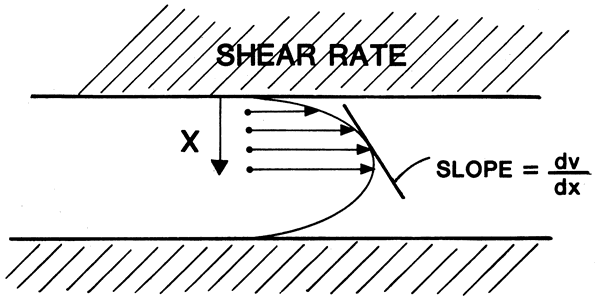
1. Hultsch E: The scope of hyaluronic acid as an experimental intraocular implant. Ophthalmology 87:706, 1980 2. Peyman GA, Ericson ES, May DR: A review of substances and techniques of vitreous replacement. Surv Ophthalmol 17:41, 1972 3. Edelhauser HF, MacRae SM: Irrigating and viscous solutions. In Sears M, Tarkkanen A (eds): Surgical Pharmacology of the Eye, pp 363–388. New York, Raven Press, 1985 4. Benson WE, Diamond JG, Tasman W: Intraocular irrigating solutions for pars plana vitrectomy. Arch Ophthalmol 99:1013, 1981 5. Moorhead LC, Redburn DA, Merritt J, Garcia CA: The effects of intravitreal irrigation during vitrectomy on the electroretinogram. Am J Ophthalmol 88:239, 1979 6. Alvarez MA, Jimeno JC: Role of intraocular irrigating solutions in the pathogenesis of the postvitrectomy
retinal edema. Curr Eye Res 6:1369, 1987 7. Intraocular Irrigating Solutions, pp 1–44. Fort Worth, Alcon Laboratories, 1992 8. Kaye GI, Mishima S, Cole JD, Kaye NW: Studies on the cornea: VII. Effects of perfusion with a Ca++-free medium on the corneal endothelium. Invest Ophthalmol Vis Sci 7:53, 1968 9. Kita M, Negi A, Marmor MF: Lowering the calcium concentration in the subretinal space in vivo loosens
retinal adhesion. Invest Ophthalmol Vis Sci 33:23, 1992 10. Negi A, Yoshihito H, Shin-ichiro K: Importance of bicarbonate ion in the vitreous space. Arch Ophthalmol 100:1839, 1982 11. Negi A, Yoshihito H, Shin-ichiro K: Effects of intraocular irrigating solutions on the electroretinographic
B-wave. Am J Ophthalmol 92:28, 1981 12. Winkler BS, Simson V, Benner J: Importance of bicarbonate in retinal function. Invest Ophthalmol Vis Sci 16:766, 1977 13. Araie M, Shirasawa E, Ohashi T: Intraocular irrigating solutions and permeability of the blood-aqueous
barrier. Arch Ophthalmol 108:882, 1990 14. Blanks JC, Pickford MS, Organisciak DT: Ascorbate treatment prevents accumulation of phagosomes in RPE in light
damage. Invest Ophthalmol Vis Sci 33:2814, 1992 15. Gonnering R, Edelhauser HF, VanHorn DL, Durant W: The pH tolerance of rabbit and human corneal endothelium. Invest Ophthalmol Vis Sci 18:373, 1979 16. Winkler BS, Trese MT: The pH of antibiotic vitreous infusion combinations: A potential cause
of retinal toxicity. Ophthalmic Surg 23:622, 1992 17. Haimann MH, Abrams GW, Edelhauser HF, Hatchell DL: The effect of intraocular irrigating solutions on lens clarity in normal
and diabetic rabbits. Am J Ophthalmol 94:594, 1982 18. Christiansen JM, Kollarits CR, Fukui H, et al: Intraocular irrigating solutions and lens clarity. Am J Ophthalmol 82:594, 1976 19. Haimann MH, Abrams GW: Prevention of lens opacification during diabetic vitrectomy. Ophthalmology 91:116, 1984 20. Novak MA, Rice TA, Michels RG, Auer C: The crystalline lens after vitrectomy for diabetic retinopathy. Ophthalmology 91:1480, 1984 21. Duffin RM, Pettit TH, Straatsma BR: Maintenance of mydriasis with epinephrine during cataract surgery. Ophthalmic Surg 14:41, 1983 22. Slack JW, Edelhauser HF, Helenek MJ: A bisulfite-free intraocular epinephrine solution. Am J Ophthalmol 110:77, 1990 23. Hull DS, Chemotti MT, Edelhauser HF, et al: Effect of epinephrine on the corneal endothelium. Am J Ophthalmol 79:245, 1975 24. Edelhauser HF, Hyndiuk RA, Zeeb A, Schultz RO: Corneal edema and the intraocular use of epinephrine. Am J Ophthalmol 93:327, 1982 25. Hammer ME, Chenoweth RG: The effect of intraocular dilating solution on cat endothelium. Ophthalmic Surg 15:585, 1984 26. Olson RJ, Kolodner H, Riddle P, Escapini H: Commonly used intraocular medications and the corneal endothelium. Arch Ophthalmol 98:2224, 1980 27. Endo EG, Yao XY, Marmor MF: Pigment adherence as a measure of retinal adhesion: Dependence on temperature. Invest Ophthalmol Vis Sci 29:1390, 1988 28. Marmor MF, Yao XY: The enhancement of retinal adhesiveness by ouabain appears to involve cellular
edema. Invest Ophthalmol Vis Sci 30:1511, 1989 29. Marmor MF, Maack T: Local environmental factors and retinal adhesion in the rabbit. Exp Eye Res 34:727, 1982 30. Balazs EA, Gibbs DA: The rheological properties and biological function of hyaluronic acid. In Balazs EA (ed): Chemistry and Molecular Biology of the Intercellular Matrix, vol 3, pp 1241–1253. London, Academic Press, 1970 31. Liesegang TJ: Viscoelastic substances in ophthalmology. Surv Ophthalmol 34:268, 1990 32. Hammer ME, Burch TG: Viscous corneal protection by sodium hyaluronate, chondroitin sulfate, and
methylcellulose. Invest Ophthalmol Vis Sci 25:1329, 1984 33. Koch DD, Liu JF, Glasser DB, et al: A comparison of corneal endothelial changes after use of Healon or Viscoat
during phacoemulsification. Am J Ophthalmol 115:188, 1993 34. Nevyas AS, Raber IM, Eagle RC, et al: Acute band keratopathy following intracameral Viscoat. Arch Ophthalmol 105:958, 1987 35. Koster R, Stilma JS: Comparison of vitreous replacement with Healon and with HPMC in rabbits' eyes. Doc Ophthalmol 61:247, 1986 36. Russell SR, Hageman GS: Chondroitin sulfate–induced generation of epiretinal membranes. Arch Ophthalmol 110:1000, 1992 37. MacRae SM, Edelhauser HF, Hyndiuk RA, et al: The effects of sodium hyaluronate, chondroitin sulfate, and methylcellulose
on the corneal endothelium and intraocular pressure. Am J Ophthalmol 95:332, 1983 38. Smith SG, Lindstrom RL: 2% Hydroxypropyl methylcellulose as a viscous surgical adjunct: A
multicenter prospective randomized trial. J Cataract Refract Surg 17:839, 1991 39. Vatne HO, Syrdalen P: The use of sodium hyaluronate (Healon) in the treatment of complicated
cases of retinal detachment. Acta Ophthalmol 64:169, 1986 40. Lane SS, Naylor DW, Kullerstrand LJ, et al: Prospective comparison of the effects of Occucoat, Viscoat, and Healon
on intraocular pressure and endothelial cell loss. J Cataract Refract Surg 17:21, 1991 41. Pape LG, Balazs EA: The use of sodium hyaluronate (Healon) in human anterior segment
surgery. Ophthalmology 87:699, 1980 42. Swartz M, Olson RJ, Pingree GC, Sakauye C: The use of anterior chamber Na-hyaluronate in a pseudophakic patient
requiring intravitreal air during retinal reattachment surgery. Ophthalmic Surg 12:98, 1981 43. Landers MB: Sodium hyaluronate (Healon) as an aid to internal fluid–gas
exchange. Am J Ophthalmol 94:557, 1982 44. Glasser DB, Katz HR, Boyd JE, et al: Protective effects of viscous solutions in phacoemulsification and traumatic
lens implantation. Arch Ophthalmol 107:1047, 1989 45. Glasser DB, Osborn DC, Nordeen JF, Min YI: Endothelial protection and viscoelastic retention during phacoemulsification
and intraocular lens implanation. Arch Ophthalmol 109:1438, 1991 46. Swartz M, Anderson DR: Use of Healon in posterior segment surgery. J Ocul Ther Surg , January-February, pp 26–28, 1984 47. Poole TA, Sudarsky RD: Suprachoroidal implantation for the treatment of retinal detachment. Ophthalmology 93:1408, 1986 48. Pruett RC, Schepens CL, Swann DA: Hyaluronic acid vitreous substitute: A six year clinical evaluation. Arch Ophthalmol 97:2325, 1979 49. Winter R: Indications for Healon instillation in microsurgery of complicated retinal
detachments. Dev Ophthalmol 14:20, 1987 50. Stenkula S, Ivert L, Gislason I, et al: The use of sodiumhyaluronate (Healon) in the treatment of retinal
detachment. Ophthalmic Surg 12:435, 1981 51. Koster R, Stilma JS: Healon as intravitreal substitute in retinal detachment surgery in 40 patients. Doc Ophthalmol 64:13, 1986 52. Blumenkranz MS, Nussbaum J, Verstraeten T, et al: Vitreoretinal applications of Hylan gel: A new crosslinked hyaluronate
polymer. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 53. Fitzgerald CR: The use of Healon in a case of rolled-over retina. Retina 1:227, 1981 54. Stenkula S: Sodium hyaluronate as a vitreous substitute and intravitreal surgical tool. In Rosen ES (ed): Viscoelastic Materials: Basic Science and Clinical Applications, pp 157–160. New York, Pergamon Press, 1989 55. Crafoord S, Stenkula S: Healon GV in posterior segment surgery: An open clinical study. Invest Ophthalmol Vis Sci 33:1313, 1992 56. Stenkula S, Ivert L, Berglin L, Crafoord S: Healon yellow as a surgical tool in maneuvering intraocular tissues. Ophthalmic Surg 23:708, 1992 57. Folk JC, Weingeist TA, Packer AJ, Howcroft MJ: Sodium hyaluronate (Healon) in closed vitrectomy. Ophthalmic Surg 17:299, 1986 58. Packer AJ, Brooks WM, Hutton WL, Ramsay RC: Procoagulant effects of intraocular sodium hyaluronate (Healon) after
phakic diabetic vitrectomy: A prospective randomized study. Ophthalmology 96:1491, 1989 59. Vygantas CM, Peyman GA, Daily MJ, Ericson ES: Octafluorocyclobutane and other gases for vitreous replacement. Arch Ophthalmol 90:235, 1973 60. Adamson AW: Physical Chemistry of Surfaces. New York, John Wiley & Sons, 1982 61. Stinson TW, Donlon JV: Interaction of intraocular air and sulfur hexafluoride with nitrous oxide: A
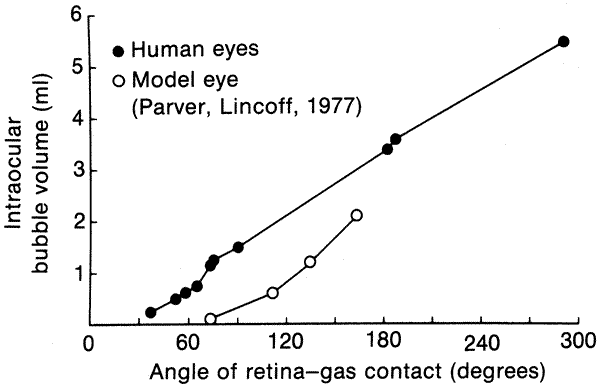
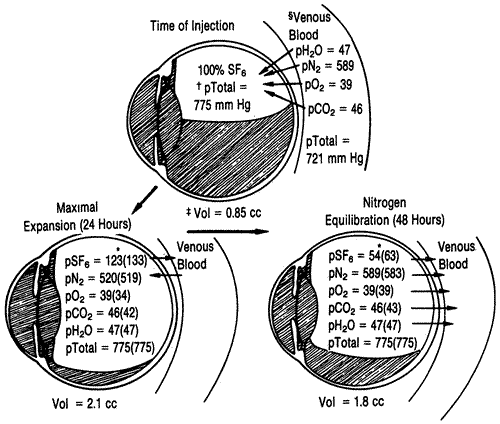
computer simulation. Anesthesiology 56:385, 1982 62. Parver LM, Lincoff H: Mechanics of intraocular gas. Invest Ophthalmol Vis Sci 17:77, 1978 63. Parver LM, Lincoff H: Geometry of intraocular gas used in retinal surgery. Mod Probl Ophthalmol 18:338, 1977 64. Hilton GF, Grizzard WS: Pneumatic Retinopexy: A twostep outpatient operation without conjunctival
incision. Ophthalmology 93:626, 1986 65. Lincoff A, Kreissig I: Intravitreal behavior of perfluorocarbons. Dev Ophthalmol 2:17, 1981 66. Fineberg E, Machemer R, Sullivan P, et al: Sulfur hexafluoride in owl monkey vitreous cavity. Am J Ophthalmol 79:67, 1975 67. Tornambe PE, Hilton GF: Pneumatic retinopexy: A multicenter randomized controlled clinical trial
comparing pneumatic retinopexy with scleral buckling. Ophthalmology 96:772, 1989 68. Fineberg E, Machemer R, Sullivan P: SF6 for retinal detachment surgery. Mod Probl Ophthalmol 12:173, 1974 69. Miller B, Lean JS, Miller H, Ryan SJ: Intravitreal expanding gas bubble: A morphologic study in the rabbit eye. Arch Ophthalmol 102:1708, 1984 70. Foulks GN, DeJuan E, Hatchell DL, et al: The effect of perfluoropropane on the cornea in rabbits and cats. Arch Ophthalmol 105:256, 1987 71. Abrams GW, Edelhauser HF, Aaberg TM, Hamilton LH: Dynamics of intravitreal sulfur hexafluoride gas. Invest Ophthalmol Vis Sci 13:863, 1974 72. Lincoff H, Mardirossian J, Lincoff A, et al: Intravitreal longevity of three perfluorocarbon gases. Arch Ophthalmol 98:1610, 1980 73. Lincoff A, Lincoff H, Iwamoto T, et al: Perfluoro-n-butane: A gas for a maximum duration retinal
tamponade. Arch Ophthalmol 101:460, 1983 74. Lincoff H, Maisel JM, Lincoff A: Intravitreal disappearance rates of four perfluorocarbon gases. Arch Ophthalmol 102:928, 1984 75. Wang RF, Thompson JT: Prediction of the kinetics of disappearance of sulfur hexafluoride and
perfluoropropane intraocular gas bubbles. Ophthalmology 95:609, 1988 76. Thompson JT: Kinetics of intraocular gases: Disappearance of air, sulfur hexafluoride, and
perfluoropropane after pars plana vitrectomy. Arch Ophthalmol 107:687, 1989 77. Lincoff H, Stergiu P, Smith R, Movshovich A: Longevity of expanding gases in vitrectomized eyes. Retina 12:364, 1992 78. Thompson JT: The absorption of mixtures of air and perfluoropropane after pars plana
vitrectomy. Arch Ophthalmol 110:1594, 1992 79. Meyers SM, Ambler JS, Fracs F, et al: Variation of perfluoropropane disappearance after vitrectomy. Retina 12:359, 1992 80. Peters MA, Abrams GW, Hamilton LH, et al: The nonexpansile, equilibrated concentration of perfluoropropane gas in
the eye. Am J Ophthalmol 100:831, 1985 81. Ligget PE: Gas bubble selection. In Tornambe PE, Grizzard WS (eds): Pneumatic Retinopexy: A Clinical Symposium, chap 6. Des Plaines, IL, Greenwood, 1989 82. Sebag J, Tang M: Pneumatic retinopexy using only air. Retina 13:8, 1993 83. Norton EW, Aaberg T, Fung W, Curtin VT: Giant retinal tears: I. Clinical management with intravitreal air. Am J Ophthalmol 68:1011, 1969 84. Machemer R, Allen AW: Retinal tears 180° and greater: Management with vitrectomy and intravitreal
gas. Arch Ophthalmol 94:1340, 1976 85. Joondeph BC, Flynn HW, Blankenship GW, et al: The surgical management of giant retinal tears with the cannulated extrusion
needle. Am J Ophthalmol 108:548, 1989 86. Blankenship GW, Ibanez-Langlois S: Treatment of myopic macular hole and detachment: Intravitreal gas exchange. Ophthalmology 94:333, 1987 87. Lincoff H, Kreissig I, Brodie S, Wilcox L: Expanding gas bubbles for the repair of tears in the posterior pole. Graefes Arch Clin Exp Ophthalmol 219:193, 1982 88. Lincoff H, Coleman J, Kreissig I, et al: The perfluorocarbon gases in the treatment of retinal detachment. Ophthalmology 90:546, 1983 89. Chang S, Coleman DJ, Lincoff H, et al: Perfluoropropane gas in the management of proliferative vitreoretinopathy. Am J Ophthalmol 98:180, 1984 90. Lopez R, Chang S: Long-term results of vitrectomy and perfluorocarbon gas for the
treatment of severe proliferative vitreoretinopathy. Am J Ophthalmol 113:424, 1992 91. Chang S, Lincoff H, Coleman DJ, et al: Perfluorocarbon gases in vitreous surgery. Ophthalmology 92:651, 1985 92. Greenspan HP: The Theory of Rotating Fluids. New York, Press Syndicate of the University of Cambridge, 1968 (reprinted with corrections 1969) 93. Friberg TR, Eller AW: Pneumatic repair of primary and secondary retinal detachments using a binocular
indirect ophthalmoscope laser delivery system. Ophthalmology 95:187, 1988 94. Hilton GF, Kelly NE, Salzano TC, et al: Pneumatic retinopexy: A collaborative report of the first 100 cases. Ophthalmology 94:307, 1987 95. McAllister IL, Meyers SM, Zegarra H, et al: Comparison of pneumatic retinopexy with alternative surgical techniques. Ophthalmology 95:877, 1988 96. Chan CK, Wessels IF: Delayed subretinal fluid absorption after pneumatic retinopexy. Ophthalmology 96:1691, 1989 97. Kelly NE, Wendel RT: Vitreous surgery for idiopathic macular holes: Results of a pilot study. Arch Ophthalmol 109:654, 1991 98. Abrams GW, Swanson DE, Sabates WI, Goldman AI: The results of sulfur hexafluoride gas in vitreous surgery. Am J Ophthalmol 94:165, 1982 99. Hart RH, Vote, BJ, Borthwick, JH, et al: Loss of Vision Caused by Expansion of Intraocuar Perfluoropropane (C3F8) Gas
During Nitrous Oxide Anesthesia. Am J Ophthalmol 134:761, 2002 100. Whitacre MM, Mainster MA: Hazards of laser beam reflections in eyes containing gas. Am J Ophthalmol 110:33, 1990 101. Diddie KR, Smith RE: Intraocular gas injection in the pseudophakic patient. Am J Ophthalmol 89:659, 1980 102. Gardner TW, Norris JL, Zakov ZN, Williams GW: A survey of intraocular gas use in North America. Arch Ophthalmol 106:1188, 1988 103. Duerksen JS, Oyakawa RT, Lemor M: A nationwide survey of the use of perfluoropropane and sulfur hexafluoride. Am J Ophthalmol 108:195, 1989 104. Cibis PA, Becker B, Okun E, Canaan S: The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol 68:590, 1962 105. Armaly MF: Ocular tolerance to silicones. Arch Ophthalmol 68:390, 1962 106. Mukai N, Lee PF, Schepens CL: Intravitreous injection of silicone: An experimental study. Ann Ophthalmol 4:273, 1972 107. Mukai N, Lee PF, Schepens CL: Intravitreous injection of silicone: An experimental study. Ann Ophthalmol 4:273, 1972 108. Haut J, Ullern M, VanEffenterre G, Chermet M: Utilisation du silicone intra-oculaire à propos de 200 cas. Soc Ophtalmol Fr 79:797, 1979 109. Zivojnovic R, Mertens DAE, Peperkamp E: Das flussige Silikon in der Amotiochirurgie (II) Bericht uber 280 Falleweitere
Entwicklung der Technik. Klin Monatsbl Augenheilkd 181:444, 1982 110. Silicone Study Group: Vitrectomy with silicone oil or perfluoropropane
gas in eyes with severe proliferative vitreoretinopathy: Results of a
randomized clinical trial: Report 2 Arch Ophthalmol 110:780, 1992 111. Kreiner CF: Chemical and physical aspects of clinically applied silicones. Dev Ophthalmol 14:1, 1987 112. Parel J-M: Silicone oils: Physicochemical properties. In Ryan SJ (ed): Retina, vol 3, pp 261–277. St. Louis, CV Mosby, 1989 113. DeJuan E, McCuen B, Tiedeman J: Intraocular tamponade and surface tension. Surv Ophthalmol 30:47, 1985 114. Crisp A, DeJuan E, Tiedeman J: Effect of silicone oil viscosity on emulsification. Arch Ophthalmol 105:546, 1987 115. Bartov E, Pennarola F, Savion N, et al: A quantitative in vitro model for silicone oil emulisfication: Role of
blood constituents. Retina 12:S23, 1992 116. Bartov E, Alhalel A, Savion N, Treister G: Role of blood components in silicone oil emulsification: In vitro and in
vivo models. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 117. Nakamura K, Refojo MF, Crabtree DV: Factors contributing to the emulsification of intraocular silicone and
fluorosilicone oils. Invest Ophthalmol Vis Sci 31:647, 1990 118. Petersen J: The physical and surgical aspects of silicone oil in the vitreous cavity. Graefes Arch Clin Exp Ophthalmol 225:452, 1987 119. Heidenkummer H, Kampik A, Thierfelder S: Emulsification of silicone oils with specific physicochemical characteristics. Graefes Arch Clin Exp Ophthalmol 229:88, 1991 120. Heidenkummer H, Kampik A, Thierfelder S: Experimental evaluation of in vitro stability of purified polydimethylsiloxanes (silicone
oil) in viscosity ranges from 1000 to 5000 centistokes. Retina 12:S28, 1992 121. Drazin PG, Reid WH: Hydrodynamic Stability. New York, Press Syndicate of the University of Cambridge, 1981 (reprinted 1984, 1985) 122. Haut J, Ullern M, Chermet M, Van Effenterre G: Complications of intraocular injections of silicone combined with vitrectomy. Ophthalmologica 180:29, 1980 123. Lean JS: Use of silicone oil as an additional technique in vitreoretinal surgery. In Ryan SJ (ed): Retina, vol 3, pp 279–292. St. Louis, CV Mosby, 1989 124. McCartney DL, Miller KM, Stark WJ, et al: Intraocular lens style and refraction in eyes treated with silicone oil. Arch Ophthalmol 105:1385, 1987 125. Haut J, Larricart JP, Van Effenterre G, Pinon-Pignero F: Some of the most important properties of silicone oil to explain its action. Ophthalmologica 191:150, 1985 126. Shugar JK, DeJuan E, McCuen BW, et al: Ultrasonic examination of the silicone filled eye: Theoretical and practical
considerations. Graefes Arch Clin Exp Ophthalmol 224:361, 1986 127. Leaver PK: Complications of intraocular silicone oil. In Ryan SJ (ed): Retina, vol 3, pp 293–306. St. Louis, CV Mosby, 1989 128. Ober RR, Blanks JC, Odgen TE, et al: Experimental retinal tolerance to liquid silicone. Retina 3:77, 1983 129. Ohira A, Wilson CA, DeJuan E, et al: Experimental retinal tolerance to emulsified silicone oil. Retina 11:259, 1991 130. Blodi FC: Injection and impregnation of liquid silicone into ocular tissues. Am J Ophthalmol 71:1044, 1971 131. Eckardt C, Nicolai U, Czank M, Schmidt D: Identification of silicone oil in the retina after intravitreal injection. Retina 12:S17, 1992 132. Mukai N, Lee P, Oguri M, Schepens CL: A long-term evaluation of silicone retinopathy in monkeys. Can J Ophthalmol 10:391, 1975 133. Green K, Cheeks L, Slagle T, et al: Comparative effects of gases and oils on corneal endothelial and blood-retinal
barrier permeability. Invest Ophthalmol Vis Sci 33:1108, 1992 134. Sparrow JR, Chang S, Vinals AF: Evaluation of the blood-aqueous barrier after vitreous replacement
with perfluoropropane gas and liquid silicone. Retina 12:370, 1992 135. Gabel VP, Kampik A, Burkhardt J: Analysis of intraocularly applied silicone oils of various origins. Graefes Arch Clin Exp Ophthalmol 225:160, 1987 136. Nakamura K, Refojo MF, Crabtree DV, Leong F: Analysis and fractionation of silicone and fluorosilicone oils for intraocular
use. Invest Ophthalmol Vis Sci 31:2059, 1990 137. Nakamura K, Refojo MF, Crabtree DV, et al: Ocular toxicity of low-molecular-weight components of silicone
and fluorosilicone oils. Invest Ophthalmol Vis Sci 32:3007, 1991 138. Fastenberg DM, Diddie KR, Delmage JM, Dorey K: Intraocular injection of silicone oil for experimental proliferative vitreoretinopathy. Am J Ophthalmol 95:663, 1983 139. Lean JS, VanDerZee WAM, Ryan SJ: Experimental model of proliferative vitreoretinopathy (PVR) in
the vitrectomised eye: Effect of silicone oil. Br J Ophthalmol 68:332, 1984 140. Lambrou FH, Burke JM, Aaberg TM: Effect of silicone oil on experimental traction retinal detachment. Arch Ophthalmol 105:1269, 1987 141. DeMolfetta V, Bottoni F, Arpa P, et al: The effect of simultaneous internal tamponade on fluid compartmentalization
and its relationship to cell proliferation. Retina 12:S40, 1992 142. Levine AM, Ellis RA: Intraocular liquid silicone implants. Am J Ophthalmol 55:939, 1963 143. Winslow R: Silicone found to spur output of antibodies, researchers say. Wall Street Journal , August 28, 1992, p B2 144. Spiera H: Scleroderma after silicone augmentation mammoplasty. JAMA 260:236, 1988 145. Seleznick MJ, Martinez-Osuna P, Espinoza LR, Vasey FB: Is silicone associated with connective tissue disease? J Fla Med Assoc 78:85, 1991 146. Nicolai U, Eckardt C, Kohlhoff M, Wottgo HU: Biocompatibility of silicone oil and high-density liquids: An immunologic
study. Invest Ophthalmol Vis Sci 33:1315, 1992 147. Tolentino FI, Refojo MF, Arroyo MH, Araiz J: Fluorosilicone oil for complex retinal detachments. Invest Ophthalmol Vis Sci 33:1314, 1992 148. Nishimura A, Wajima R, Kaneko T, et al: Effects of longterm vitreous replacement with fluorosilicone oil: An electrophysiological
study in vivo. Invest Ophthalmol Vis Sci 33:1314, 1992 149. Gabel VP, Kampik A, Gabel CH, Spiegel D: Silicone oil with high specific gravity for intraocular use. Br J Ophthalmol 71:262, 1987 150. La Heij EC, Hendriske F, Kessels AG: Results and Complications of Temporary Silicon Oil Tamponade in Patients
with Complicated Retinal Detachments. Retina 21:107, 2001 151. Kampik A, Hoing C, Heidenkummer H: Problems and timing in the removal of silicone oil. Retina 12:S11, 1992 152. Nguyen QH, Lloyd MA, Heuer DK, et al: Incidence and management of glaucoma after intravitreal silicone oil injection
for complicated retinal detachments. Ophthalmology 99:1520, 1992 153. Lucke K, Bopp S, Laqua H: Long-term fate of eyes with and without silicone oil removal. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 154. Dabil, H, Akduman, L, Olk, RJ: Comparison of silicone oil removal with passive drainage alone versus passive
drainage combined with air-fluid exchange. Retina 22:597, 2002 155. Watzke RC: Silicone retinopiesis for retinal detachment: A long-term clinical
evaluation. Arch Ophthalmol 77:185, 1967 156. Scott JD: Treatment of massive vitreous retraction. Trans Ophthalmol Soc UK 93:417, 1973 157. Constable I, Mohamed S, Tan PL: Super viscous silicone liquid in retinal surgery. Aust J Ophthalmol 10:5, 1982 158. Manschot WA: Intravitreal silicone injection. Adv Ophthalmol 36:197, 1978 159. Alexandridis E, Daniel H: Results of silicone oil injection into the vitreous. Dev Ophthalmol 2:24, 1981 160. Scott JD: Use of liquid silicone in vitrectomised eyes. Dev Ophthalmol 2:185, 1981 161. Silicone Study Group: Vitrectomy with silicone oil or sulfur hexafluoride
gas in eyes with severe proliferative vitreoretinopathy: Results of
a randomized clinical trial: Report 1. Arch Ophthalmol 110:770, 1992 162. Abrams GW, Azen SP, McCuen II BW, et al: Vitrectomy With Siliucone Oil or Long-Acting Gas in Eyes With Severe
Proliferative Vitreoretinopathy: Results of Additional and Long-Term
Follow-Up. Arch Ophthalmol 115:335, 1997 163. Hammer ME, Grizzard WS, Pavan PR: Silicone oil is a better vitreous tamponade than sulfurhexafluoride for
difficult retinal detachments: A prospective randomized trial. Invest Ophthalmol Vis Sci 34:952, 1993 164. Cox MS, Trese MT, Murphy PL: Silicone oil for advanced proliferative vitreoretinopathy. Ophthalmology 93:646, 1986 165. Kampik A, Gabel VP, Spiegel D: Intraokulare Tamponade mit Hochviskosem silikonol bei massiver proliferativer
Vitreo-retinopathie. Klin Monatsbl Augenheilkd 185:368, 1984 166. Lean JS, Leaver PK, Cooling RJ, McLeod D: Management of complex retinal detachments by vitrectomy and fluid/silicone
exchange. Trans Ophthalmol Soc UK 102:203, 1982 167. Lucke KH, Foerster MH, Laqua H: Long-term results of vitrectomy and silicone oil in 500 cases of
complicated retinal detachments. Am J Ophthalmol 104:624, 1987 168. Gonvers M: Temporary use of intraocular silicone oil in the treatment of detachment
with massive periretinal proliferation: Preliminary report. Ophthalmologica 184:210, 1982 169. Gonvers M: Temporary silicone oil tamponade in the management of retinal detachment
with proliferative vitreoretinopathy. Am J Ophthalmol 100:239, 1985 170. Federman JL, Eagle RC: Extensive peripheral retinectomy combined with posterior 360° retinotomy
for retinal reattachment in advanced proliferative vitreoretinopathy
cases. Ophthalmology 97:1305, 1990 171. Charles S: Proliferative vitreoretinopathy management 1,081 consecutive cases. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 172. Leaver PK, Lean JS: Management of giant retinal tears using vitrectomy and silicone oil/fluid
exchange. Trans Ophthalmol Soc UK 101:189, 1981 173. Leaver PK, Billington BM: Vitrectomy and fluid/silicone oil exchange for giant retinal tears: 5 years
follow-up. Graefes Arch Clin Exp Ophthalmol 227:323, 1989 174. Antoszyk AN, McCuen BW, DeJuan E, Machemer R: Silicone oil injection after failed primary vitreous surgery in severe
ocular trauma. Am J Ophthalmol 107:537, 1989 175. Lemmen KD, Heimann K: Fruh-Vitrektomie mit primarer Silikonolinjektion bei schwerstverletzten
Augen. Klin Monatsbl Augenheilkd 193:594, 1988 176. Rinkoff JS, DeJuan E, McCuen BW: Silicone oil for retinal detachment with advanced proliferative vitreoretinopathy
following failed vitrectomy for proliferative diabetic retinopathy. Am J Ophthalmol 101:181, 1986 177. McLeod D: Silicone oil injection during closed microsurgery for diabetic retinal
detachment. Graefes Arch Clin Exp Ophthalmol 224:55, 1986 178. Brourman ND, Blumenkranz MS, Cox MS, Trese MT: Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology 96:759, 1989 179. Gonvers M: Temporary silicone oil tamponade in the treatment of complicated diabetic
retinal detachments. Graefes Arch Clin Exp Ophthalmol 228:415, 1990 180. Karel I, Kalvodova B: Long-termed results of pars plana vitrectomy with silicone oil implantation
for diabetic retinopathy complications. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 181. Azzolini C, Brancato R, Camesasca FI, Codenotti M: Influence of silicone oil on iris microangiopathy in diabetic vitrectomized
eyes. Ophthalmology 100:1152, 1993 182. Haut J, Van Effenterre G, Flamand M: Treatment of macular hole retinal detachment with silicone oil, with or
without argon laser photocoagulation. Ophthalmologica 187:25, 1983 183. Goldbaum MH, McCuen BW, Hanneken AM, et al: Silicone oil tamponade to seal macular holes without position restrictions. Ophthalmology 105:2140, 1998 184. Gopal L, Kini MM, Badrinath SS, Sharma T: Management of retinal detachment with choroidal coloboma. Ophthalmology 98:1622, 1991 185. Morse LS, McCuen BW: The use of silicone oil in uveitis and hypotony. Retina 11:399, 1991 186. Sidikaro Y, Silver L, Holland GN, Kreiger AE: Rhegmatogenous retinal detachments in patients with AIDS and necrotizing
retinal infections. Ophthalmology 98:129, 1991 187. Orellana J, Teich SA, Leiberman RM, et al: Treatment of retinal detachments in patients with the acquired immune deficiency
syndrome. Ophthalmology 98:939, 1991 188. Jabs DA, Enger C, Haller J, deBustros S: Retinal detachments in patients with cytomegalovirus retinitis. Arch Ophthalmol 109:794, 1991 189. Chan C, Okun E: The question of ocular tolerance to intravitreal liquid silicone: A long-term
analysis. Ophthalmology 93:651, 1986 190. Leaver PK, Grey RHB, Garner A: Silicone oil injection in the treatment of massive preretinal retraction: II. Late
complications in 93 eyes. Br J Ophthalmol 63:361, 1979 191. Moisseiev J, Bartov E, Cahane M, et al: Cataract extraction in eyes filled with silicone oil. Arch Ophthalmol 110:1649, 1992 192. Ghoraba HH, El-Dorghamy AA, Atia AF, et al: The problems of biometry in combined silicone removal and cataract extraction. Retina 22:589, 2002 193. Eaton AM, Jaffe GJ, McCuen II BW, et al: Condenation on the Posterior Surface of Silicone Intraocular Lenses During
Fluid-Air Exchange. Ophthalmol 102:733, 1995 194. Apple, DJ, Federman, JL, Krolicki, TJ, et al: Irreversible Silicone Adhesion to Silicone Intraocular Lenses. Ophthalmol 103:1555, 1996 195. Burk LL, Shields MB, Proia AD, McCuen BW: Intraocular pressure following intravitreal silicone oil injection. Ophthalmic Surg 19:565, 1988 196. Ando F: Intraocular hypertension resulting from pupillary block by silicone oil. Am J Ophthalmol 99:87, 1985 197. Haut J, Ullern M, Chermet M, Van Effenterre G: Complications of intraocular injections of silicone combined with vitrectomy. Ophthalmologica 180:29, 1980 198. Avitabile, T, Bonfiglio, V, Cicero, A, et al: Correlation between quantity of silicone oil emulsified in the anterior
chamber and high pressure in vitrectomized eyes. Retina 22:443, 2002 199. Parwar, BL, Coleman, AL, Small, KW: Silicone oil migration through an Ahmed valve. Retina 22: 657, 2002 200. Budenz, DL, Taba, KE, Feuer, WJ, et al: Surgical management of secondary glaucoma after pars plana vitrectomy and
silicone oil injection for complex retinal detachment. Ophthalmol 108:1628, 2001 201. Norman BC, Oliver J, Cheeks L, et al: Corneal endothelial permeability after anterior chamber silicone oil. Ophthalmology 97:1671, 1990 202. Sternberg P, Hatchell DL, Foulks GN, Landers MB: The effect of silicone oil on the cornea. Arch Ophthalmol 103:90, 1985 203. Noorily SW, Foulks GN, McCuen BW: Results of penetrating keratoplasty associated with silicone oil retinal
tamponade. Ophthalmology 98:1186, 1991 204. Lewis H, Burke JM, Abrams GW, Aaberg TM: Perisilicone proliferation after vitrectomy for proliferative vitreoretinopathy. Ophthalmology 95:583, 1988 205. Steffansen B, Verstraeten T, Hartzer M, et al: Fluorouracil prodrugs suspended in silicone oil as an intraocular slow
release system. Invest Ophthalmol Vis Sci 33:728, 1992 206. Araiz J, Arroyo M, Refojo MF, et al: Antiproliferative effect of retinoic acid in intravitreous silicone oil
in an animal model of PVR. Invest Ophthalmol Vis Sci 33:1057, 1992 207. Weidemann P, Leinung C, Hilgers RD, et al: Daunomycin and silicone oil for the treatment of proliferative vitreoretinopathy. Graefes Arch Clin Exp Opthalmol 229:150, 1991 208. Asaria RH, Kon CH, Bunce C, et al: Adjuvant 5-fluorourcil and heparin prevents proliferative vitreoretinopathy. Ophthalmol 108:1179, 2001 209. Budde M: Am J Ophthalmol 131:392, 2001 210. Eller AW, Friberg TR, Mah F: Migration of silicone oil into the brain: A complication of intraocular
silicone oil for retinal tamponade. Am J Ophthalmol 129:685, 2000 211. Donahue SP, Friberg TR, Johnson BL: Intraconjunctival cavitary inclusions of silicone oil complicating retinal
detachment repair. Am J Ophthalmol 114:639, 1992 212. Eller AW, Gardner TW, D'Antonio JA: A survey of intraocular silicone oil use in the United States. Ophthalmology 99:1174, 1992 213. Lakits A, Nennadal T, Scholda C, et al: Chemical stability of silicone oil in the human eye after prolonged clinical
use. Ophthalmol 106:1091, 1999 214. Clark LC Jr , Gollan F: Survival of mammals breathing organic liquids equilibrated with oxygen
at atmospheric pressure. Science 152:1755, 1966 215. Haidt SJ, Clark LC Jr , Ginsberg J: Liquid perfluorocarbon replacement of the eye. Invest Ophthalmol Vis Sci 22(suppl):233, 1982 216. Clark LC Jr : Methods of treating disorders of an eye with liquid perfluorocarbons. US Patent #4,490,351, 1984 217. Zimmerman NJ, Faris D: The use of N-perfluorocarbon amines in complicated retinal detachments. Invest Ophthalmol Vis Sci 25(suppl):258, 1984 218. Chang S: Low viscosity liquid fluorochemicals in vitreous surgery. Am J Ophthalmol 103:38, 1987 219. Nabih M, Peyman GA, Clark LC Jr , et al: Experimental evaluation of perfluorophenanthrene as a high specific gravity
vitreous substitute: A preliminary report. Ophthalmic Surg 20:286, 1989 220. Sparrow JR, Matthews GP, Iwamoto T, et al: Retinal tolerance to intravitreal perfluoroethylcyclohexane liquid in the
rabbit. Retina 13:56, 1993 221. Berrocal M, Chang S, Sun J, Simoni GJ: Ultrasonographic findings after removal of liquid vitreous replacements. Presented before the Retina Society Meeting, New York, 1992 222. Azzolini C, Docchio F, Brancato R, Trabucchi G: Interactions between light and vitreous fluid substitutes. Arch Ophthalmol 110:1468, 1992 223. Flores-Aguilar M, Crapotta JA, Munguia D, et al: Perfluorooctylbromide (PFOB) as a temporary vitreous substitute. Invest Ophthalmol Vis Sci 32(suppl):1225, 1991 224. Berkowitz BA, Wilson CA, Hatchell DL: Oxygen kinetics in the vitreous substitute perfluorotributylamine: A 19FNMR study. Invest Ophthalmol Vis Sci 32(suppl):1225, 1991 225. Meinert H, Mader J, Reuter P: Effect of purification of perfluorodecalin on toxicity to human HEP2 cells. Presented before the Jules Gonin Meeting, Lausanne, Switzerland, 1990 226. Griffith FD: 3M Material Safety Data Sheet. 3M Co, 1991 227. Grafstein D: Detection, estimation, and removal of impurities in fluorocarbon liquids. Analy Chem 26:523, 1954 228. Sparrow JR, Ortiz R, MacLeish PR, Chang S: Fibroblast behavior at aqueous interfaces with perfluorocarbon, silicone, and
fluorosilicone liquids. Invest Ophthalmol Vis Sci 31:638, 1990 229. Chang S, Sparrow JR, Iwamoto T, et al: Experimental studies of tolerance to intravitreal perfluoro-n-octane
liquid. Retina 11:367, 1991 230. Miyamoto K, Refojo MF, Tolentino FI, et al: Perfluoroether liquid as a long-term vitreous substitute. Retina 4:264, 1984 231. Clark LC Jr , Wesseler EP, Miller ML, Kaplan S: Ring versus straight chain perfluorocarbon emulsions for perfusion media. Microvasc Res 8:320, 1974 232. Han DP, Murray TG, Boldt HC, et al: Perfluoro-n-octane: Evaluation of acute anterior segment
toxicity with intravitreal use. Invest Ophthalmol Vis Sci 33:1313, 1992 233. Hammer ME, Rinder DF, Hicks EL, et al: Tolerance of perfluorocarbons, fluorosilicone, and silicone liquids in
the vitreous. In Freeman HM, Tolentino FK (eds): Proliferative Vitreoretinopathy, pp 156–161. New York, Springer-Verlag, 1988 234. LoCascio JA, Hammer ME, Rinder DF, et al: Effect of selected liquid perfluorochemicals compared to high and low viscosity
silicone oil on corneal stroma and endothelium. Invest Ophthalmol Vis Sci 26:145, 1985 235. Velikay M, Binder S, Wedrich A, et al: Perfluorodecalin and perfluorooctylbromide highly purified versus industrial
products: An experimental study for short and extended time tolerance. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 236. Wagner DG, Ferreira MA, Campos M: Perfluorotributylamine retinal toxicity after removal evaluated by serial
electroretinogram. Invest Ophthalmol Vis Sci 33:1313, 1992 237. Bryan JS, Friedman SM, Mames RN, et al: Experimental vitreous replacement with perfluorotri-n-propylamine. Arch Ophthalmol 112:1098, 1994 238. Aguilar MF, Munguia D, Loeb E, et al: Intraocular tolerance of perfluorooctylbromide (perflubron). Retina 15:3, 1995 239. Chang S, Zimmerman NJ, Iwamoto T, et al: Experimental vitreous replacement with perfluorotributylamine. Am J Ophthalmol 103:29, 1987 240. Velikay M, Wedrich A, Stolba U, et al: Experimental long-term vitreous replacement with purifies ans nonpurified
perfluorodecalin. Am J Ophthalmol 116:565, 1993 241. Conway MD, Peyman GA, Karacorlu M, et al: Perfluorooctylbromide (PFOB) as a vitreous substitute in non-human
primates. Invest Ophthalmol Vis Sci 33(suppl):1314, 1992 242. Eckardt C, Nicolai U, Winter M, Knop E: Experimental intraocular tolerance to liquid perfluorooctane and perfluoropolyether. Retina 11:375, 1991 243. Peyman GA, Conway MD, Soike KF, Clark LC Jr : Longterm vitreous replacement in primates with intravitreal vitreon or
vitreon plus silicone. Ophthalmic Surg 22:657, 1991 244. Sparrow JR, Jayakumar A, Berrocal M, et al: Experimental studies of the combined use of vitreous substitutes of high
and low specific gravity. Retina 12:134, 1992 245. Berrocal MH, Sparrow JR, Jayakumar A, et al: The combined use of vitreous substitutes of high and low specific gravity. Invest Ophthalmol Vis Sci 33:1057, 1992 246. Bottoni F, Sborgia M, Vinciguerra P, et al: Perfluorodecalin and perfluorophenanthrene as postoperative shortterm vitreous
substitutes. Invest Ophthalmol Vis Sci 33:899, 1992 247. Soike K, Peyman GA, Conway MD: Long-term vitreous replacement in primates with intravitreal vitreon
or vitreon plus silicone. Invest Ophthalmol Vis Sci 32:1225, 1991 248. Verma LK, Peyman GA, Wafapoor H, et al: An analysis of posterior segment complications after vitrectomy using the
perfluorocarbon perfluoroperhydrophenanthrene (Vitreon). Ophthalmic Surgery 26:29, 1995 249. Viebahn M, Buettner H: Perfluorophenanthrene Unsuitable for Postoperative Retinal Tamponade. Am J Ophthalmol 118:124, 1994 250. Batman C, Cekic O: Effects of the long-term use of perfluoroperhydrophenanthrene on
the retina. Ophthalmic Surgery and Lasers 29:144, 1998 251. DeQueiroz JM, Blanks JC, Ozler SA, et al: Subretinal perfluorocarbon liquids. Retina 12:S33, 1992 252. Berglin L, Ren J, Algvere P: Retinal detachment and degeneration following subretinal perfluorodecalin
in the rabbit eye. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 253. Berglin L, Ren J, Algvere P: Progressive retinal detachment and degeneration following subretinal perfluorodecalin
in the rabbit eye. Invest Ophthalmol Vis Sci 33:1314, 1992 254. Chang S, Lincoff H, Zimmerman NJ, Fuchs W: Giant retinal tears: Surgical techniques and results using perfluorocarbon
liquids. Arch Ophthalmol 107:761, 1989 255. Elsing SH, Fekrat S, Green WR, et al: Clinicopathologic findings in eyes with retained perfluoro-n-octane
liquid. Ophthalmol 108:45, 2001 256. Brochure on Perfluoron by Infinitech, 1992 257. Chang S, Reppucci V, Zimmerman NJ, et al: Perfluorocarbon liquids in the management of traumatic retinal detachments. Ophthalmology 96:785, 1989 258. Blinder KJ, Peyman GA, Paris CL, et al: Vitreon, a new perfluorocarbon. Br J Ophthalmol 75:240, 1991 259. Chang S, Ozmert E, Simoni GJ: Controlled delivery of perfluorocarbon liquids. Am J Ophthalmol 107:299, 1989 260. Ie D, Glaser BM, Sjaarda RN, et al: The use of perfluoro-octane in the management of giant retinal tears
without proliferative vitreoretinopathy. Retina 14:323, 1994 261. Scott IU, Murray TG, Flynn HW, et al: Outcomes and complications associated with giant retinal tear management
using perfluoro-n-octane. Ophthalmol 109:1828, 2002 262. Kreiger AE, Lewis H: Management of giant retinal tears without scleral buckling: Use of radical
dissection of the vitreous base and perfluoro-octane and intraocular
tamponade. Ophthalmology 99:491, 1992 263. Verstraeten T, Williams GA, Chang S, et al: Lens-sparing vitrectomy with perfluorocarbon liquid for the primary
treatment of giant retinal tears. Ophthalmol 102:17, 1995 264. Mathis A, Pagot V, Gazagne C, Malecaze F: Giant retinal tears: Surgical techniques and results using perfluorodecalin
and silicone oil tamponade. Retina 12:S7, 1992 265. Bottoni F, Bailo G, Arpa P, et al: Management of giant retinal tears using perfluorodecalin as a postoperative
short-term vitreoretinal tamponade: A long-term follow-up
study. Ophthalmic Surg 25:365, 1994 266. Chang S, Ozmert E, Zimmerman NJ: Intraoperative perfluorocarbon liquids in the management of proliferative
vitreoretinopathy. Am J Ophthalmol 106:668, 1988 267. Coll GE, Chang S, Sun J, et al: Perfluorocarbon liquid in the management of retinal detachment with proliferative
vitreoretinopathy. Ophthalmology 102:630, 1995 268. Corcostegui B, Mateo C, Arumi JG, et al: Surgical treatment of PVR using perfluorocarbons liquids. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 269. Han DP, Rychwalski PJ, Mieler WF, et al: Management of complex retinal detachment with combined relaxing retinotomy
and intravitreal perfluoro-n-octane injection. Am J Ophthalmol 118:24, 1994 270. Desai UR, Peyman GA, Harper CA: Perfluorocarbon liquid in traumatic vitreous hemorrhage and retinal detachment. Ophthalmic Surg 24:537, 1993 271. Desai UR, Peyman GA, Harper CA, Alturki WA: The use of vitreon in the removal of massive vitreous hemorrhage caused
by perforating ocular trauma. Invest Ophthalmol Vis Sci 33:1314, 1992 272. Lewis H, Blumenkranz MS, Chang S: Treatment of dislocated crystalline lens and retinal detachment with perfluorocarbon
liquids. Retina 12:299, 1992 273. Movshovich A, Berrocal MH, Chang S: The protective properties of liquid perfluorocarbons in phacofragmentation
of dislocated lenses. Retina 14:457, 1994 274. Wallace RT, McNamara JA, Brown G, et al: The use of perfluorophenanthrene in the removal of intravitreal lens fragments. Am J Ophthalmol 116:196, 1993 275. Greve MD, Peyman GA, Mehta NJ, et al: Use of perfluoroperhydrophenanthrene in the mangement of posteriorly dislocated
crystalline and intraocular lenses. Ophthalmic Surg 24:593, 1993 276. Lewis H, Sanchez G: The use of perfluorocarbon liquids in the repositioning of posteriorly
dislocated intraocular lenses. Ophthalmology 100:1055, 1993 277. Liu K, Peyman GA, Chen M, Chang K: Use of high-density vitreous substitutes in the removal of posteriorly
dislocated lenses or intraocular lenses. Ophthalmic Surg 22:503, 1991 278. Mathis A, Pagot V, Idder A, Malecaze F: The use of perfluorodecalin in pars plana vitrectomy for complications
of proliferative diabetic retinopathy. Presented before the Jules Gonin Meeting, Vienna, Austria, 1992 279. Eckardt C, Eckardt U, Conrad HG: Macular rotation with and without counter-rotation of the globe
in patients with age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 237:313, 1999 280. Toth CA, Freedman SF: Macular translocation with 360-degree peripheral retinectomy. Retina 21:293, 2001 281. Lai JC, Lapolice DJ, Stinnett SS, et al: Visual outcomes following macular translocation with 360-degree
peripheral retinectomy. Arch Ophthalmol 120:1317, 2002 282. Pertile G, Claes C: Macular translocation with 360 degree retinotomy for management of age-related
macular degeneration with subfoveal choroidal neovascularization. Am J Ophthalmol 134:560, 2002 283. Paris CL, Peyman GA, Blinder KJ, et al: Surgical technique for managing rhegmatogenous retinal detachment following
prosthokeratoplasty. Retina 11:301, 1991 284. Peyman GA, Alturki WA, Nelson NC: Surgical management of incarcerated retina in the sclerotomy. Ophthalmic Surg 23:628, 1992 285. Vander JF: Tissue plasminogen activator irrigation to facilitate removal of subretinal
hemorrhage during vitrectomy. Ophthalmic Surg 23:361, 1992 286. Heimann K: Combined intraocular PFCL plus silicone oil tamponade for control of intraoperative
hemorrhages (sandwich technique). Vitreoretinal Surg Technol 5:8, 1993 287. Adile SL, Peyman GA, Greve MD, et al: Postoperative chronic pressure abnormalities in the vitreon study. Ophthalmic Surg 25:584, 1994 288. Scott IU, Murray TG, Flynn HW, et al: Outcomes and complications associated with perfluoro-n-octane
and perfluoroperhydrophenanthrene in complex retinal detachment repair. Ophthalmol 107:860, 2000 289. Loewenstein A, Humayun MS, De Juan E, et al: Perfluoroperhydrophenanthrene versus perfluoro-n-octane in
vitreoretinal surgery. Ophthalmology 107:1078, 2000 290. Meinert H, Roy T: Semifluorinated alkanes- A new class of compounds with outstanding
properties for use in ophthalmology. European J Ophthalmol 10:189, 2000 291. Hoerauf H, Kobuch K, Dresp J, et al: Combined use of partially fluorinated alkanes, perfluorocarbon liquids
and silicone oil: an experimental study. Graefe's Arch Clin Exp Ophthalmol 239:373, 2001 292. Zeana D, Becker J, Kuckelkorn R, et al: Perfluorohexyloctane as a long-term vitreous tamponade in the experimental
animal. International Ophthalmol 23:17, 1999 293. Kobuch K, Menz DH, Hoerauf H, et al: New substances for intraocular tamponades: perfluorocarbon liquids and
hydrofluorocarbon-oligomers in vitreoretinal surgery. Graefe's Arch Clin Exp Ophthalmol 239:635, 2001 294. Hiscott P, Magee RM, Colthurst M, et al: Clinicopathological correlation of epiretinal membranes and posterior lens
opacification following perfluorohexyloctane tamponade. Br J Ophthalmol 85:179, 2001 295. Roider J, Hoerauf H, Kobuch K, et al: Clinical findings on the use of long-term heavy tamponades (semifluorinated
alkanes and their oligomers) in complicated retinal
detachment surgery. Graefe's Clin Exp Ophthalmol 240:965, 2002 296. Aisenbrey S, Lafaut BA, Szurman P, et al: Macular translocation with 360-degree retinotomy for exudative age-related
macular degeneration. Arch Ophthalmol 120:451, 2002 297. Zeana D, Schrage N, Kirchhof B, et al: Silicone oil removal from a silicone intraocular lens with perfluorohexyloctane. J Cataract Refract Surg 26:301, 2000 298. Kirchhof B, Wong D, Meurs JV, et al: Use of perfluorohexyloctane as a long-term internal tamponade agent
in complicated retinal detachment surgery. Am J Ophthalmol 133:95, 2002 |