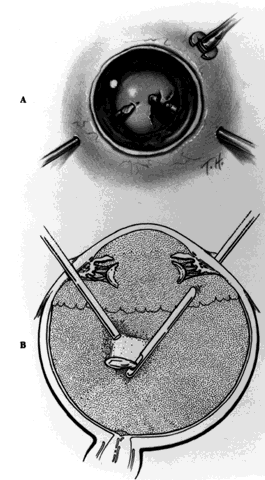
that are important because of their potentially devastating consequences. PRIMARY SURGICAL REPAIR Exploration of the Globe A careful exploration of the globe is indicated to determine the extent
of the defects in the eye wall whenever ocular penetration is established
or suspected. Although often the first step, exploration is deferred
until obvious anterior lacerations or ruptures are repaired so that
manipulations required to expose the posterior segment of the eye cause
no additional prolapse of ocular contents. A complete conjunctival
peritomy is performed and all quadrants of the globe inspected. Traction
on temporary sutures, looped around the rectus muscles, combined with
the retraction of conjunctiva and Tenon's fascia facilitates
visualization of the sclera beneath the extraocular muscles and posteriorly. Care
must be taken, when passing sutures or a muscle hook beneath
rectus muscles, to avoid inadvertent penetration through an undetected
defect in the sclera. Wound Closure Restoration of the structural integrity of the globe by careful anatomic
reapposition and creation of a watertight wound closure is the main
goal of the primary surgical repair of penetrating injuries. Corneal lacerations
are closed with deeply placed interrupted 10-0 nylon sutures. Interrupted 7-0 or 8-0 nylon sutures are used to repair scleral wounds
to withstand the stress of subsequent vitrectomy should a second operation
become necessary. Homologous corneal or scleral grafts or glue
may be used to close defects caused by the loss of tissue. Posterior
exit wounds are usually self-sealing and not repaired unless they are
large.18 Prolapsed lens material is removed and vitreous excised by use of Weck-cel
spears (Weck) and scissors, or a vitreous suction-cutter. Unless
necrotic and exposed for more than 24 hours, prolapsed uveal tissue is
reposited. Extruded retina is also reposited taking care to avoid incarceration. Reconstruction of the Anterior Chamber Reconstruction of the anterior chamber and restoration of the pupil are
important goals of the primary surgical repair. Iris and vitreous incarceration
in a corneal laceration causes chronic inflammation, peripheral
anterior synechiae, and closure of the anterior chamber angle. Incarcerated
vitreous provides a scaffold for fibrous ingrowth that creates
membranes, the contraction of which causes tractional retinal detachments
and retinal tears. With use of a cyclodialysis spatula, iris is
reposited through the wound or swept from it via a limbal incision on
the opposite side of the globe. Alternatively, sodium hyaluronate may
be used to reform the anterior chamber, protect a clear lens, if present, reposit
tissues, and keep them out of the wound. An anteriorly displaced
cataractous lens or lens fragments are removed with a vitrectomy
probe through the limbus or, if large choroidal detachments have been
excluded, the pars plana. Phacofragmentation may be needed to remove
hard lens material from the eyes of elderly patients, in which case
suction must be carefully monitored to avoid vitreous aspiration and
consequent traction. An anterior vitrectomy is performed and air is placed
in the anterior chamber to prevent recurrent iridocorneal adhesions
and reincarceration of the vitreous. Removal of Foreign Material Foreign material should be removed from the eye during the primary repair
of penetrating injuries. The management of intraocular foreign bodies
will be discussed later. If infection is present, a vitrectomy is performed
during the primary operation to remove organisms, exotoxins, and
inflammatory debris. In most cases, a traumatic cataract is removed during a second operation
unless its presence interferes with wound closure. The removal of blood
from the anterior chamber, should it become necessary to visualize
the posterior segment of the eye, is also best deferred until a secondary
repair is undertaken. Prophylactic Cryopexy The value of prophylactic cryopexy to surround the sutured wound is controversial. Although
the retina is lacerated by all perforations of the
eye wall that extend posterior to the ora serrata, many are self-sealed
by incarcerated vitreous and subsequent surrounding chorioretinal
scarring. Furthermore, experimental studies indicate that cryopexy breaks
down the blood-retina barrier and stimulates cellular proliferation
and migration, thereby causing membrane formation and tractional retinal
detachment.19 On the other hand, retinal lacerations were responsible for 20% of the
rhegmatogenous retinal detachments reported in one study of detachments
caused by penetrating injuries.20 We currently treat retinal lacerations with ophthalmoscopically monitored
light cryoretinopexy, when possible, but not if blood in the vitreous
prevents their visualization. Prophylactic Antibiotics Penetrating ocular injuries are complicated by infectious endophthalmitis
in 2% to 7% of cases.21 Its presence is often masked by the pain and inflammation of the injury, resulting
in a disastrous delay of the diagnosis. Approximately 25% of
cases of endophthalmitis after penetrating injuries are caused by Bacillus species, the virulence of which results in a poor prognosis.22,23 Systemic prophylaxis with intravenous antibiotics is the accepted standard
of medical care, although the low drug level obtained within the
vitreous cavity is of questionable value. For intravenous prophylaxis, we
prefer vancomycin for the coverage of increasingly prevalent strains
of penicillin- and cephalosporin-resistant gram-positive organisms. A
third-generation cephalosporin (ceftazidime) is used for gram-negative
coverage instead of parenteral aminoglycosides, which may cause renal
complications. Intravenous prophylaxis is continued for 3 to 5 days
depending on the level of concern generated by the nature of the penetrating
injury. The recently introduced antibiotic ciprofloxacin may
be of prophylactic value because of its reported penetrance of the eye
with systemic administration.24–26 Prophylactic antibiotics are injected into the vitreous cavity of eyes
at high risk for infection during the primary surgical repair if the needle
can be safely introduced and monitored. Foreign bodies contaminated
by soil cause approximately 90% of cases of post-traumatic Bacillus endophthalmitis.27 These virulent organisms are sensitive to vancomycin. Although gram-negative
organisms are rarely the cause of post-traumatic endophthalmitis, they
are covered more safely by ceftazidime than by gentamicin.28,29 We recommend the intraocular injection of 1 mg of vancomycin as well as 250 or 400 μg
of amikacin or 2.25 mg of ceftazidime when the history
and clinical findings indicate a high risk of infection. RETINAL BREAKS AND RHEGMATOGENOUS RETINAL DETACHMENT Traction retinal detachments due to fibrocellular proliferation, membrane
formation, and contracture are a characteristic feature of penetrating
ocular trauma. Nevertheless, approximately 75% of retinal detachments
after penetrating injuries are rhegmatogenous in origin.20 Retinal breaks are produced at the time of the injury and occur later
as sequelae of intraocular scarring. Acute Retinal Breaks The contusional component of penetrating injuries produces retinal breaks
identical to those caused by blunt, nonperforating trauma to the eye. Retinal
dialyses are found in 55% to 64% of eyes with retinal detachments
caused by ocular penetration.20,30 Horseshoe or opercular tears due to traction on the posterior edge of
the vitreous base and on isolated vitreoretinal adhesions occur in approximately 30% of
cases with detectable retinal breaks.20 Atrophic holes are less frequent (18%).20 Retinal lacerations are produced whenever the eye is penetrated posterior
to the ora serrata. They occur at the site of scleral perforation and
at the point of impact or exit of foreign bodies. Lacerations caused
retinal detachments in 12 (20%) of 60 eyes reported by Cox and Freeman.20 In 9 eyes, the lacerations occurred at foreign-body impact sites (Fig. 5). Faulty technique during magnet extraction caused iatrogenic retinal
lacerations in 2 eyes. Only 1 eye developed a retinal detachment from
a laceration at the point of scleral perforation because of the previously
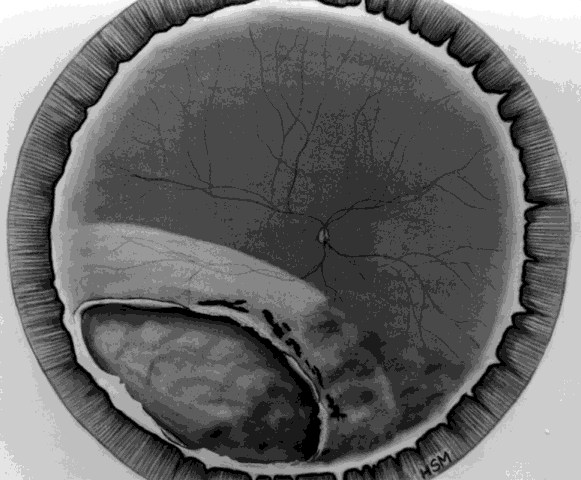
described self-sealing characteristics of these breaks. 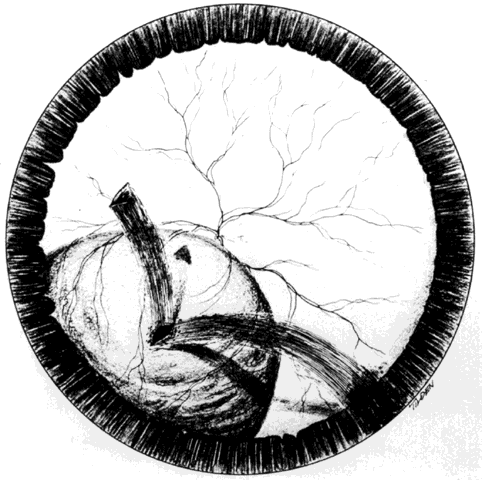 Fig. 5. Retinal laceration from impact of foreign body. Retinal detachment due
to ocular penetration. (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1357, 1978. Copyright 1978, American Medical Association.) Fig. 5. Retinal laceration from impact of foreign body. Retinal detachment due
to ocular penetration. (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1357, 1978. Copyright 1978, American Medical Association.)
|
Acute retinal breaks are treated during the primary repair of the injury, if
possible, to prevent subsequent detachment of the retina. The detection
of retinal tears is, therefore, a major goal of the preoperative
and intraoperative examinations. Manipulation of the eye, including
scleral depression, is deferred until all wounds have been securely closed. The
fundus is then examined with the indirect ophthalmoscope by
use of scleral depression, and particular attention is given to the region
of the vitreous base. All discovered retinal breaks are treated
with the same techniques previously described for tears caused by ocular
contusion. Posterior breaks are treated with either the indirect laser
or cryopexy if the breaks are accessible to the probe. Anterior breaks
can be treated through clear media with the indirect laser by use
of scleral depression. Cryopexy is preferred when breaks are partially
obscured by opacities of the media or when the diagnostic capabilities
of cryotherapy are used to freeze and thereby whiten the edges of an
otherwise occult tear. As previously described, we apply light cryopexy
to the edges of retinal lacerations at the site of scleral perforations
only if such treatment can be monitored ophthalmoscopically. Lacerations
caused by the impact of foreign bodies commonly cause detachments
of the retina and are therefore treated with cryopexy or the indirect
laser during the primary surgical repair.20 Opacities of the media, such as cataract and hemorrhage, often prevent
early diagnosis of breaks; this is a fact cited by advocates of early
vitrectomy.31 In our experience, acute breaks in the retina seldom cause detachments
immediately. The ocular media are cleared 7 to 14 days postinjury, during
the secondary surgical repair, so previously undetected retinal breaks
are discovered and treated at that time. Retinal detachments caused by the contusional component of penetrating
injuries are identical to those caused by blunt trauma. At this stage, they
are not complicated by intraocular scarring and therefore respond
favorably to conventional scleral buckling techniques. Detachments caused
by lacerations in the posterior retina are treated by vitrectomy, internal
drainage of subretinal fluid through the break with simultaneous
fluid-air exchange, endolaser, and long-acting gas tamponade. Giant
retinal tears and associated detachments require vitrectomy techniques
and perfluorocarbon liquids. Late Retinal Breaks Caused by Contracting Bands and Membranes Retinal detachments caused by contracting membranes are characteristic
of penetrating injuries.20 Shrinking transvitreal membranes initially cause traction detachments
of the retina opposite the site of scleral perforation (Fig. 6). In approximately 40% of cases, continued traction causes a dialysis
at the vitreous base and consequent rhegmatogenous retinal detachment (Fig. 7).20 Retinal incarceration in a scleral perforation produces a less common
but equally characteristic traction retinal detachment (Fig. 8). Retinal folds radiate from the site of incarceration. Associated vitreous
prolapse and entrapment cause traction on the adjacent vitreous
base with consequent detachment of the underlying peripheral retina and
pars plana epithelium. A retinal fold is created at the posterior border
of the vitreous base, which becomes increasingly prominent because
of the contracture of membranes interposed between the vitreous base
and the incarceration site. Progressive traction by this membrane may
cause breaks in the folded retina and consequent rhegmatogenous detachment (Fig. 9). 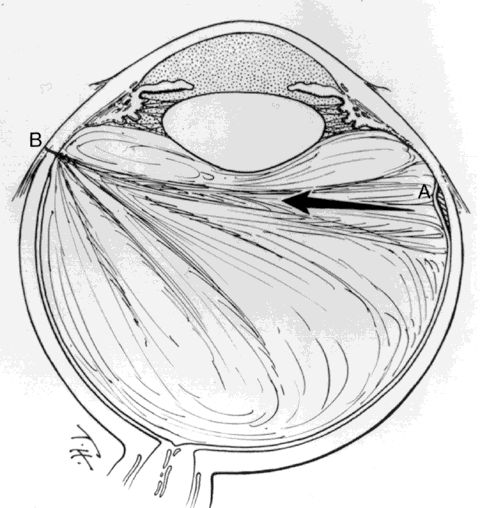 Fig. 6. Transvitreal traction causes tractional retinal detachment (A) opposite
perforation site (B). (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 210. St. Louis, CV
Mosby, 1990) Fig. 6. Transvitreal traction causes tractional retinal detachment (A) opposite
perforation site (B). (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 210. St. Louis, CV
Mosby, 1990)
|
 Fig. 7. Retinal dialysis caused by traction of shrinking membrane. Location of
scleral laceration (A). Vitreous membrane (B). Dialysis at vitreous base
border (C). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.) Fig. 7. Retinal dialysis caused by traction of shrinking membrane. Location of
scleral laceration (A). Vitreous membrane (B). Dialysis at vitreous base
border (C). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.)
|
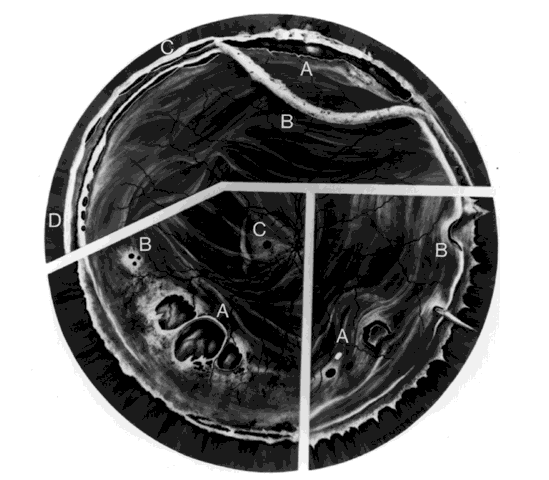
 Fig. 8. Retinal incarceration in scleral perforation. Fibrous proliferation from
wound (A). Vitreous membrane (B). Retinal folds (C). Detached pars plana
epithelium (D). Fold at posterior vitreous base border (E). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.) Fig. 8. Retinal incarceration in scleral perforation. Fibrous proliferation from
wound (A). Vitreous membrane (B). Retinal folds (C). Detached pars plana
epithelium (D). Fold at posterior vitreous base border (E). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.)
|
 Fig. 9. Retinal incarceration in scleral perforation. Dialyses in sharp fold at
posterior vitreous base border (A). Dialysis at anterior vitreous base
border (B). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.) Fig. 9. Retinal incarceration in scleral perforation. Dialyses in sharp fold at
posterior vitreous base border (A). Dialysis at anterior vitreous base
border (B). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1355, 1978. Copyright 1978, American Medical Association.)
|
Although some retinal detachments with breaks caused by membranous contracture
were successfully treated with broad, high, encircling scleral
buckles before the advent of vitreous microsurgery, approximately 50% failed
because of progressive postoperative traction.20 These cases are best treated by vitrectomy techniques, often combined
with scleral buckling, which are further discussed with other late complications
of penetrating injuries. GENERAL APPLICATIONS OF VITRECTOMY The role of vitreous microsurgery in the treatment of penetrating eye injuries
is not completely defined, but general principles and applications
have emerged with the refinement of instruments and techniques and
nearly two decades of experience. Although the value of these applications
has not been established by controlled trial, there is agreement
about the general principles of management. Removal of Foreign Material and Substances That Stimulate Intraocular Fibrocellular
Proliferation Nonmagnetic foreign bodies, and magnetic foreign bodies that are difficult
or dangerous to extract with an externally applied magnet, are removed
by forceps after vitrectomy. Vitrectomy is used in cases of post-traumatic
endophthalmitis to remove organisms, toxins, and inflammatory
debris. Material is also obtained for microscopic identification and
culture. Vitrectomy is used to remove blood and lens material from the
vitreous of eyes with posterior segment wounds to reduce the stimulus
of intraocular scarring. Removal of Damaged Vitreous To eliminate the scaffold for fibrocellular ingrowth, as much of the vitreous
gel as possible, including the cortex posterior to the equator, is
removed from eyes with severe posterior segment injuries. Removal of Aberrant Tissue and Correction of Abnormal Tissue Relationships Pars plana vitrectomy techniques effectively remove or reposit uveal tissue
from anterior segment wounds. Incarcerated lens and vitreous is also
excised. Relaxing retinotomies are performed to correct the distortion, folding, and
tension caused by retinal incarceration in posterior
segment wounds. Clearance of Opacities From thedOcular Media Cataractous lens and dense vitreous hemorrhage are removed to restore the
optical pathway and re-establish visualization of the fundus. Vitrectomy
thereby permits identification and treatment of retinal breaks. INDICATIONS FOR VITRECTOMY The selection of cases of ocular penetration for vitreous microsurgery
correlates with the general principles and applications described above. Vitrectomy
is performed when the risk of secondary complications is
high and the prognosis for recovery without vitreous surgery is historically
poor. Forceps extraction of reactive nonmagnetic foreign bodies
exposed by vitrectomy is clearly the method of choice in such cases. The
same approach is preferred over magnet extraction in some eyes with
magnetic intraocular foreign bodies. Vitrectomy is performed on eyes
with post-traumatic infectious endophthalmitis. Vitreous microsurgical
instruments are used, through limbal or pars plana incisions, to restore
the integrity of the anterior chamber when iris, lens, and vitreous
are incarcerated in corneal wounds. The historically poor prognosis
for eyes with large posterior wounds, vitreous prolapse, and vitreous
hemorrhage, particularly in globes perforated by medium to large foreign
bodies, indicates a need for vitreous surgery. Vitrectomy must be
performed when a retinal detachment is obscured by vitreous hemorrhage
of sufficient density to prevent repair by conventional techniques. TIMING OF VITRECTOMY Immediate Vitrectomy Vitreous microsurgery is performed during the primary repair of eyes with
reactive nonmagnetic intraocular foreign bodies. Immediate vitrectomy
is also preferred over external magnet extraction when metallic objects
are incarcerated in the retina and sclera or obscured by opaque media. An
immediate vitrectomy is performed on eyes infected by penetrating
injuries. Delayed Vitrectomy Vitrectomy is performed 7 to 14 days after the injury in cases where such
surgery is indicated but not an essential part of the primary repair. The
delay reduces the risk of recurrent hemorrhage from congested uvea, allows
clearing of corneal edema and hyphema, provides time for additional
evaluation and diagnostic testing, and permits complete prognostic
discussion and disclosure with the patient and family. Serial ultrasonography
during this interval provides a better preoperative understanding
of structural relationships such as detachment of the choroid, retina, and
vitreous. It is particularly helpful to identify the posterior
separation of the vitreous from the retina, the occurrence of
which greatly facilitates complete removal of the vitreous cortex posterior
to the equator of the globe. Liquefaction of blood in the suprachoroidal
space can be identified by ultrasound, thereby establishing
the proper timing of evacuation.32 Vitreous surgery is not delayed beyond 14 days. A timely enucleation can
be performed to reduce the risk of sympathetic ophthalmia if exploratory
vitrectomy discloses irreparable damage. The excision of damaged
vitreous at this stage removes the scaffold for fibrocellular ingrowth
before membranes form and traction begins. The maturation of membranes
after 14 days can produce scars with a thickness and density that make
their removal difficult or impossible. Late Vitrectomy Vitrectomies are performed after the 7- to 14-day window when secondary
complications of ocular penetration develop. Traction or rhegmatogenous
retinal detachment caused by contracting membranes requires vitreous
microsurgical repair. Vitrectomy techniques are also needed if late
or recurrent intraocular scarring leads to macular pucker or proliferative
vitreoretinopathy. MANAGEMENT OF POST-TRAUMATIC ENDOPHTHALMITIS Epidemiology Infection is a devastating complication of penetrating ocular trauma. It
occurs in 1% to 10% of eyes and is more prevalent in cases with intraocular
foreign bodies.21,33,34 The visual prognosis of post-traumatic endophthalmitis is less favorable
than that of other postoperative infections because of traumatic damage
and the virulent profile of the organisms involved.21,35,36 The diagnosis is frequently masked, and therefore delayed, by the inflammation
of the injury itself. The relatively benign course of cases with Staphylococcus epidermidis endophthalmitis after surgery occurs less consistently in traumatized
eyes infected with the same organism.37 The prognosis is very poor when gram-negative organisms and Bacillus species are involved, which occurs in 10% to 20% of cases, respectively.21,38 Bacillus species cause a rapid onset of panophthalmitis associated with severe
pain, proptosis, fever, and leukocytosis. A characteristic ring corneal
ulcer develops in 18 to 24 hours.39 The virulence of this species results from the production of potent exotoxins--an
enterotoxin and a hemolysin.40 Once produced, these exotoxins may cause destruction of tissue, even after
the eye is sterilized by antibiotics, and bacterialysis may result
in their further release. Collagenase may enhance the diffusion of toxins
and bacteria throughout the eye.39 The production of destructive proteases and endotoxin causes the virulence
of gram-negative organisms.41 Endotoxin is released after bacterial cell death and lysis. Removal of
bacteria by vitrectomy may decrease endotoxin-mediated tissue destruction
and further dilute and wash out exotoxins. Treatment After secure wound closure, undiluted aqueous and, if possible, vitreous
samples are obtained for the identification of pathogenic organisms
by microscopic inspection and culture. One tenth of a milliliter of aqueous
is aspirated into a tuberculin syringe through a 27- or 30-gauge
needle passed through the limbus. Prior to infusion, vitreous samples (0.3–0.5 mL) are
obtained by manual aspiration after the vitrectomy
probe is introduced into the vitreous cavity. If possible, vitreous
sampling is deferred and infusion delayed until the suction-cutter
and infusion cannula can be visualized. Aqueous and vitreous samples are
immediately placed on slides for Gram and Giemsa stains, and plates
of chocolate agar, blood agar, and Sabouraud's medium are inoculated. The
possibility of anaerobic infection is investigated by inoculating
chopped meat glucose broth or thioglycolate broth. A vitrectomy is performed on all eyes with obvious infectious vitritis. In
some cases, infection of the vitreous is not apparent until opacities
of the media, such as cataractous lens and hemorrhage, are removed. A
pars plana approach is delayed when the posterior segment of the eye
cannot be visualized. Also, instruments cannot be safely passed into
the vitreous cavity through the pars plana when ultrasound reveals large
choroidal detachments. In such cases, the vitrectomy probe and infusion
system (a 21-gauge needle or lighted infusion needle) are first
introduced into the anterior chamber through limbal incisions. Opacities
in the anterior segment are cleared. After the drainage of choroidal
hemorrhage or the posterior reposition of detached retina, if present, by
sodium hyaluronate, gas, or perfluorocarbon liquid, a standard
infusion cannula is introduced into the vitreous cavity through the pars
plana. The extent of the vitrectomy is determined by the clarity of
the cornea. A generous core vitrectomy is performed when visibility permits
such an undertaking. No effort is made to separate the cortex from
the retina or remove the peripheral vitreous because infection-induced
retinal necrosis increases the likelihood of retinal breaks. In one
series, all eyes with retinal breaks and endophthalmitis were lost.21 Presumably, iatrogenic breaks would lead to the same outcome. A more complete
vitrectomy is safely performed in eyes with posterior vitreous
separation from the retina. When visibility is very limited, a small
core is removed from the central vitreous cavity as long as detachment
of the retina has been excluded by preoperative ultrasonography. Intravitreal Antibiotics Antibiotics are injected into the vitreous cavity after samples have been
obtained for culture and the vitrectomy has been completed. Because
of the recent emergence of gram-positive organisms resistant to penicillin
and first-generation cephalosporins, particularly Bacillus species, we substitute vancomycin for the previously recommended combination
of cefazolin and clindamycin.23 We also use amikacin in place of gentamicin to reduce the likelihood of
retinal toxicity. Our current regimen of intravitreal antibiotics, therefore, consists
of vancomycin 1 mg/0.1 mL and amikacin 250 to 400 μg/0.1 mL, as
recommended by the Endophthalmitis Vitrectomy Study Protocol
for the treatment of postoperative endophthalmitis.42 Rarely, ceftazidime 2.25 mg/0.1 mL is substituted for amikacin. Postoperative Antibiotics We use regular-strength broad-spectrum topical antibiotics (trimethoprim
sulfate-polymyxin B, bacitracin-polymyxin B, ciprofloxacin, or gentamicin) four
to six times daily when corneal infection is absent. Infectious
corneal infiltrates are treated more aggressively with frequent “fortified” gentamicin and vancomycin. Topical steroids (prednisolone 1%) are
used four times daily, or more frequently, depending
on the amount of intraocular inflammation. As previously described, the value of systemic antibiotics is questionable
because of poor penetrance of the blood-ocular barrier and the resultant
low intravitreal concentration achieved. We continue to use intravenous
vancomycin or cefazolin in combination with a third-generation
cephalosporin effective for gram-negative organisms (ceftazidime). Postoperative
intravenous antibiotics will remain the standard of medical
care unless proved to be of no value by a controlled trial such as
the Endophthalmitis Vitrectomy Study.42 MANAGEMENT OF INTRAOCULAR FOREIGN BODIES The prognosis for eyes with intraocular foreign bodies (IOFBs) is better
than the prognosis for other types of serious penetrating trauma. A
recent series reported a final visual outcome of 20/40 or better in 60% of
eyes and 5/200 or better in 86% of eyes.43 Before the advent of vitreous microsurgery, magnetic foreign bodies carried
a more favorable prognosis than nonmagnetic ones because good results
were obtained by electromagnetic extraction. With the introduction
of foreign-body forceps, the controlled removal of all types of foreign
material became possible. Preoperative Evaluation The case history often reveals the foreign-body composition and the relative
risk of post-traumatic infection. Samples of the suspect material
are obtained, if possible, to determine its toxicity and magnetic properties. In
eyes with clear media, the exact composition, size, and location
of an IOFB are revealed by ophthalmoscopy. Magnetic properties
can be verified prior to extraction by observing movement in response
to an electromagnet that is positioned increasingly closer to the eye
until motion occurs or is excluded. In eyes with cloudy media, ancillary tests are needed to determine the
composition, size, shape, location, accessibility, and magnetic properties
of an IOFB. Plain x-ray films, with Caldwell or Waters projections, may
demonstrate the presence, but not the location, of radiopaque foreign
objects, and will not detect radiolucent objects such as wood or
glass. Ultrasound provides better foreign-body localization and is essential
to determine other structural changes such as retinal and choroidal
detachment. Care must be taken to avoid undue pressure on eyes
with large wounds to prevent additional prolapse of ocular contents. Computed
tomography (CT) scanning is the diagnostic study of choice because
it detects and localizes radiolucent and radiopaque foreign objects
in three dimensions.44 A rough determination of IOFB composition is provided by its density, because
wood is less dense than plastic, which is less dense than glass, which, in
turn, is less dense than metal. Unfortunately, scatter from
metallic foreign bodies can make localization difficult and erroneous. Magnetic
resonance imaging (MRI) is contraindicated because the motion
of a magnetic IOFB can cause significant intraocular damage.45 Indications for Removal All foreign bodies are removed during the primary repair of penetrating
injuries because of their potential toxicity and relatively frequent
association with post-traumatic endophthalmitis. Some IOFBs are inert, such
as glass, gold, silver, platinum, and aluminum, and cause little
continuing damage. Lead and zinc cause a mild nongranulomatous inflammation
of the eye. The toxicity of copper-containing foreign bodies is
determined by the concentration of copper. Metals with less than 70% copper
are relatively inert, whereas those with 70% to 90% copper lead
to ocular chalcosis. An acute suppurative inflammation is produced by
pure copper foreign bodies. The chronic toxicity of iron-containing metals
causes ocular siderosis. To avoid the risk of operative complications, encapsulated IOFBs that have
been present for a long time without causing ocular toxicity can be
observed as an alternative to removal. Serial electroretinograms are
obtained to monitor possible ocular siderosis in eyes with retained iron-containing
foreign bodies. Surgical Technique Foreign bodies in the anterior segment of the eye are removed by diamond-coated
foreign-body forceps through the wound before its repair or through
a limbal incision of appropriate size. Small inert intralenticular
IOFBs may be observed if the lens is clear. Toxic intralenticular
foreign bodies and those that have caused a cataract are removed through
the limbus by forceps or a rare earth magnet during extracapsular cataract
extraction. A posterior chamber intraocular lens may be simultaneously
inserted into an intact capsular bag or in the sulcus anterior
to the capsule when the case is otherwise uncomplicated. In eyes with clear media, small to medium-sized magnetic IOFBs in the vitreous
cavity are removed by an electromagnet through the pars plana
after closure of the entrance wound
(Fig. 10). A full-thickness scleral incision, down to but not through the pars
plana epithelium, is made 3.5 to 4 mm posterior and parallel to the limbus
on the side of the globe opposite the IOFB. The length of the incision
must permit an unobstructed exit of the foreign object from the
eye so that it does not become trapped in the vitreous base. In some cases, the
incision is extended anteriorly from its center to form a T-shaped
exit wound for relatively large IOFBs. The exposed pars plana epithelium
is treated with light diathermy to minimize subsequent hemorrhage
and cause slight retraction of the edges of the incision. A nonabsorbable
mattress suture is preplaced to permit prompt wound closure
after the IOFB is removed, thereby minimizing vitreous loss. The magnet
must be aligned with its long axis pointing directly at the foreign
body because its power is focused at the base, not the tip, of the conical
attachment. Misalignment will attract the IOFB toward tissues adjacent
to the sclerotomy. With proper technique, the foreign body rapidly
transits the vitreous cavity and works its way through the pars plana
epithelium because of the pulsed attractions of the magnet. Failure
to exit is usually due to a small incision rather than resistance of
the uveal tissue. It is seldom necessary to penetrate the pars plana epithelium
with a knife. 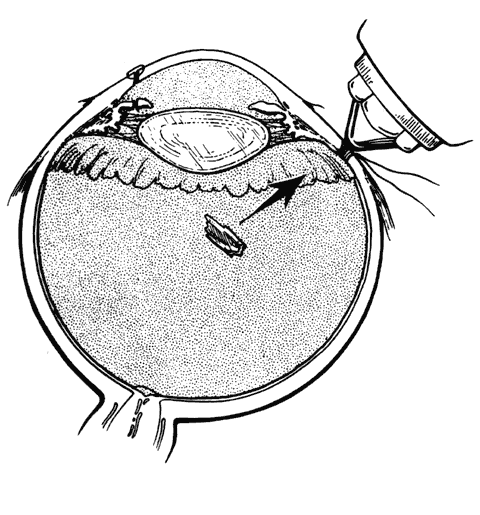 Fig. 10. Electromagnetic extraction of small to medium-sized magnetic foreign body. (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 480. St. Louis, CV Mosby, 1989) Fig. 10. Electromagnetic extraction of small to medium-sized magnetic foreign body. (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 480. St. Louis, CV Mosby, 1989)
|
After extraction of the IOFB, the fundus is inspected and retinal breaks, if
present, are treated by the methods previously described. With scleral
depression, the exit site is visualized. No treatment is applied
except in the rare instance where tractional elevation or breaks are
detected in the adjacent retina or pars plana epithelium, in which case
light cryopexy is used to surround the defects with chorioretinal or
cilioretinal adhesions. In eyes with clear media, small magnetic IOFBs embedded in the retina can
be extracted through the underlying sclera by an externally applied
magnet if they are anteriorly located and, therefore, accessible. The
embedded foreign body is surrounded preoperatively by slit lamp-delivered
argon laser or intraoperatively by indirect laser, after which its
position is carefully localized ophthalmoscopically. A sclerotomy is
performed where the IOFB is located after nonabsorbable sutures are positioned
to create a localized scleral buckle. The magnetic extraction
of the foreign body is immediately followed by placement of a silicone
sponge explant. Traction on bridle sutures is simultaneously relaxed
to reduce the intraocular pressure, thereby minimizing vitreous loss
and the risk of retinal incarceration, which is the principal complication
of this technique. Medium- to large-sized magnetic IOFBs embedded
in the retina are removed by vitreous microsurgical techniques even
from eyes with clear media. Posterior transscleral extraction of such
objects creates large exit wounds with attending prolapse and incarceration
of vitreous and retina. Nonmagnetic foreign bodies and magnetic IOFBs in eyes with opaque media
are removed by vitrectomy techniques. The integrity of the globe is first
secured by watertight closure of entrance wounds. A three-port pars
plana approach is used but must be deferred in severely disorganized
eyes when blood or a cataractous lens in the anterior segment prevents
visualization of instruments passed through the pars plana. In such
cases, the anterior opacities are first cleared by a vitrectomy probe, and
an infusion needle is placed through limbal incisions, after which
the instruments are safely transferred to appropriate pars plana incisions. When
ultrasound clearly demonstrates the absence of retinal or
choroidal detachment, the removal of anterior opacities may begin primarily
through pars plana incisions placed 3 to 3.5 mm posterior to the
limbus. A lighted infusion needle can often be visualized through cloudy
media and is, therefore, used to eliminate the possibility of subretinal
or suprachoroidal infusion during the initial removal of the
opaque anterior vitreous. It is replaced by a standard infusion cannula
and separate endoilluminator when the anterior vitreous cavity has been
cleared. In the eyes of young patients, soft cataracts are easily
removed by the vitrectomy probe, but phacofragmentation may be needed
when hard lenses are encountered in the eyes of older patients. Proceeding posteriorly, opaque vitreous is removed until the IOFB is identified
and exposed, after which it is grasped by foreign-body forceps. Surrounding
adherent vitreous is removed by the vitrectomy probe before
the foreign body is elevated to the midvitreous cavity; from there
it is passed through a pars plana incision of appropriate size. The
IOFB may be exchanged between forceps in the vitreous cavity to alter
its alignment, thereby presenting the smallest diameter to the exit wound. Alternatively, large
foreign bodies in aphakic eyes may be passed
through a limbal incision. An open-sky technique may be the only possible
means of removing very large foreign objects. The cornea is usually
severely damaged by the entrance of such IOFBs and must be removed
to visualize and facilitate their extraction. Eyes with very large foreign
bodies are usually severely disorganized, are seldom salvaged, and
generally come to enucleation. Intraretinal nonmagnetic foreign bodies and embedded magnetic IOFBs unsuitable
for posterior transscleral magnet extraction because of their
large size, posterior location, or the presence of opaque media are removed
by vitreous microsurgery. Intraretinal magnetic IOFBs are not removed
by magnet through the pars plana because their passage through intervening
retina can produce large lacerations and detachments (Fig. 11).20 Approached and exposed in the same manner described above, they are gently
dislodged, grasped with foreign-body forceps, and removed. A lighted
pick can be used to manipulate the IOFB into a position or orientation
favorable for a secure grip by diamond-coated forceps. Alternatively, magnetic
intraretinal foreign bodies may be elevated to the midvitreous
by a rare earth magnet, after which they are removed by forceps (Fig. 12) to avoid being dislodged in the vitreous base, as often occurs when extraction
by the magnet itself is attempted. Intraretinal foreign bodies
of 1 to 2 weeks' duration may become encapsulated (Fig. 13A), in which case the capsule is incised by a myringotomy knife or sharp
needle before it can be grasped by forceps or dislodged by the rare earth
magnet (see Fig. 13B). 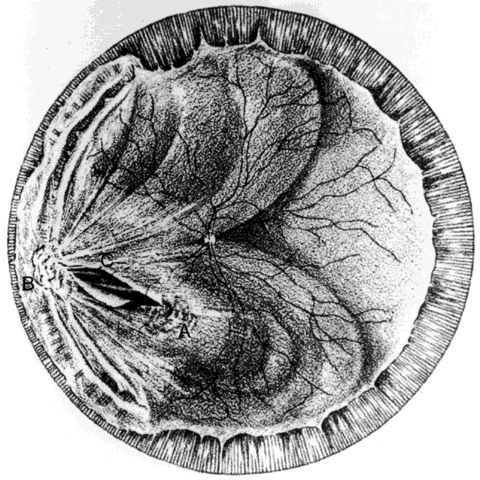 Fig. 11. Laceration by foreign body dragged through retina during magnet extraction. Initial
location of foreign body (A). Extraction site (B). Retinal
laceration (C). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1358: 1978. Copyright 1978, American Medical Association.) Fig. 11. Laceration by foreign body dragged through retina during magnet extraction. Initial
location of foreign body (A). Extraction site (B). Retinal
laceration (C). (Cox MS, Freeman HM: Retinal detachment due to ocular penetration. I. Clinical
characteristics and surgical results. Arch Ophthalmol 96:1358: 1978. Copyright 1978, American Medical Association.)
|
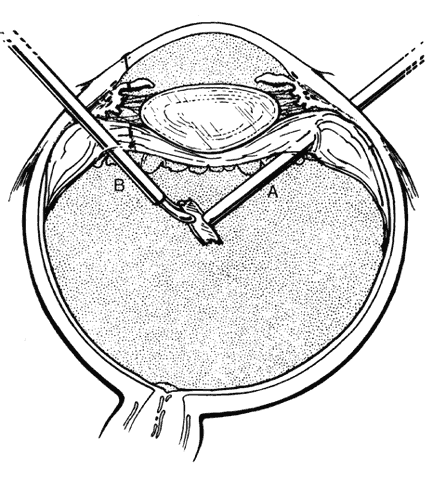 Fig. 12. Midvitreous transfer of foreign body from rare earth magnet (A) to intraocular
forceps (B). (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 481. St. Louis, CV Mosby, 1989) Fig. 12. Midvitreous transfer of foreign body from rare earth magnet (A) to intraocular
forceps (B). (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 481. St. Louis, CV Mosby, 1989)
|
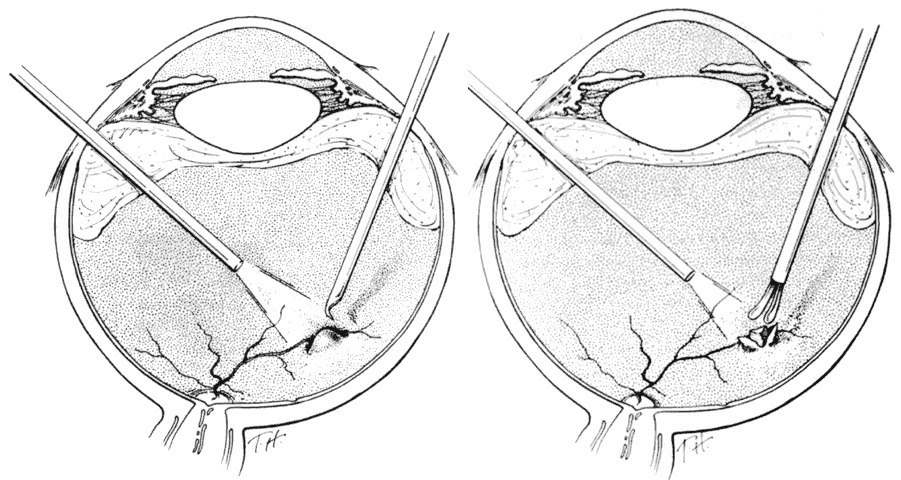 Fig. 13. Removal of encapsulated intraocular foreign body. A. Capsule surrounding
foreign body is opened with sharp blade or pick after removal of vitreous
gel. B. Exposed foreign body is grasped by forceps and removed. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 837. St. Louis, CV
Mosby, 1990)A Fig. 13. Removal of encapsulated intraocular foreign body. A. Capsule surrounding
foreign body is opened with sharp blade or pick after removal of vitreous
gel. B. Exposed foreign body is grasped by forceps and removed. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 837. St. Louis, CV
Mosby, 1990)A
|
The vitreous cortex posterior to the equator is removed, if possible, from
eyes with intraretinal foreign bodies. If not detached spontaneously, it
is separated with a retinal pick (Fig. 14) or by suction with a soft-tipped cannula. The retinal laceration at the
site of foreign-body incarceration is surrounded by laser and supported
by a localized scleral buckle when the surrounding cortical vitreous
cannot be removed. If present, subretinal fluid surrounding the incarceration
site is drained internally through the retinal laceration
while a fluid-air exchange is performed. Endolaser is applied around the
break and the air is replaced by a short-acting gas tamponade. 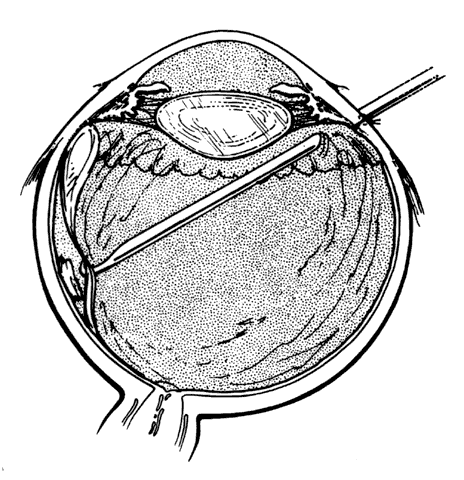 Fig. 14. Creation of posterior vitreous detachment by a vit-reoretinal pick (gentle
aspiration with soft-tipped cannula is an alternative). (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 484. St. Louis, CV Mosby, 1989)A Fig. 14. Creation of posterior vitreous detachment by a vit-reoretinal pick (gentle
aspiration with soft-tipped cannula is an alternative). (Sternberg P: Trauma: Principles and techniques of treatment. In Ryan SJ (ed): Retina, Vol 3, p 484. St. Louis, CV Mosby, 1989)A
|
Although retinal lacerations at foreign-body impact and incarceration sites
are frequently self-sealing, they lead to subsequent retinal detachments
in some cases. Therefore, we treat these lacerations, and retinal
breaks located elsewhere in the fundus, as previously described. An
encircling scleral buckle is almost routinely used to support the residual
peripheral vitreous, particularly in phakic eyes, where peripheral
dissection is limited, and in cases where residual opacities interfere
with visualization of the vitreous base. SECONDARY SURGICAL REPAIR After the primary closure of wounds, some eyes require vitreous microsurgery
performed 7 to 14 days after the injury. The purpose of the vitrectomy
is to prevent or minimize secondary complications of the ocular
penetration. As previously described, iris, vitreous, and lens incarcerated
in anterior segment wounds are removed to restore the anterior
chamber, clear the pupil, and eliminate the scaffold for fibrous ingrowth. The
vitrectomy is performed on eyes with large wounds of the posterior
segment, vitreous prolapse, and vitreous hemorrhage to remove blood, disrupted
lens, and damaged vitreous, thereby reducing the stimulus
and eliminating the scaffold for fibrocellular ingrowth. As previously
described, the anterior segment is cleared and all the vitreous, including
the cortex, is removed posterior to the equator. Globes perforated (double penetration) by medium to large foreign bodies, in
particular, require a second operation (Fig. 15A). Although a complete vitrectomy is performed, the vitreous over a large
self-sealed exit wound is isolated from adjacent structures and trimmed
but not completely removed. A small vitreous plug is left behind
to avoid reopening the wound (see Fig. 15B). 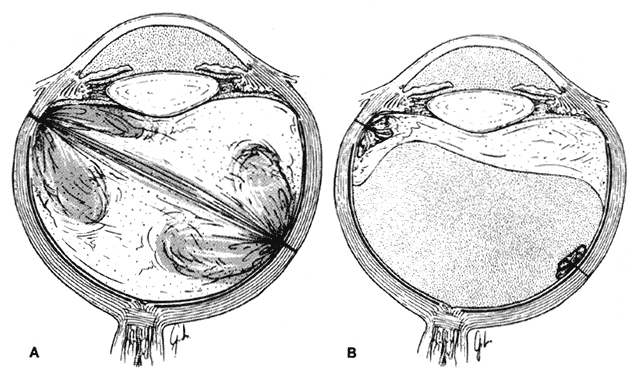 Fig. 15. Treatment of eye with double perforation. A. Transvitreal path of foreign
body and vitreous hemorrhage. B. Vitreous gel excised, including cortical
vitreous posterior to equator. Plug of vitreous gel remains over
exit wound. (Michels RG, Wilkinson CP, Rice TA: Vitreous surgery. Retinal Detachment, p 837. St. Louis, CV
Mosby, 1990)A Fig. 15. Treatment of eye with double perforation. A. Transvitreal path of foreign
body and vitreous hemorrhage. B. Vitreous gel excised, including cortical
vitreous posterior to equator. Plug of vitreous gel remains over
exit wound. (Michels RG, Wilkinson CP, Rice TA: Vitreous surgery. Retinal Detachment, p 837. St. Louis, CV
Mosby, 1990)A
|
Retinal incarcerations causing fixed radiating folds are sometimes encountered
during the secondary repair. Small incarcerations require no treatment
unless they prevent retinal reattachment or the folds distort
the macula. Peripheral retinal incarcerations, of medium size, can be
supported by a scleral buckle if necessary. When successful, the radiating
folds flatten with time. Scleral buckles fail when the peripheral
incarceration is large and are less beneficial when incarcerations are
posterior. Relaxing retinotomies are performed when fixed folds, unresponsive
to scleral buckling, prevent retinal reattachment or distort
the macula.46 Focal incarcerations of the posterior retina are treated by a relaxing
retinotomy that circles the site (Fig. 16). Endodiathermy is preplaced on the proposed path of the retinal incision
to minimize bleeding. The retina is reattached by internal drainage
of subretinal fluid through the retinotomy and simultaneous fluid-air
exchange. The retinotomy is surrounded by two to three rows of endolaser, after
which the air is replaced by an isoexpansive concentration
of perfluoropropane gas. 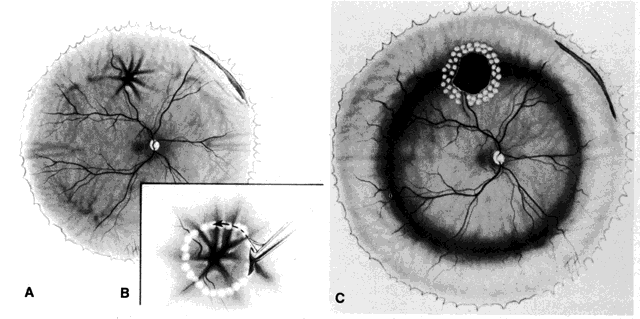 Fig. 16. Release of focal retinal incarceration. A. Superior penetrating injury
with retinal incarceration, retinal dialysis, and retinal detachment. B. Endodiathermy
surrounding incarceration. Retina cut 360° around
incarceration site. C. Postoperative appearance. Laser endophotocoagulations
applied to edges of retinotomy. (Abrams GW: Retinotomies and retinectomies. In Ryan SJ (ed): Retina, Vol 3, p 326. St. Louis, CV
Mosby, 1989)A Fig. 16. Release of focal retinal incarceration. A. Superior penetrating injury
with retinal incarceration, retinal dialysis, and retinal detachment. B. Endodiathermy
surrounding incarceration. Retina cut 360° around
incarceration site. C. Postoperative appearance. Laser endophotocoagulations
applied to edges of retinotomy. (Abrams GW: Retinotomies and retinectomies. In Ryan SJ (ed): Retina, Vol 3, p 326. St. Louis, CV
Mosby, 1989)A
|
The folds radiating from a peripheral incarceration of the retina are relaxed
by an equatorial retinotomy placed posterior to the location of
the incarceration, the two ends of which are carried anteriorly to eliminate
all folds (Figs. 17A and B). For hemostasis, the incision is preceded by endodiathermy. If the retina
is highly detached and mobile, 2 to 3 mL of perfluorocarbon liquid
placed in the posterior vitreous cavity will stabilize the retina and
thereby simplify the retinotomy. All traction on posterior retinal breaks, if
present, should be eliminated before the infusion of perfluorocarbon
so the material does not flow under the retina. When the retinotomy
is small and the posterior edge does not roll over, the retina
is reattached by fluid-air exchange with perfluorocarbon liquid removal. The
flattened retinotomy is surrounded by laser, supported by an encircling
scleral buckle, and the air is replaced by long-acting gas. 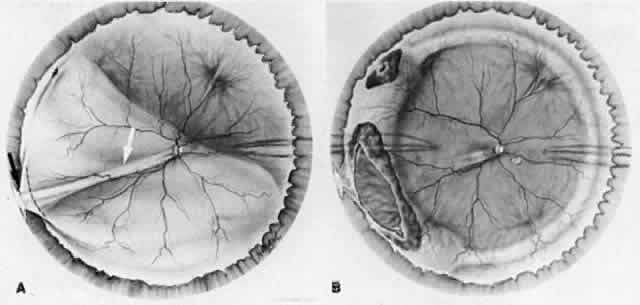 Fig. 17. Release of peripheral retinal incarceration. A. Rhegmatogenous retinal
detachment with retinal fold (white arrow) radiating from incarceration
site (black arrow). Fold relaxed by equatorial retinotomy (dotted line). B. Retina
reattached. Encircling buckle supports retinotomy and peripheral
break. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 209. St. Louis, CV
Mosby, 1990)A Fig. 17. Release of peripheral retinal incarceration. A. Rhegmatogenous retinal
detachment with retinal fold (white arrow) radiating from incarceration
site (black arrow). Fold relaxed by equatorial retinotomy (dotted line). B. Retina
reattached. Encircling buckle supports retinotomy and peripheral
break. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 209. St. Louis, CV
Mosby, 1990)A
|
The retina is sometimes massively incarcerated in distant scleral ruptures (Fig. 18A). Such prolapse is encouraged by hemorrhage into the suprachoroidal space
opposite the wound. It is necessary to perform a large retinotomy
of 180° to 270° to release the retina, in which case a giant tear
with a rolled posterior edge is created. Reattachment of the retina
is accomplished by the injection of perfluorocarbon liquid into the
posterior vitreous cavity as fluid is released anteriorly. The peripheral
retina is supported by a broad scleral buckle of moderate height, and
laser is applied 360° (see Fig. 18B). The perfluorocarbon is replaced by air, which in turn is replaced by
silicone oil. An inferior iridectomy is performed in aphakic eyes (the
lens is lost in virtually all of these cases) to prevent the prolapse
of oil into the anterior chamber postoperatively.47 We prefer silicone oil over long-acting gas in eyes with large retinotomies
because a tamponade of long duration is achieved without the need
for reinjection postoperatively. Postoperative positioning is also less
restrictive with oil than with gas, and the transparency of oil permits
early assessment of the visual acuity of the eye, which is helpful
in determining the potential value of subsequent reoperations should
they become necessary. In young patients, the long-term complications
of silicone oil are significant, and therefore it is removed approximately 6 months
postoperatively when the retina is secure. 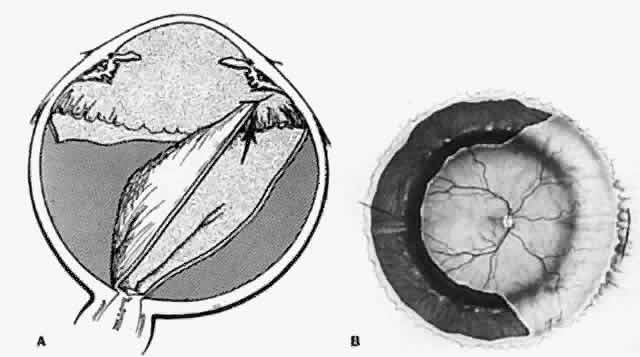 Fig. 18. Release of distant retinal incarceration. A. Torn retina incarcerated in
wound on opposite side of eye. B. Retina reattached after release from
wound by large retinotomy. (Abrams GW: Retinotomies and retinectomies. In Ryan SJ (ed): Retina, Vol 3, p 327. St. Louis, CV
Mosby, 1989)A Fig. 18. Release of distant retinal incarceration. A. Torn retina incarcerated in
wound on opposite side of eye. B. Retina reattached after release from
wound by large retinotomy. (Abrams GW: Retinotomies and retinectomies. In Ryan SJ (ed): Retina, Vol 3, p 327. St. Louis, CV
Mosby, 1989)A
|
Hemorrhage is sometimes encountered during the secondary repair. Temporary
hemostasis is frequently achieved by raising the level of the infusion
bottle, which increases the intraocular pressure and occludes both
injured and normal vessels. Subsequent lowering of the infusate may
reopen and thereby identify the injured vessel, which is cauterized with
endodiathermy. Focal bleeding vessels in attached retina may be closed
with endoargon laser, which has the added advantage of avoiding the
adhesions that sometimes form between the target vessel and an endodiathermy
probe. Often, bleeding sites cannot be identified or are not accessible to focal
cautery. In these cases, a fluid-air or fluid-silicone oil exchange
may be helpful. Vascular compression and concentration of clotting factors
have been proposed as possible explanations for the hemostatic effect
of these agents.48 The addition of thrombin to the infusate in a concentration of 100 U/mL
may control occult or diffuse bleeding sites.49,50 It should be washed out at the end of surgery to avoid postoperative inflammation
and fibrin formation. A damaged cornea may severely limit the visibility and, consequently, the
secondary repair of pathology in the posterior segment of the eye. Edematous
corneal epithelium is removed and the interference of striae
reduced by the application of sodium hyaluronate to the endothelial surface. Nevertheless, it
is sometimes necessary to use a temporary keratoprosthesis
in difficult cases. The Eckardt device is preferred because
it permits a good view of the fundus periphery.51 The prosthesis is replaced by donor cornea at the end of the operation. Choroidal hemorrhage is commonly caused by penetrating trauma. Although
usually small and self-limiting, massive bleeding into the suprachoroidal
space can expel ocular contents through an open wound or force the
apposition of inner retinal surfaces in the center of the vitreous cavity (kissing
choroidal detachment). The untreated prognosis of small
to medium-sized choroidal hemorrhages is good. Large choroidal hemorrhages
must be treated during the secondary repair of penetrating injuries
when they interfere with the correction of associated pathology such
as retinal detachments. Uncontrollable elevation of the intraocular
pressure is also an indication for drainage of large choroidal hemorrhages. Hemorrhagic choroidal detachments are often first diagnosed by ultrasound, which
is a good method of following their evolution and resolution. Liquefaction
of clotted blood, which occurs between 7 and 14 days, can
be demonstrated ultrasonographically. Thus, the optimal time for drainage, if
necessary, is established. Drainage is accomplished through
several sclerotomies placed to correspond to the major accumulations
of suprachoroidal blood. Air is simultaneously infused into the restricted
preretinal space by an air pump, which automatically controls the
intraocular pressure. To avoid penetration of the retina, the infusion
needle is first placed into the anterior chamber through the limbus. When
sufficient drainage has been accomplished, infusion is transferred
to a standard pars plana port, air is replaced by fluid, and the remaining
repairs are performed. Alternatively, sodium hyaluronate or silicone
oil may be used as the primary infusate to facilitate visualization
of intraocular structures as the operation proceeds posteriorly. The
cohesive quality of silicone oil, although limited, tends to maintain
the integrity of the expanding preretinal space better than sodium
hyaluronate, but the view is better through Healon (Kabi Pharmacia). The
intraocular pressure is maintained throughout these maneuvers to avoid
hypotony and consequent recurrent choroidal hemorrhaging. Recently, we have treated choroidal hemorrhages by infusing perfluorocarbon
liquid into the vitreous cavity instead of gas, sodium hyaluronate, or
silicone oil. The heavy expanding mass of perfluorocarbon in the
posterior vitreous cavity displaces the blood in the suprachoroidal space
anteriorly toward the sclerotomies, thereby facilitating its removal. The
same technique can be used advantageously to force subretinal
blood from the posterior pole toward anterior retinal breaks or retinotomies, through
which it is aspirated and eliminated. LATE VITRECTOMY Vitreous microsurgery can be performed whenever reparable late complications
arise. These include complicated traction and rhegmatogenous retinal
detachments caused by incarceration and contraction of intraocular
scars, and the effects of reproliferation, such as macular pucker and
proliferative vitreoretinopathy. Complicated Traction and Rhegmatogenous Retinal Detachments The detachments most characteristic of penetrating trauma are the traction
elevations of the vitreous base and peripheral retina opposite a scleral
wound (see Figs. 6 to 9). Progressive contracture of transvitreal membranes causes late dialyses
at the vitreous base borders as previously described. These membranes
are excised with a vitrectomy probe or, if mature and difficult to
remove, by sharp dissection with a combination of scissors, knife, and
forceps. The vitreous cortex posterior to the equator is removed. It
has usually separated from the retina spontaneously, in which case the
excision is accomplished without difficulty. When posterior vitreous
detachment is absent, the cortex must be dissected from the retina. Epiretinal
membranes, which may have formed after the injury, must also
be removed. Traction detachments of the peripheral retina flatten after
membrane removal and are additionally supported by a broad encircling
scleral buckle of moderate height (Fig. 19). Rhegmatogenous detachments with large dialyses are reattached with perfluorocarbon
liquid, as previously described, after the release of traction. A
broad encircling scleral buckle is also used in these cases, but
the height is limited to prevent radial folding of the retina.  Fig. 19. Encircling scleral buckle supporting peripheral traction detachment after
membrane removal. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 834. St. Louis, CV
Mosby, 1990) Fig. 19. Encircling scleral buckle supporting peripheral traction detachment after
membrane removal. (Michels RG, Wilkinson CP, Rice TA: Vitreous Surgery. Retinal Detachment, p 834. St. Louis, CV
Mosby, 1990)
|
Repair of retinal detachments complicated by incarceration and scar contracture
requires relaxation by retinotomy and membrane dissection as
described above. An encircling scleral buckle is used to support the peripheral
retinotomy and the vitreous base. In cases of posterior incarceration
and subsequent relaxing retinotomy, a circumferential scleral
buckle is also routinely used to support the peripheral vitreous. Cellular Reproliferation and Recurrent Intraocular Scarring Are Common
After Ocular Penetration The treatment of proliferative vitreoretinopathy and macular pucker, a
minor manifestation of the same process, is described elsewhere in these
volumes. |