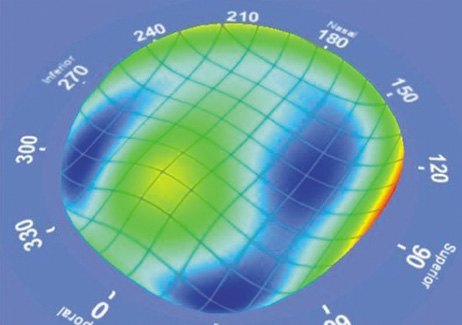
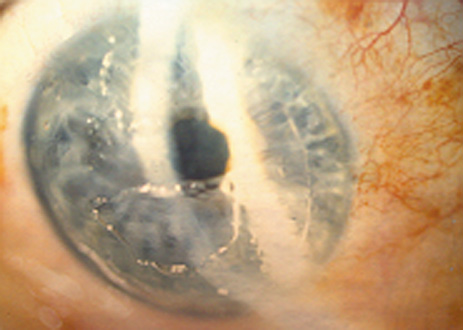
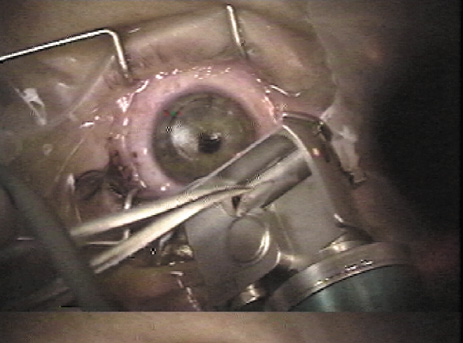
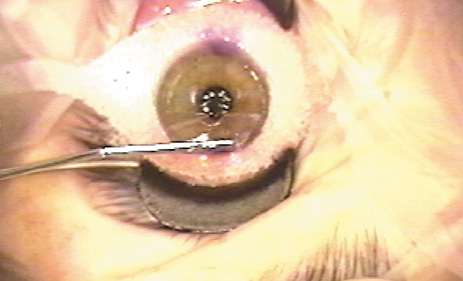
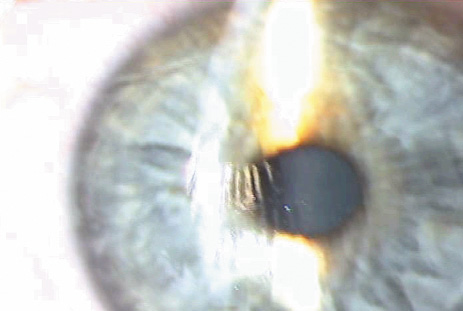
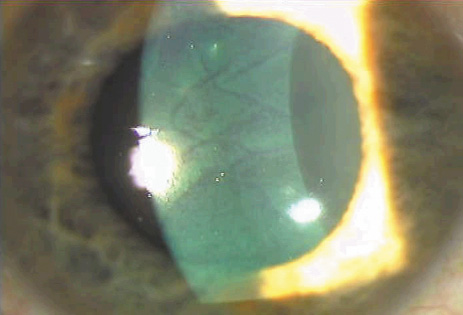
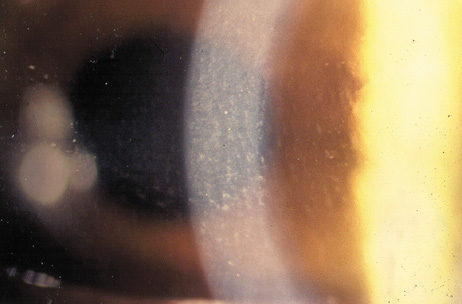
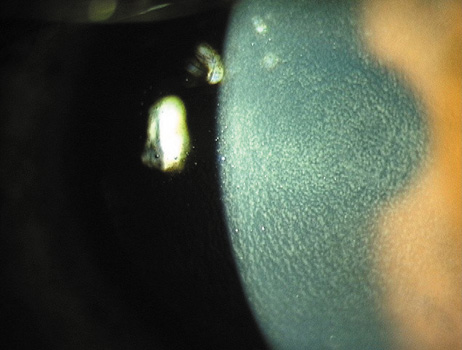
1. Duffey RJ, Leaming D: Trends in refractive surgery in the United States. J Cataract Refract Surg 30:1781, 2004 2. Barraquer Moner JI: Refractive keratoplasty. Est Inf Oftal 10:2, 1949 3. Barraquer JI: Method of cutting lamellar grafts in frozen corneas. New orientations for
refractive surgery. Arch Soc Am Ophthalmol 1:237, 1958 4. Barraquer JI: Method of cutting lamellar grafts in frozen corneas. New orientations for
refractive surgery. Arch Soc Am Ophthalmol 1:237, 1958 5. Swinger CA, Krumeich JH, Cassiday D: Planar lamellar refractive keratoplasty. J Refract Surg 2:17, 1986 6. Ruiz LA, Rowsey JJ: In situ myopic keratomileusis. Invest Ophthalmol Vis Sci 29:S39, 1988 7. Ruiz LA: Cheratomileusi automatizzata in situ. In: Chirurgia della Miopia Assile Mediante Cheratomileusi. Milan: CAMO, 1993:137 8. Pallikaris IG, Papatzanaki ME, Stathi EZ, et al: Laser in situ keratomileusis. Lasers Surg Med 10:463, 1990 9. Pallikaris IG, Papatzanaki ME, Siganos DS, et al: A corneal flap technique for laser in situ keratomileusis. Human study. Arch Ophthalmol 145:1699, 1991 10. Lee KW, Joo CK: Clinical results of laser in situ keratomileusis with superior and nasal
hinges. J Cataract Surg 29:457, 2003 11. Kumano Y, Matsui H, Zushi I, et al: Recovery of corneal sensation after myopic correction by laser in situ
keratomileusis with a nasal or superior hinge. J Cataract Refract Surg 29:757, 2003 12. Nordan LT, Slade SG, Baker RN, et al: Femtosecond laser flap creation for laser in situ keratomileusis: 6 month
follow-up of initial U.S. clinical series. J Refract Surg 19:8, 2003 13. Seiler T, Quurke AW: Iatrogenic kerectasis after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg 24:1007, 1998 14. Fogla R, Rao SK, Padmanabhan P: Keratoectasia in 2 cases with pellucid marginal corneal degeneration after
laser in situ keratomileusis. J Cataract Refract Surg 29:788, 2003 15. Ambrosio R Jr, Klyce SD, Wilson SE: Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg 19:24, 2003 16. Wang Z, Chen J, Yang B: Comparison of laser in situ keratomileusis and photorefractive keratectomy
to correct myopia from −1.25 to −6.00 diopters. J Refract Surg 13:528, 1997 17. El Danasoury MA, El-Maghraby A, Klyce SD, Mehrez K: Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis
in correcting low myopia (from −2.00 to −5.50 diopters). A
randomized study. Ophthalmology 106:411, 1999 18. Salah T, Waring III GO, El Maghraby A, Moadel K, Grimm SB: Excimer laser in situ keratomileusis under a corneal flap for myopia of 2 to 20 diopters. Am J Ophthalmol 121:143, 1996 19. Maldonado Bas A, Onnis R: Results of laser in situ keratomileusis in different degrees of myopia. Ophthalmology 105:606, 1998 20. El-Maghraby A, Salah T, Waring III GO, Klyce S, Ibrahim O: Randomized bilateral comparison of excimer laser in situ keratomileusis
and photorefractive keratectomy for 2.50 to 8.00 diopters of myopia. Ophthalmology 106:447, 1999 21. McDonald MB, Carr JD, Frantz JM, et al: Laser in situ keratomileusis for myopia up to −11 diopters with up
to −5 diopters of astigmatism with the Summit Autonomous LADARVision
excimer laser system. Ophthalmology 108:309, 2001 22. Pop M, Payette Y: Photorefractive keratectomy versus laser in situ keratomileusis: A control-matched
study. Ophthalmology 107:251, 2000 23. Pesando PM, Ghiringhello MP, Tagliavacche P: Excimer laser in situ keratomileusis for myopia. J Refract Surg 13:521, 1997 24. Knorz MC, Wiesinger B, Liermann A, Seiberth V, Liesenhoff H: Laser in situ keratomileusis for moderate and high myopia and myopic astigmatism. Ophthalmology 105:932, 1998 25. Knorz MC, Liermann A, Seiberth V, Steiner H, Wiesinger B: Laser in situ keratomileusis to correct myopia of −6.00 to −29.00 diopters. J Refract Surg 12:575, 1996 26. Steinert RF, Hersh PS, the Summit Technology PRK-LASIK Study Group: Spherical and aspherical photorefractive
keratectomy and laser in-situ keratomileusis for moderate
to high myopia: Two prospective, randomized clinical trials. Trans Am Ophthalmol Soc XCVI:197, 1998 27. Hersh PS, Brint SF, Maloney RK, et al: Photorefractive keratectomy versus laser in situ keratomileusis for moderate
to high myopia. A randomized prospective study. Ophthalmology 105:1512, 1998 28. Guell JL, Muller A: Laser in situ keratomileusis (LASIK) for myopia from −7 to −18 diopters. J Refract Surg 12(2):222, 1999 29. Tsai RJ: Laser in situ keratomileusis for myopia of −2 to −25 diopters. J Refract Surg 13:S427, 1997 30. Perez-Santonja JJ, Bellot J, Claramonte P, Ismail MM, Alio JL: Laser in situ keratomileusis to correct high myopia. J Cataract Refract Surg 23:372, 1997 31. Marinho A, Pinto MC, Pinto R, Vaz F, Neves MC: LASIK for high myopia: one year experience. Ophthal Surg Lasers 27:S517, 1996 32. Kim HM, Jung HR: Laser assisted in situ keratomileusis for high myopia. Ophthal Surg Lasers 27:S508, 1996 33. Williams DK: Multizone photorefractive keratectomy for high and very high myopia: Long-term
results. J Cataract Refract Surg 23:1034, 1997 34. El Danasoury MA, Waring III GO, El Maghraby A, Mehrez K: Excimer laser in situ keratomileusis to correct compound myopic astigmatism. J Refract Surg 13:511, 1997 35. Condon PI, Mulhern M, Fulcher T, Foley-Nolan A, O'Keefe M: Laser intrastromal keratomileusis for high myopia and myopic astigmatism. Br J Ophthalmol 81:199, 1997 36. Salchow DJ, Zirm ME, Stieldorf C, Parisi A: Laser in situ keratomileusis for myopia and myopic astigmatism. J Cataract Refract Surg 24:175, 1998 37. McDonald MB, Carr JD, Frantz JM, et al: Laser in situ keratomileusis for myopia up to −11 diopters with up
to −5 diopters of astigmatism with the Summit Autonomous LADARVision
excimer laser system. Ophthalmology 108:309, 2001 38. Zaldivar R, Davidorf JM, Oscherow S: Laser in situ keratomileusis for myopia from −5.50 to −11.50 diopters
with astigmatism. J Refract Surg 14:19, 1998 39. El Danasoury MA, Waring GO, El Maghraby E, Mehrez K: Excimer laser in situ keratomileusis to correct compound myopic astigmatism. J Refract Surg 13:511, 1997 40. Fraenkel GE, Webber SK, Sutton GL, Lawless MA, Rogers CM: Toric laser in situ keratomileusis for myopic astigmatism using an ablatable
mask. J Refract Surg 15:111, 1999 41. Ibrahim O: Laser in situ keratomileusis for hyperopia and hyperopic astigmatism. J Refract Surg 14:S179, 1998 42. Ojeimi G, Waked N: Laser in situ keratomileusis for hyperopia. J Refract Surg 13:S432, 1997 43. Davidorf JM, Eghbali F, Onclinx T, Maloney RK: Effect of varying the optical zone diameter on the results of hyperopic
laser in situ keratomileusis. Ophthalmology 108:1261, 2001 44. Goker S, Er H, Kahvecioglu C: Laser in situ keratomileusis to correct hyperopia from +4.25 to +8.0 diopters. J Refract Surg 14:26, 1998 45. Reviglio VE, Bossana EL, Luna JD, et al: Laser in situ keratomileusis for myopia and hyperopia using the LaserSite 200 laser
in 300 consecutive cases. J Refract Surg 16:716, 2000 46. Salz JJ, Stevens CA, LADARVision LASIK Hyperopia Study Group: LASIK correction of spherical
hyperopia, hyperopic astigmatism, and mixed astigmatism with the LADARVision
excimer laser system. Ophthalmology 109:1647, 2002 47. Arbelaez MC, Knorz MC: Laser in situ keratomileusis for hyperopia and hyperopic astigmatism. J Refract Surg 15:406, 1999 48. Durrie DS, Aziz AA: Lift-flap retreatment after laser in situ keratomileusis. J Refract Surg 15:150, 1999 49. Perez-Santonja JJ, Ayala MJ, Sakla HF, Ruiz-Moreno JM, Alio JL: Retreatment after laser in situ keratomileusis. Ophthalmology 106:21, 1999 50. Hersh PS, Fry KL, Bishop DS: Incidence and associations of retreatment after LASIK. Ophthalmology 110:748, 2003 51. Davis EA, Hardten DR, Lindstrom M, Samuelson TW, Lindstrom RL: LASIK enhancements: a comparison of lifting to recutting the flap. Ophthalmology 109:2308, 2002 52. Netto MV, Wison SE: Flap lift for LASIK retreatment in eyes with myopia. Ophthalmology 111:1362, 2004 53. Carones F, Vigo L, Carones A, Brancato R: Evaluation of photorefractive keratectomy retreatments after regressed
myopic laser in situ keratomileusis. Ophthalmology 108:1732, 2001 54. Weisenthal RW, Salz J, Sugar A, et al: Photorefractive keratectomy for treatment of flap complications in laser
in situ keratomileusis. Cornea 22:399, 2003 55. Ane HA, Swale JA, Majmudar PA: Prophylactic use of mitomycin-C in the management of buttonholed LASIK
flaps. J Cataract Refract Surg 29:390, 2003 56. Soong HK, Dastjerdi MH: Lenticular myopia from oil-droplet cataract: A cautionary note in laser
in situ keratomileusis. J Cataract Refract Surg 30:2438, 2004 57. Lee YC, Hu FR, Wang IJ: Quality of vision after laser in situ keratomileusis. Influence of dioptric
correction and pupil size on vision function. J Catarct Refract Surg 29:769, 2003 58. Boxer Wachler BS: Effect of pupil size on visual function under monocular and binocular conditions
in LASIK and non-LASIK patients. J Cataract Refract Surg 29:275, 2003 59. Nuijts RM, Nabar VA, Hament WJ, Eggink FA: Wavefront-guided versus standard laser in situ keratomileusis to correct
low to moderate myopia. J Cataract Refract Surg 28:1907, 2002 60. Phusitphoykai N, Tungsiripat T, Siriboonkoom J, Vongthongsri A: Comparison of conventional versus wavefront-guided laser in situ keratomileusis
in the same patient. J Refract Surg 19:S217, 2003 61. Durrie DS, Stahl J: Randomized comparison of custom laser in situ keratomileusis with the Alcon
CustomCornea and the Bausch & Lomb Zyoptix systems: One month
results. J Refract Surg 20:S614, 2004 62. Awwad ST, El-Kateb M, Bowman RW, Cavanagh HD, McCulley JP: Wavefront-guided laser in situ keratomileusis with the Alcon CustomCornea
and the VISX Custom Vue: Three month results. J Cataract Refract Surg 20:S606, 2004 63. Slade S: Contralateral comparison of Alcon CustomCornea and VISX Custom Vue wavefront-guided
laser in situ keratomileusis: one month results. J Refract Surg 20:S601, 2004 64. Chalita MR, Xu M, Krueger RR: Alcon CustomCornea wavefront-guided retreatments after laser in situ keratomileusis. J Refract Surg 20:S624, 2004 65. Nakano K, Nakano E, Oliveira M, Portellinha W, Alvarenga L: Intraoperative microkeratome complications in 47,094 laser in situ keratomileusis
surgeries. J Refract Surg 20:S723, 2004 66. Gimbel HV, Anderson Penno EE, van Westenbrugge JA, Ferensowicz M, Furlong MT: Incidence and management of intraoperative and early postoperative complications
in 1000 consecutive laser in situ keratomileusis cases. Ophthalmology 105:1839, 1998 67. Stulting RD, Carr JD, Thompson KP, et al: Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology 106:13, 1999 68. Lin RT, Maloney RK: Flap complications associated with lamellar refractive surgery. Am J Ophthalmol 127:129, 1999 69. Lane HA, Swale JA, Majmudar PA: Prophylactic use of mitomycin-C in the management of a buttonholed LASIK
flap. J Cataract Refract Surg 29:390, 2003 70. Chalita MR, Roth AS, Krueger RR: Wavefront guided surface ablation with prophylactic use of mitomycin C
after a buttonhole laser in situ keratomileusis flap. J Refract Surg 20:176, 2004 71. Joo CK, Kim TG: Corneal perforation during laser in situ keratomileusis. J Cataract Refract Surg 25:1165, 1999 72. Battat L, Macri A, Dursun D, Pflugfelder SC: Effects of laser in situ keratomileusis on tear production, clearance, and
the ocular surface. Ophthalmology 108:1230, 2001 73. Michaeli A, Slomovic AR, Sakhichand K, Rootman DS: Effect of laser in situ keratomileusis on tear secretion and corneal sensitivity. J Refract Surg 20:379, 2004 74. Calvillo MP, McLaren JW, Hofge DO, Bourne WM: Corneal reinnervation after LASIK: Prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci 45:3991, 2004 75. Tanaka M, Takano Y, Dogru M, et al: Effect of preoperative tear function on early functional visual acuity
after laser in situ keratomileusis. J Cataract Refract Surg 30:2311, 2004 76. Jackson DW, Hamill MB, Koch DD: Laser in situ keratomileusis flap suturing to treat recalcitrant flap striae. J Cataract Refract Surg 29:264, 2003 77. Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae
after LASIK. Ophthalmology 111:740, 2004 78. Noack J, Tonnies R, Hohla K, Birngruber R, Vogel A: Influence of ablation plume dynamics on the formation of central islands
in excimer laser photorefractive keratectomy. Ophthalmology 104:823, 1997 79. Oshika T, Klyce SD, Smolek MK, McDonald MB: Corneal hydration and central islands after excimer laser photorefractive
keratectomy. J Cataract Refract Surg 24:1575, 1998 80. Alkara N, Genth U, Seiler T: Diametral ablation: A technique to manage decentered photorefractive keratectomy
for myopia. J Refract Surg 15:436, 1999 81. Lim-Bon-Siong R, Williams JM, Steinert RS, Pepose JS: Retreatment of decentered excimer photorefractive keratectomy ablations. Am J Ophthalmol 123:122, 1997 82. Talamo JH, Wagoner MD, Lee SY: Management of ablation decentration following excimer laser photorefractive
keratectomy. Arch Ophthalmol 113:706, 1995 83. Pallikaris I, Siganos D: LASIK complications management. In The Eximer Manual. Boston: Little, Brown, 1997:227 84. Steinert RF The: 2004 Binkhorst Lecture. The pursuit of perfect vision: Ophthalmology's
Holy Grail? J Cataract Refract Surg 2005, in press 85. Kaufman SC, Maitchouk DY, Chiou AGY, Beuerman RW: Interface inflammation after laser in situ keratomileusis. Sands of the
Sahara syndrome. J Cataract Refract Surg 24:1589, 1998 86. Smith RJ, Maloney RK: Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology 105:1721, 1998 87. Steinert RF, McColgin AZ, White A, Horsburgh GM: Diffuse interface keratitis after LASIK: A non-specific syndrome. Am J Ophthalmol 129:380, 2000 88. Holland SP, Mathias RG, Morck DW, Chiiu J, Slade SG: Diffuse lamellar keratitis related to endotoxins released from sterilizer
reservoir biofilms. Ophthalmology 107:1227, 2000 89. Karp CL, Tuli SS, Yoo SH, et al: Infectious Keratitis after LASIK. Ophthalmology 110:503, 2003 90. Freitas D, Alvarenga L, Sampaio J, et al: An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology 110:276, 2003 91. Asano-Kato N, Toda I, Hori-Komai Y, Takano Y, Tsubota K: Epithelial ingrowth after laser in situ keratomileusis: Clinical features
and possible mechanisms. Am J Ophthalmol 134:801, 2002 92. Rojas MC, Lumba JD, Manche EE: Treatment of epithelial ingrowth after laser in situ keratomileusis with
mechanical debridement and flap suturing. Arch Ophthalmol 122:997, 2004 93. Vajpayee RB, Gupta V, Sharma N: PRK for epithelial ingrowth after LASIK. Cornea 22:259, 2003 94. Rad AS, Jabbarvand M, Saifi N: Progressive keratectasia after laser in situ keratomileusis. J Reefract Surg 20:S718, 2004 95. Randleman JB, Russell B, Ward MA, Thompson DP, Stulting RD: Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 110:267, 2003 96. Ou RJ, Shaw EL, Glasgow BJ: Keratectasia after laser in situ keratomileusis (LASIK): Evaluation
of the calculated residual stromal bed thickness. Am J Ophthalmol 134:771, 2002 97. Pokroy R, Levinger S, Hirsh A: Single Intacs segment for post-laser in situ keratomileusis keratectasia. J Cataract Refract Surg 30:1685, 2004 |